Abstract
The aim of our study was to evaluate the differences in protein and amino acid metabolism after subcutaneous turpentine administration in the soleus muscle (SOL), predominantly composed of red fibres, and the extensor digitorum longus muscle (EDL) composed of white fibres. Young rats (40–60 g) were injected subcutaneously with 0.2 ml of turpentine oil/100 g body weight (inflammation) or with the same volume of saline solution (control). Twenty-four hours later SOL and EDL were dissected and incubated in modified Krebs–Heinseleit buffer to estimate total and myofibrillar proteolysis, chymotrypsin-like activity of proteasome (CHTLA), leucine oxidation, protein synthesis and amino acid release into the medium. The data obtained demonstrate that in intact rats, all parameters measured except protein synthesis are significantly higher in SOL than in EDL. In turpentine treated animals, CHTLA increased and protein synthesis decreased significantly more in EDL. Release of leucine was inhibited significantly more in SOL. We conclude that turpentine-induced inflammation affects more CHTLA, protein synthesis and leucine release in EDL compared to SOL.
Keywords: amino acids, fast-twitch, inflammation, leucine, proteasome, protein synthesis, proteolysis, slow-twitch
There are two basic types of skeletal muscle fibres (I and II) which differ in both physical and biochemical properties due to alternate expression of myosin heavy chain (MHC) isoforms (Rivero et al. 1996). According to the dominantly expressed MHC isoform, they may be further divided into subtypes I, IC, IIC, IIA, IIAD, IID, IIDB and IIB. However, many fibres co-express different MHC isoforms in variable relative amounts, forming a continuum (Sant’ana Pereira et al. 1995). In general, type I fibres have small diameters (Rivero et al. 1998), they contain higher levels of mitochondria and myoglobin, their contraction is more prolonged, their twitch rate is slower (Schiaffino & Reggiani 1996), they have more capillaries than type II fibres do and the main source of energy is blood glucose and free fatty acids. Type II fibres are thicker, they contain higher levels of myosin ATPase, their contraction is stronger and faster, muscle glycogen serves as the basic source of energy and the resistance to fatigue is not so high as in the case of type I fibres. With respect to these properties, type I fibres are also called red, oxidative or slow-twitch fibres and type II fibres white, glycolytic or fast-twitch fibres.
Skeletal muscles have variable numbers of fast-twitch and slow-twitch fibres, the relative amounts are appropriately related to function, training and genetic set (Gollnick et al. 1972; Simoneau & Bouchard 1995; Kernell et al. 1998). The large back muscles, which are necessary for maintaining erect posture or breathing, or leg muscles, used for walking, such as the soleus muscle (SOL), are predominantly composed of slow-twitch fibres (Gupta et al. 1989), which allow them prolonged, steady contractions. In contrast, muscles which ensure delicate and rapid movements, e.g. muscles of the eye or muscles of the fingers, such as the extensor digitorum longus (EDL) muscles, are predominantly composed of fast-twitch fibres (Soukup et al. 2002).
Skeletal muscles are known to serve as the main source of amino acids in various pathological conditions, such as starvation, cancer, sepsis, or trauma providing amino acids, especially for acute-phase protein synthesis and gluconeogenesis. In severe illnesses, the enhanced amino acid release into the circulation is caused by increased proteolysis and decreased protein synthesis, resulting in muscle cachexia. A number of animal models provide evidence of the different responses of SOL and EDL to inflammation, but many of these have important limitations, particularly associated with the trigger for inflammation. Endotoxin administration is usually performed by constant infusion to prevent its rapid elimination from the body (Fish & Spitzer 1984). A high variability of response (Mela-Riker et al. 1988) is a disadvantage of bacteria application. Additionally, if bacteria are injected in bolus form, shock is often developed (Hau & Simmons 1977). Cecal ligation and puncture (CLP) is a complicated and invasive method requiring anaesthesia (Safranek et al. 2006).
It has been demonstrated that a simple and reproducible model, which causes localized inflammation (Laflamme & Rivest 1999), is subcutaneous (s.c.) turpentine administration. This agent has been used to study hormonal changes, hepatic regeneration and production of acute-phase mediators following acute inflammation (Kulkarni et al. 1985; Birch & Schreiber 1986; Woloski & Jamieson 1987; Okajima et al. 1997). To date however, little is known about effect of turpentine-induced inflammation on protein and amino acid metabolism in various types of skeletal muscle.
Considering the different fibre compositions and correspondingly different functions and metabolic features of SOL and EDL, different responses of SOL and EDL during turpentine-induced inflammation should be expected. The aim of our study was to evaluate the differences in protein and amino acid metabolism after s.c. turpentine administration in SOL (slow-twitch, red muscle) and EDL (fast-twitch, white muscle).
