Abstract
Azathioprine (AZA) is a cytotoxic immunosuppressive drug used in the prevention of rejection in organ transplants and the treatment of auto-immune diseases. However, AZA is haemotoxic causing significant bone marrow depression. The present studies were to characterize the haemotoxicity of AZA in the female CD-1 mouse. In Experiment 1, a dose-ranging study, with AZA gavaged daily for 10 days, clinical evidence of toxicity was evident at 125 mg/kg and above. Experiment 2 was a dose–response study with AZA gavaged daily for 10 days at 40–120 mg/kg. At day 1 after the final dose, AZA induced a dose-related pancytopaenia, reduced femoral marrow cellularity, increases in serum levels of the cytokine fms-like tyrosine kinase 3 ligand, reduction in granulocyte-monocyte colony-forming units and erythroid colonies, and increased bone marrow apoptosis. Histology demonstrated hepatocyte hypertrophy, thymic atrophy, reduced splenic extramedullary haemopoiesis, and reduced cellularity of sternal bone marrow. In Experiment 3, AZA was dosed for 10 days at 100 mg/kg with autopsies at 1, 3, 9, 22, 29, 43 and 57 days postdosing. At 1, 3 and 9 days, haematological parameters reflected changes in Experiment 2. At 22/29 days, many blood parameters were returning towards normal; at 43/57 days, most parameters compared with controls. However, there was some evidence of a persistent (i.e. residual/late-stage) mild reduction in RBC and erythroid progenitor cell counts at day 43/57. We conclude that the CD-1 mouse provides an acceptable model for the haemotoxicity of AZA in man.
Keywords: azathioprine, haemotoxicity, mouse, myelotoxicity, toxicity
Purine analogues, first synthesized in the early 1940s, were initially developed for therapeutic use in diseases of a proliferative nature, and particularly the leukaemias (Elion et al. 1960, 1961; Rundles et al. 1961; Elion 1989), and recently these agents have been successfully used in a wide range of leukaemic conditions, e.g. acute myelocytic leukaemia, acute lymphoblastic leukaemia, acute granulocytic leukaemia and chronic granulocytic leukaemia (Bostrom & Erdmann 1993; Dollery 1999). The original mechanistic rationale for developing these agents [e.g. 6-mercaptopurine (6-MP) and 6-thioguanine nucleotides (6-TGNs)] was to interfere with DNA synthesis and thus hinder cell proliferation. The pro-drug, azathioprine (AZA; B.W. 57–322; Imuran; 6(1-methyl-4-nitro-5-imidazolyl)thiopurine) was developed after 6-MP and 6-TGN as a slow-release form of 6-MP by the addition of a nitro-imidazole side chain to the 6-MP molecule (Elion et al. 1961; Rundles et al. 1961; Elion 1989). The pharmacological effects of AZA are therefore associated with the cleavage of the drug in vivo to 6-MP, principally by red blood cell glutathione (Marino & Doyle 1994).
Following these initial investigations, it was subsequently discovered that 6-MP and AZA were each capable of inhibiting the immune response of rabbits injected with foreign antigens, as the animals were unable to mount an immune response to the administered antigen (Schwartz et al. 1958; Schwartz & Dameshek 1959). Also, both 6-MP and later AZA, were found to modulate the immune response and were capable of preventing the rejection of renal homografts in the dog (Calne 1960, 1961; Calne et al. 1962). The successful transplantation of kidneys to unrelated human recipients was first reported in 1961/1962, with regimens of immunosuppression consisting of AZA and corticosteriods (e.g. prednisone) (Goodwin et al. 1963; Murray et al. 1963; Elion 1989). In this way the therapeutic use of AZA revolutionized the practice of organ transplantation by preventing graft rejection. This, in turn, led to the rapid development of kidney and other organ transplantation techniques and opened up the possibility of the successful transplantation of other organs and tissues, e.g. liver, heart and lung (Elion 1989; Marino & Doyle 1994).
For approximately 20 years, AZA, in combination with corticosteriods, remained the principal post-transplantation immunosuppressant treatment until the discovery of cyclosporin A (CsA) (Borel et al. 1976). CsA was introduced into clinical practice in 1979 (Calne et al. 1979; Calne 2004) following experiments with renal transplants in the dog and with orthotopic heart transplants in the pig (Calne et al. 1978). CsA, in turn, had a profound effect in the field of organ transplantation by reducing the incidence of graft rejection and increasing long-term survival, again when used in combination with corticosteriods (Calne 2004).