Methods
Animals
Male Wistar rats (body weight 40–60 g) obtained from BioTest (Konarovice, Czech Republic) were used in this study. The rats were housed under controlled conditions (12-h light-dark cycle, 22 °C, 55–65% relative humidity) with free access to standard laboratory chow and water. All procedures involving animals were performed according to guidelines set by the Institutional Animal Use and Care Committee of Charles University, Prague, Czech Republic.
Materials
l-[1-14C]Leucine was purchased from MP Biomedicals (Irvine, CA, USA); amino acids, cycloheximide, Folin–Ciocalteu’s phenol reagent, turpentine oil, LLVY-MCA and albumin from Sigma Chemical (St Louis, MO, USA); hydroxide of hyamine from Packard Instruments (Meriden, CT, USA); Aminoplasmal 15 from B. Braun Medicals (Melsungen, Germany); MG-132 from Biomol (Hamburg, Germany). Other chemicals were purchased from Sigma Chemicals, Waters (Milford, MA, USA) and Lachema (Brno, Czech Republic).
Experimental design
The rats were injected by 0.2 ml of turpentine/100 g body weight in the dorsolumbar area. Control animals were injected with the same volume of 0.9% saline solution. After 24 h, the rats were anaesthetized with pentobarbital (6 mg/100 g body weight, intraperitoneally) and blood was withdrawn from the bifurcation of aorta. Administration of turpentine oil s.c. causes localized inflammation via necrosis (Wusteman et al. 1990). The dose of turpentine was determined on the basis of the study of Wusteman et al. (1990). The time interval was selected according to the experiments of Zarrabian et al. (1998) and Birch and Schreiber (1986) who reported the maximal levels of several acute phase proteins after 24 h following turpentine insult. SOL and EDL muscles of both legs were dissected according to Maizels et al. (1977). Isolated muscles were fixed to stainless steel clips to provide slight tension and immediately transferred to 2.5 ml of modified Krebs–Heinseleit bicarbonate buffer with 6 mm glucose and 2 mU/ml insulin (pH 7.4, 37 °C). Other components present in the medium were dependent on the measured parameter [protein synthesis and leucine oxidation or proteolysis and chymotrypsin-like activity (CHTLA) of proteasome]. The medium was saturated with O2/CO2 (19:1). Muscles were preincubated for 30 min in a thermostatically controlled bath (37 °C) with a shaking device (70 cycles/min). After preincubation, muscles were quickly rinsed in 0.9% NaCl, blotted and transferred to a second set of vials containing fresh incubation media identical in composition and volume with the preincubation one. The viability of incubated muscles was previously confirmed in our laboratory (Safranek et al. 2003) as well as by other authors (Fang et al. 2005). Muscles from the right leg were always used for proteolysis and CHTLA determination, muscles from the left leg for protein synthesis and leucine oxidation assay.
Protein synthesis and leucine oxidation
Protein synthesis and leucine oxidation was measured after 1-h incubation of the muscle in 2.5 ml of medium enriched with amino acids in approximately physiological concentrations (Aminoplasmal 15 with added glutamine and tyrosine, total concentration 2.8 mm) and l-[1-14C]leucine (0.6 μCi/ml). At the end of the incubation period, 0.4 ml of hydroxide of hyamine was added to the well hanging above the incubation medium, the reaction was stopped by the addition of 35% (v/v) perchloric acid solution (0.2 ml) into the incubation medium and the flasks were shaken for 1 h to ensure complete absorption of 14CO2 into the hyamine hydroxide. Muscles were removed from the incubation flasks, quickly rinsed in cold 6% (v/v) HClO4, blotted, and homogenized in 0.6 ml of 6% (v/v) HClO4. The homogenate was centrifuged for 5 min at 12,000 g and the pellet was used for other measurements. l-[1-14C]Leucine incorporation into precipitated proteins was estimated after their hydrolysis in 1 m NaOH. Protein synthesis and leucine oxidation was calculated using leucine specific activity in the incubation medium and expressed as nmol of incorporated leucine/g protein/h and nmol of oxidized leucine/g wet weight/h respectively. The radioactivity of the samples was measured with the liquid scintillation radioactivity counter LS 6000 (Beckman Instruments, CA, USA). The protein content of the samples was estimated according to Lowry et al. (1951).
Total and myofibrillar proteolysis
Total and myofibrillar protein breakdown was estimated after 2-h incubation of muscle in medium enriched with cycloheximide (0.5 mm), which prevented the reincorporation of released amino acids into proteins. As tyrosine is not metabolized in the muscle, the amount released into the medium reflects total proteolysis in the muscle. The amount of 3-methylhistidine (3MH), a characteristic product of myofibrillar breakdown, released into the medium served for myofibrillar proteolysis calculation. The rates of amino acid release were estimated on the basis of their appropriate concentrations in the medium and weight of muscle. 3MH was quantified using a high performance liquid chromatography (HPLC) method (Waters) based on reaction with fluorescamine (Wassner et al. 1980; Lowell et al. 1986); other amino acid concentrations in the medium were determined by the HPLC method after precolumn derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (Cohen & Michaud 1993; Reverter et al. 1997).
Chymotrypsin-like activity of proteasome
The CHTLA of proteasome was determined using fluorogenic substrate SucLLVY-MCA (0.1 mm) according to the method of Gomes-Marcondes and Tisdale (2002) with the following modifications. Muscles were homogenized after incubation and the homogenate was centrifuged for 10 min at 18,000 g at 4 °C. Cellular supernatant (0.1 ml) was incubated with substrate (0.05 mm) in a total volume of 0.2 ml, with and without inhibitor, MG-132 (0.02 mm), for 1 h on ice. Fluorescence was determined with an excitation wavelength of 340 nm and an emission wavelength of 440 nm (Perkin Elmer luminescence spectrometer LS 50B). The standard curve was established for 7-amino-4-methylcoumarin (AMC) which permitted the expression of CHTLA activity as nmol AMC/g protein/h. The activity was adjusted for the protein concentration of the supernatant estimated according to Lowry et al. (1951). The differences after subtraction of inhibited from non-inhibited activities were used for the calculations.
Statistical analysis
Results are expressed as the mean ± SEM. F-test and two-sample t-test or anova followed by Tukey–Kramer test (statistical software ncss 2001; NCSS Raysville, UT, USA) were used for analysis of the data. Differences were considered significant at P < 0.05.
Results
Protein synthesis and leucine oxidation
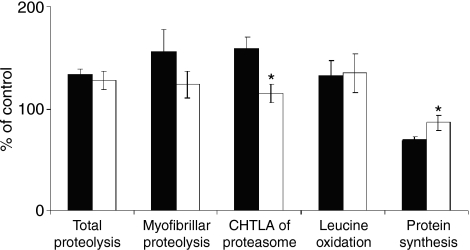
Leucine oxidation was significantly higher in SOL of both intact and turpentine treated rats compared to EDL. The difference in protein synthesis between SOL and EDL of intact animals was not significant while in SOL of turpentine treated animals, this parameter was significantly higher. There were no statistical differences between control and turpentine treated rats neither in SOL nor in EDL (Table 1). The relative decrease in protein synthesis in turpentine group against the control group was significantly higher in EDL than in SOL while leucine oxidation changes were not statistically significant (Figure 1).
Table 1.
Basic parameters of protein and amino acid metabolism in EDL and SOL
| Control | Turpentine | |||
|---|---|---|---|---|
| EDLc (n = 7) | SOLc (n = 7) | EDLt (n = 7) | SOLt (n = 7) | |
| Total proteolysis (nmol TYR/g wet wt/h) | 109 ± 6 | 171 ± 7* | 145 ± 5 | 219 ± 16†‡ |
| Myofibrillar proteolysis (nmol 3MH/g wet wt/h) | 0.542 ± 0.033 | 1.167 ± 0.086* | 0.845 ± 0.119 | 1.447 ± 0.157† |
| CHTLA of proteasome (nmol AMC/g of protein/h) | 1034 ± 71 | 2125 ± 125* | 1640 ± 106 | 2436 ± 174† |
| Leucine oxidation (nmol LEU/g wet wt/h) | 45 ± 3 | 86 ± 7* | 59 ± 9 | 116 ± 12† |
| Protein synthesis (nmol LEU/g of protein/h) | 2870 ± 136 | 3269 ± 166 | 1982 ± 77 | 2804 ± 271† |
SOL, the soleus muscle; EDL, the extensor digitorum longus muscle; TYR, tyrosine; 3MH, 3-methylhistidine; CHTLA, chymotrypsin-like activity; AMC, 7-amino-4-methylcoumarin; LEU, leucine.
Values are expressed as mean ± SEM.
anova followed by Tukey–Kramer test.
P < 0.05 SOLcvs. EDLc.
P < 0.05 SOLtvs. EDLt.
P < 0.05 SOLtvs. SOLc.
Figure 1.
Relative effect of inflammation on basic parameters of protein and amino acid metabolism in EDL (filled bars) and SOL (open bars). Means of appropriate control values were used as 100%. Values are means ± SEM, n = 7 in each group. F-test followed by two-sample t-test; •P < 0.05 vs. EDL. SOL, the soleus muscle; EDL, the extensor digitorum longus muscle; CHTLA, chymotrypsin-like activity.