However, today, AZA remains widely used as an immunosuppressant in transplant recipients, particularly for the prevention of renal graft rejection, and is also employed in cardiac and hepatic transplantation (Elion 1989; MC 2003; Sweetman 2005); for these indications AZA is generally used in combination with corticosteriods. Furthermore, AZA is also frequently prescribed as a steroid sparing agent in auto-immune diseases and in conditions that are considered to have an auto-immune component. For example, AZA is used today in the treatment of blood disorders (e.g. auto-immune haemolytic anaemia and thrombocytopenic purpura), connective tissue/muscular disorders (e.g. systemic lupus erythematosus and polymyositis), inflammatory bowel disease (e.g. Crohn’s disease and ulcerative colitis), liver disorders (e.g. chronic active hepatitis and primary biliary cirrhosis), and lung disorders (e.g. cryptogenic fibrosing alveolitis); AZA is also currently used in rheumatoid arthritis, myasthenia gravis, multiple sclerosis, severe psoriasis and psoriatic arthritis (Dollery 1999; MC 2003; BNF 2004; Sweetman 2005). The dose level of AZA generally recommended for the prevention of rejection in organ and tissue transplantation is 1–5 mg/kg daily, and in the treatment of auto-immune diseases the dose of 1–3 mg/kg daily is often used (Dollery 1999; MC 2003; Sweetman 2005).
The therapeutic use of AZA is, however, not without complications. AZA induces a range of toxic effects that may ultimately result in the discontinuation of treatment. These changes include gastrointestinal disturbances, pancreatitis, reversible alopecia, rashes, muscle and joint pains, chills, fever, tachycardia, pneumonitis, hypotension and renal dysfunction (BNF 2004; Sweetman 2005); some or all of these effects may represent hypersensitivity reactions. Liver toxicity is also frequently reported; here the changes are grouped into three syndromes, an idiosyncratic cholestatic reaction, vascular disorders including endothelial cell injury and possible veno-occlusive disease (Lemley et al. 1989), and hypersensitivity responses (MC 2003; Sweetman 2005). Also, AZA may be associated with an increased risk of neoplastic disease (lymphomas, skin cancers), and it is considered that the use of AZA should not be initiated in patients who may be pregnant. Furthermore, in AZA-treated patients there may be an increased susceptibility to infection (e.g. tuberculosis, and bacterial, viral, fungal and parasitic diseases).
However, the toxic effect generally giving the most cause for concern in the AZA-treated patient is the risk of severe, dose-related myelosuppression, resulting in a significant decrease in all the cellular elements of the blood (Bacon et al. 1981; Jeurissen et al. 1988; Burke et al. 1989; Kerstens et al. 1993, 1995). The leucopenia, anaemia and thrombocytopenia induced are all dose-dependent, and may result in a profound pancytopenia in a percentage of patients (Hohlfeld et al. 1988; Connell et al. 1993; Creemers et al. 1993; Leipold et al. 1997; Colombel et al. 2000). The effect on granulopoiesis (i.e. the induction of neutropenia) is the most significant change, and generally there may be a relative sparing of megakaryocytes, and hence platelet formation (Whisnant & Pelkey 1982). Macrocytic, including megaloblstic, anaemia has also been widely reported (Klippel & Decker 1974; Wickramasinghe et al. 1974; McGrath et al. 1975; Nicholls & Davidson 1979; DeClerck et al. 1980). For these reasons, the regular monitoring of blood counts is required in the AZA-treated patient (BNF 2004; Sweetman 2005).
Few experimental studies have been reported which investigate aspects of the haemotoxicity and myelotoxicity of AZA in laboratory animals (i.e. rodents, and the rabbit, cat, dog and primate). Literature searches have identified no recent papers recording investigations involving repeat dose studies with AZA in the mouse, although a recent report by Smith et al. (2003) sets out results obtained in a 30-day repeat dose study with AZA in the inbred F344 rat. The present experiments were therefore carried out to characterize the effects of AZA treatment on the haematopoietic system of the mouse, and compare these changes to those reported in man. AZA was selected as a drug of choice for these studies because, as well as causing a profound pancytopenia, AZA may also induce a pure red cell aplasia (Old et al. 1978; Pruijt et al. 1996; Thompson & Gales 1996; Al-Uzri et al. 2003), and aplastic anaemia (Lawson et al. 1984; Sudhir et al. 2002). Furthermore, we have been planning experiments directed towards the development of a mouse model of drug-induced aplastic anaemia, and are considering studies with AZA administered as a second haemotoxic agent to mice pretreated with the anti-cancer agent busulphan (Gibson et al. 2003; Turton et al. 2006).