Total and myofibrillar proteolysis
Total and myofibrillar proteolysis was significantly higher in SOL of both intact and turpentine treated rats compared to EDL. Application of turpentine oil caused significant increase in total proteolysis in SOL only. Myofibrillar proteolysis in the septic group remained unchanged against control in both types of muscle (Table 1). Relative changes in total and myofibrillar proteolysis in the turpentine group against the control were not statistically significant in both SOL and EDL (Figure 1).
Chymotrypsin-like activity of proteasome
The CHTLA of proteasome was statistically higher in SOL of both intact and turpentine treated rats compared to EDL. We detected no significant changes between the control and septic groups (Table 1). However, the relative increase in CHTLA in EDL of septic rats against control was statistically higher than in SOL (Figure 1).
Amino acid release into the incubation medium
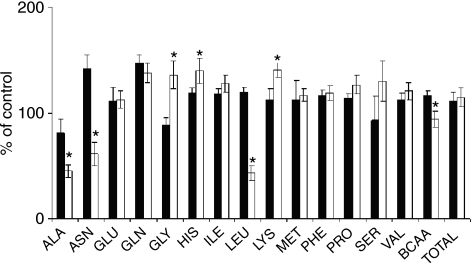
Table 2 represents rates of amino acid release from SOL and EDL of turpentine treated and control animals. Most amino acids shared a significantly greater release into the medium from SOL than from EDL in both experimental groups. In SOL of turpentine treated animals, we observed a significant decrease in leucine release compared to the control while EDL was not affected. Figure 2 demonstrates relative changes in amino acid release from EDL and SOL of turpentine treated rats against the control. In EDL, release of asparagine, leucine and BCAA (branched chain amino acid – valine, leucine, isoleucine) increased while decreasing in SOL, resulting in a significant difference. In contrast, release of glycine decreased in EDL and increased in SOL. In SOL, release of alanine decreased while release of histidine and lysine increased significantly more than in EDL.
Table 2.
Release of amino acids into the medium (nmol/g wet wt/h)
| Control | Turpentine | |||
|---|---|---|---|---|
| EDLc (n = 7) | SOLc (n = 7) | EDLt (n = 7) | SOLt (n = 7) | |
| ALA | 739 ± 117 | 856 ± 108 | 595 ± 22 | 471 ± 25 |
| ASN | 105 ± 19 | 196 ± 27* | 150 ± 11 | 135 ± 13 |
| GLU | 130 ± 23 | 330 ± 37* | 143 ± 18 | 370 ± 36† |
| GLN | 1038 ± 139 | 1541 ± 218* | 1517 ± 72 | 2116 ± 176† |
| GLY | 807 ± 197 | 785 ± 102 | 712 ± 57 | 1063 ± 123† |
| HIS | 143 ± 13 | 202 ± 21 | 170 ± 7 | 282 ± 29† |
| ILE | 132 ± 20 | 208 ± 17* | 156 ± 6 | 266 ± 21† |
| LEU | 261 ± 32 | 413 ± 32* | 311 ± 11 | 232 ± 18†‡ |
| LYS | 360 ± 40 | 417 ± 30 | 404 ± 29 | 584 ± 39† |
| MET | 73 ± 8 | 125 ± 10* | 83 ± 12 | 146 ± 8† |
| PHE | 142 ± 19 | 222 ± 17* | 167 ± 7 | 264 ± 20† |
| PRO | 282 ± 21 | 359 ± 14* | 319 ± 14 | 457 ± 42† |
| SER | 376 ± 51 | 570 ± 39* | 352 ± 76 | 740 ± 108† |
| VAL | 217 ± 30 | 332 ± 23* | 245 ± 11 | 401 ± 33† |
| BCAA | 610 ± 82 | 953 ± 72* | 712 ± 28 | 899 ± 72† |
| TOTAL | 4805 ± 729 | 6556 ± 695* | 5324 ± 353 | 7527 ± 691† |
SOL, the soleus muscle; EDL, the extensor digitorum longus muscle; BCAA, branched chain amino acids (valine, leucine, isoleucine); TOTAL, sum of all measured amino acids; ALA, ASN, GLU, GLN, GLY, HIS, ILE, LEU, LYS, MET, PHE, PRO, SER, VAL – standard amino acid abbreviations.
Values are expressed as mean ± SEM.
anova followed by Tukey–Kramer test.
P < 0.05 SOLcvs. EDLc.
P < 0.05 SOLtvs. EDLt.
P < 0.05 SOLtvs. SOLc
Figure 2.
Relative effect of inflammation on amino acid release from EDL (filled bars) and SOL (open bars). Means of appropriate control values were used as 100%. Values are means ± SEM, n = 7 in each group. F-test followed by two-sample t-test; •P < 0.05 vs. EDL. SOL, the soleus muscle; EDL, the extensor digitorum longus muscle; BCAA, branched chain amino acids (valine, leucine, isoleucine); TOTAL, sum of all measured amino acids; ALA, ASN, GLU, GLN, GLY, HIS, ILE, LEU, LYS, MET, PHE, PRO, SER, VAL – standard amino acid abbreviations.
Discussion
We found that total proteolysis during inflammation increased comparably in SOL (28%) and EDL (34%), increase in myofibrillar proteolysis in SOL (24%) was lower than in EDL (56%). The differences in myofibrillar proteolysis could reflect the changes in CHTLA of proteasome which increased by 14% in SOL and 58% in EDL, while the total proteolysis could be influenced by other (e.g. lysosomal) mechanisms. Myofibrils cannot be cleaved by the proteasome itself (Salomon & Goldberg 1996). However, various catabolic conditions activate other proteases (e.g. caspase-3), which disintegrate myofibrils and make the actin and myosin accessible for proteasomal cleaving (Du et al. 2005). Our findings are in agreement with those of Hasselgren et al. (1989) who demonstrated that an increase in total and myofibrillar proteolysis during sepsis is significantly more pronounced in EDL than in SOL. Similar results were published by Tiao et al. (1997) and also after burn injury (Fang et al. 1998). We suggest that different response of EDL and SOL to inflammation could be caused by different sensitivities of white and red fibres to various humoral factors, e.g. hormones (Larbaud et al. 2001) and cytokines (Fang et al. 1995). In addition, glucocorticoids (Wang et al. 1998), proteolysis inducing factor-PIF (Lorite et al. 2001) and cytokines (Li et al. 1998) comprise some of the most potent substances which are able to affect the expression of genes of the ATP-dependent ubiquitin-proteasome system.
In agreement with the enhanced catabolic activity, the release of amino acids from muscle to incubation medium during inflammation tended to increase in both SOL and EDL. Considering the important role of leucine in muscle energy and protein metabolism, the relative changes in its release are very interesting. In SOL, the release of leucine decreased while in EDL, it increased in response to turpentine treatment, resulting in a significant difference. The decrease in leucine release in SOL was observed without a corresponding increase in leucine oxidation. We hypothesize that leucine accumulates in the muscle, and/or that its intermediate –α-ketoisocaproate – is more released into the medium or transformed to β-hydroxy-β-methylbutyrate. This suggestion is partly based on an observation of increased leucine intramuscular concentration after stress hormone administration (Hammarqvist et al. 2001).
In our study, we observed a significant difference between the decrease in protein synthesis in EDL (31%) and SOL (14%) following turpentine treatment. Our findings are in agreement with those of Vary & Kimball (1992), who observed that the protein synthetic rate in fast-twitch muscle was reduced by sepsis, whereas slow-twitch muscles were not affected. With regard to changes in CHTLA of proteasome in SOL and EDL during inflammation, it may be suggested that the decrease in protein synthesis is related to the changes in activity of the proteasomal system. This suggestion is based on findings that some translation initiation factors, such as eIF4G or eIF3a are cleaved in the proteasome (Baugh & Pilipenko 2004). Moreover, as the removal of leucine reduces protein synthesis through changes in both eIF2B and eIF4E (Vary et al. 1999) and leucine is unique among the BCAA in its ability to stimulate protein synthesis in muscle (Anthony et al. 2000), we suggest that possible cumulation of leucine in SOL could affect protein synthesis.
Total proteolysis increased and leucine release decreased significantly while other parameters did not change in SOL of septic rats against control. EDL remained unaffected. As reported by Chai et al. (2002) significant increase in muscle proteolysis following burning was connected with rise of mRNA of several proteasomal subunits. Additionally, the protesomal complex is highly stimulated by some cytokines. Chai et al. (2003) detected increases in protesomal subunit expression and enhanced TNF-α and IL-6 levels after Escherichia coli endotoxin administration. Finally, Bazel et al. (1999) found, that CLP increases IL-6 levels in liver while s.c. turpentine application does not. With regard to protein synthesis, there is no agreement considering the effect of inflammation on skeletal muscle. Kadlcikova et al. (2004) observed decrease in protein synthesis in skeletal muscle after CLP while Hasselgren et al. (1986) and Wusteman et al. (1990) reported no significant changes in protein synthesis in skeletal muscle during sepsis after CLP or turpentine administration respectively.
We conclude that there are differences in protein and amino acid metabolism between SOL (slow, red muscle) and EDL (fast, white muscle) both in physiological conditions and after turpentine administration. The rate of catabolic and synthetic reactions is higher in SOL than in EDL. During inflammation, CHTLA of proteasome and protein synthesis are less affected in SOL compared to EDL, as well as the rate of release of leucine and some other amino acids.
Acknowledgments
This study was funded by Research Project MSM 0021620820. We are grateful for the methodical consultation to J. Chladek, MSc, PhD, for the proof reading to C. McGrath, PhD and for the technical support to R. Rysava and H. Buzkova.
References
- Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J. Nutr. 2000;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- Baugh JM, Pilipenko EV. 20S proteasome differentially alters translation of different mRNAs via the cleavage of eIF4F and eIF3. Mol. Cell. 2004;16:575–586. doi: 10.1016/j.molcel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Bazel S, Andrejko KM, Chen J, Deutschman CS. Hepatic gene expression and cytokine responses to sterile inflammation: comparison with cecal ligation and puncture sepsis in the rat. Shock. 1999;11:347–355. [PubMed] [Google Scholar]
- Birch HE, Schreiber G. Transcriptional regulation of plasma protein synthesis during inflammation. J. Biol. Chem. 1986;261:8077–8080. [PubMed] [Google Scholar]
- Chai J, Wu Y, Sheng ZZ. The relationship between skeletal muscle proteolysis and ubiquitin - proteasome proteolytic pathway in burned rats. Burns. 2002;28:527–533. doi: 10.1016/s0305-4179(02)00049-9. [DOI] [PubMed] [Google Scholar]
- Chai J, Wu Y, Sheng ZZ. Role of ubiquitin-proteasome pathway in skeletal muscle wasting in rats with endotoxemia. Crit. Care Med. 2003;31:1802–1807. doi: 10.1097/01.CCM.0000069728.49939.E4. [DOI] [PubMed] [Google Scholar]
- Cohen SA, Michaud DP. Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal. Biochem. 1993;211:279–287. doi: 10.1006/abio.1993.1270. [DOI] [PubMed] [Google Scholar]
- Du J, Hu Z, Mitch WE. Molecular mechanisms activating muscle protein degradation in chronic kidney disease and other catabolic conditions. Eur. J. Clin. Invest. 2005;35:157–163. doi: 10.1111/j.1365-2362.2005.01473.x. [DOI] [PubMed] [Google Scholar]
- Fang CH, James HJ, Ogle C, Fischer JE, Hasselgren PO. Influence of burn injury on protein metabolism in different types of skeletal muscle and the role of glucocorticoids. J. Am. Coll. Surg. 1995;180:33–42. [PubMed] [Google Scholar]
- Fang CH, Li BG, Tiao G, Wang JJ, Fischer JE, Hasselgren PO. The molecular regulation of protein breakdown following burn injury is different in fast- and slow-twitch skeletal muscle. Int. J. Mol. Med. 1998;1:163–169. doi: 10.3892/ijmm.1.1.163. [DOI] [PubMed] [Google Scholar]
- Fang CH, Li BG, James JH, et al. Protein breakdown in muscle from burned rats is blocked by insulin-like growth factor and glycogen synthase kinase-3beta inhibitors. Endocrinology. 2005;146:3141–3149. doi: 10.1210/en.2004-0869. [DOI] [PubMed] [Google Scholar]
- Fish RE, Spitzer JA. Continuous infusion of endotoxin from an osmotic pump in the conscious unrestrained rat: a unique model of endotoxemia. Circ. Shock. 1984;12:135–149. [PubMed] [Google Scholar]
- Gollnick PD, Armstrong RB, Saubert CW, IV, Piehl K, Saltin B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J. Appl. Physiol. 1972;33:312–319. doi: 10.1152/jappl.1972.33.3.312. [DOI] [PubMed] [Google Scholar]
- Gomes-Marcondes MCC, Tisdale MJ. Induction of protein catabolism and the ubiquitin-proteasome pathway by mild oxidative stress. Cancer Lett. 2002;180:69–74. doi: 10.1016/s0304-3835(02)00006-x. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Misulis KE, Dettbarn WD. Activity dependent characteristics of fast and slow muscle: biochemical and histochemical considerations. Neurochem. Res. 1989;14:647–655. doi: 10.1007/BF00964874. [DOI] [PubMed] [Google Scholar]
- Hammarqvist F, Ejesson B, Wernerman J. Stress hormones initiate prolonged changes in the muscle amino acid pattern. Clin. Physiol. 2001;21:44–50. doi: 10.1046/j.1365-2281.2001.00291.x. [DOI] [PubMed] [Google Scholar]
- Hasselgren PO, Talamini M, James JH, Fischer JE. Protein metabolism in different types of skeletal muscle during early and late sepsis in rats. Arch. Surg. 1986;121:918–923. doi: 10.1001/archsurg.1986.01400080064011. [DOI] [PubMed] [Google Scholar]
- Hasselgren PO, James JH, Benson DW, et al. Total and myofibrillar protein breakdown in different types of rat skeletal muscle: effects of sepsis and regulation by insulin. Metabolism. 1989;38:634–640. doi: 10.1016/0026-0495(89)90100-5. [DOI] [PubMed] [Google Scholar]
- Hau T, Simmons RL. Animal models of peritonitis. Surg. Gynecol. Obstet. 1977;144:755–756. [PubMed] [Google Scholar]
- Kadlcikova J, Holecek M, Safranek R, Tilser I, Kessler BM. Effects of proteasome inhibitors MG132, ZL3VS and AdaAhx3L3VS on protein metabolism in septic rats. Int. J. Exp. Pathol. 2004;85:365–371. doi: 10.1111/j.0959-9673.2004.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D, Hensbergen E, Lind A, Eerbeek O. Relation between fibre composition and daily duration of spontaneous activity in ankle muscles of the cat. Arch. Ital. Biol. 1998;136:191–203. [PubMed] [Google Scholar]
- Kulkarni AB, Reinke R, Feigelson P. Acute phase mediators and glucocorticoids elevate α1-acid glycoprotein gene transcription. J. Biol. Chem. 1985;260:15386–15389. [PubMed] [Google Scholar]
- Laflamme N, Rivest S. Effects of systemic immunogenic insults and circulating proinflammatory cytokines on the transcription of the inhibitory factor kappaB alpha within specific cellular populations of the rat brain. J. Neurochem. 1999;73:309–321. doi: 10.1046/j.1471-4159.1999.0730309.x. [DOI] [PubMed] [Google Scholar]
- Larbaud D, Balage M, Taillandier D, Combaret L, Grizard J, Attaix D. Differential regulation of the lysosomal, Ca2+-dependent and ubiquitin/proteasome-dependent proteolytic pathways in fast-twitch and slow-twitch rat muscle following hyperinsulinaemia. Clin. Sci. 2001;101:551–558. [PubMed] [Google Scholar]
- Li Y-P, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumour necrosis factor alpha. FASEB J. 1998;12:871–880. doi: 10.1096/fasebj.12.10.971. [DOI] [PubMed] [Google Scholar]
- Lorite MJ, Smith HJ, Arnold JA, Morris A, Thompson MG, Tisdale MJ. Activation of ATP-ubiquitin-dependent proteolysis in skeletal muscle in vivo and murine myoblasts in vitro by a proteolysis-inducing factor (PIF) Br. J. Cancer. 2001;85:297–302. doi: 10.1054/bjoc.2001.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Ruderman NB, Goodman MN. Regulation of myofibrillar protein degradation in rat skeletal muscle during brief and prolonged starvation. Metabolism. 1986;35:1121–1127. doi: 10.1016/0026-0495(86)90025-9. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maizels EZ, Ruderman NB, Goodman MN, Lau D. Effect of acetoacetate on glucose metabolism in the soleus and extensor digitorum longus muscles of the rat. Biochem. J. 1977;162:557–568. doi: 10.1042/bj1620557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mela-Riker L, Alexander P, Bartos D, et al. Chronic hyperdynamic sepsis in the rat: I. Characterization of the animal model. Circ. Shock. 1988;25:231–244. [PubMed] [Google Scholar]
- Okajima A, Miyazawa K, Naitoh Y, Inoue K, Kitamura N. Induction of hepatocyte growth factor activator messenger RNA in the liver following tissue injury and acute inflammation. Hepatology. 1997;25:97–102. doi: 10.1053/jhep.1997.v25.pm0008985272. [DOI] [PubMed] [Google Scholar]
- Reverter M, Lundh T, Lindberg JA. Determination of free amino acids in pig plasma by precolumn derivatization with 6-N- hydroxysuccinimidyl carbamate and high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1997;696:1–8. doi: 10.1016/s0378-4347(97)00217-x. [DOI] [PubMed] [Google Scholar]
- Rivero JL, Talmadge RJ, Edgerton VR. Correlation between myofibrillar ATPase activity and myosin heavy chain composition in equine skeletal muscle and the influence of training. Anat. Rec. 1996;246:195–207. doi: 10.1002/(SICI)1097-0185(199610)246:2<195::AID-AR6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Rivero JL, Talmadge RJ, Edgerton VR. Fibre size and metabolic properties of myosin heavy chain-based fibre types in rat skeletal muscle. J. Muscle Res. Cell Motil. 1998;19:733–742. doi: 10.1023/a:1005482816442. [DOI] [PubMed] [Google Scholar]
- Safranek R, Holecek M, Kadlcikova J, et al. Effect of acute acidosis on protein and amino acid metabolism in rats. Clin. Nutr. 2003;22:437–443. doi: 10.1016/s0261-5614(03)00041-4. [DOI] [PubMed] [Google Scholar]
- Safranek R, Holecek M, Sispera L, Muthny T. Aspects of protein and amino acid metabolism in a model of severe glutamine deficiency in sepsis. Ann. Nutr. Metab. 2006;50:361–367. doi: 10.1159/000094300. [DOI] [PubMed] [Google Scholar]
- Salomon V, Goldberg AL. Importance of the ATP-ubiquitin-proteasome pathway in degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J. Biol. Chem. 1996;271:26690–26697. doi: 10.1074/jbc.271.43.26690. [DOI] [PubMed] [Google Scholar]
- Sant’ana Pereira JA, Wessels A, Nijtmans L, Moorman AF, Sargeant AJ. New method for the accurate characterization of single human skeletal muscle fibres demonstrates a relation between mATPase and MyHC expression in pure and hybrid fibre types. J. Muscle Res. Cell Motil. 1995;16:21–34. doi: 10.1007/BF00125307. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol. Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Simoneau JA, Bouchard C. Genetic determinism of fiber type proportion in human skeletal muscle. FASEB J. 1995;9:1091–1095. doi: 10.1096/fasebj.9.11.7649409. [DOI] [PubMed] [Google Scholar]
- Soukup T, Zacharova G, Smerdu V. Fibre type composition of soleus and extensor digitorum longus muscles in normal female inbred Lewis rats. Acta Histochem. 2002;104:399–405. doi: 10.1078/0065-1281-00660. [DOI] [PubMed] [Google Scholar]
- Tiao G, Lieberman M, Fischer JE, Hasselgren PO. Intracellular regulation of protein degradation during sepsis is different in fast- and slow-twitch muscle. Am. J. Physiol. 1997;272(3 Pt 2):R849–R856. doi: 10.1152/ajpregu.1997.272.3.R849. [DOI] [PubMed] [Google Scholar]
- Vary TC, Kimball SR. Sepsis-induced changes in protein synthesis: differential effects on fast- and slow-twitch muscles. Am. J. Physiol. 1992;262(6 Pt 1):C1513–C1519. doi: 10.1152/ajpcell.1992.262.6.C1513. [DOI] [PubMed] [Google Scholar]
- Vary TC, Jefferson LS, Kimball SR. Amino acid-induced stimulation of translation initiation in rat skeletal muscle. Am. J. Physiol. 1999;277(6 Pt 1):E1077–E1086. doi: 10.1152/ajpendo.1999.277.6.E1077. [DOI] [PubMed] [Google Scholar]
- Wang L, Lus G-J, Wang JJ, Hasselgren PO. Dexamethasone stimulates proteasome- and calcium-dependent proteolysis in cultured L6 myotubes. Shock. 1998;10:298–306. doi: 10.1097/00024382-199810000-00011. [DOI] [PubMed] [Google Scholar]
- Wassner SJ, Schlitzer L, Li JB. A rapid, sensitive method for the determination of 3-methylhistidine levels in urine and plasma using high-pressure liquid chromatography. Anal. Biochem. 1980;104:284–289. doi: 10.1016/0003-2697(80)90076-7. [DOI] [PubMed] [Google Scholar]
- Woloski BMRNJ, Jamieson JC. Rat corticotrophin, insulin and thyroid hormone levels during the acut phase response to inflammation. Comp. Biochem. Physiol. 1987;86:15–19. doi: 10.1016/0300-9629(87)90269-6. [DOI] [PubMed] [Google Scholar]
- Wusteman M, Wight DG, Elia M. Protein metabolism after injury with turpentine: a rat model for clinical trauma. Am. J. Physiol. 1990;259(6 Pt 1):E763–E769. doi: 10.1152/ajpendo.1990.259.6.E763. [DOI] [PubMed] [Google Scholar]
- Zarrabian S, Attaix D, Marcy J, et al. Effects of alimentary whole proteins versus their small peptide hydrolysates on liver and skeletal muscle during the acute inflammation phase in the rat. Clin. Nutr. 1998;17:169–176. doi: 10.1016/s0261-5614(98)80053-8. [DOI] [PubMed] [Google Scholar]