In the present experiments we also wished to investigate the possible development of AZA-induced changes in the serum concentrations of the cytokine fms-like tyrosine kinase 3 (FLT-3) ligand (FL). Studies in patients undergoing chemotherapy for the treatment of malignant conditions, or in preparation for bone marrow transplantation, have significantly raised plasma FL levels (Chklovskaia et al. 1999), and the increase in plasma Fl corresponds to the time of bone marrow aplasia. Recent studies have also demonstrated that the plasma level of FL is increased immediately following chemotherapeutic treatment, but then returns to pretreatment levels after haematological recovery (Blumenthal et al. 2000; Huchet et al. 2003). Plasma FL concentrations are also increased in patients with conditions characterized by bone marrow failure, for example, aplastic anaemia and Fanconi anaemia (Lyman et al. 1995).
The present investigations on AZA haemotoxicity/myelotoxicity therefore include the characterization of changes in peripheral blood and bone marrow, the assessment of the number of committed bone marrow progenitor cells, levels of bone marrow apoptosis, measurement of serum FL and the investigation of histological changes in various tissues. Three experiments were carried out: a preliminary dose-ranging pilot study (Experiment 1), a dose–response study (Experiment 2), and a Main study to investigate the time course of the return of blood/marrow parameters towards normal in the postdosing period, and identification of any ‘residual’ or ‘late-stage’ effects (Experiment 3). A preliminary report in abstract form has been published (Molyneux et al. 2004).
Materials and methods
Mice
Outbred female CD-1 mice (Crl:CD-1, ICR; Charles River Laboratories Ltd, Margate, Kent, UK) and female ICR (CD-1) mice (Harlan Ltd, Bicester, Oxon, UK) were caged in groups of 5–7 with free access to diet (Rat and Mouse No.1; SDS Ltd, Witham, Essex, UK) and mains drinking water ad libitum. A temperature of 19–22 °C was maintained, with relative humidity of 45–65% and a light:dark cycle of 12:12 h (lights on at 07.00 h). Before the initiation of the experimental procedures, animals were allowed at least 7 days to acclimatize. During the dosing periods, animals were observed at least daily for signs of declining health, and body weights were determined at appropriate times throughout the course of the experiments. Where a mouse became ill and it was considered that recovery was unlikely, the animal was killed. All animal procedures were conducted under local Ethical Committee guidelines and approval for Home Office Project and Personal Licences, and followed the UK Home Office (1989)‘Code of Practice for the Housing and Care of Animals used in Scientific Procedures’.
Azathioprine administration
Azathioprine (Sigma Chemical Co., Poole, Dorset, UK) was suspended in vegetable oil and administered by gavage at dose levels of 25–400 mg/kg in a volume of approximately 0.20 ml/mouse. Control animals were treated with vehicle (vegetable oil) at the same dose volume.
Experimental design
Experiment 1: Preliminary dose-ranging pilot study
Forty-eight female CD-1 mice (mean body weight 20.4 g) (Charles River) were divided into 12 groups of vehicle control (n = 4) and AZA-treated animals (25, 50, 75, 100, 125, 150, 200, 250, 300, 350 and 400 mg/kg; n = 4 per dose level). Animals were dosed with vehicle or AZA daily for 10 days and studied for 19 days postdosing. During the dosing and postdosing periods, animals were observed for clinical evidence of drug toxicity twice daily (or more frequently), and weighed daily or every 2 or 3 days.
Experiment 2: Dose–response study
Sixty female CD-1 mice (mean body weight 25.7 g) (Charles River) were divided into six groups of vehicle control (n = 10) and AZA-treated (40, 60, 80, 100 and 120 mg/kg; n = 10). Animals were dosed with vehicle or AZA daily for 10 days. On day 1 postdosing, animals were killed by an intraperitoneal (i.p.) injection of pentobarbitone sodium (Sagatal, Rhône Mérieux Ltd, Harlow, Essex, UK) for the examination of blood, bone marrow and other tissues and organs.
Experiment 3: Main study
A total of 136 female ICR (CD-1) mice (mean body weight 21.7 g) (Harlan) were treated with vehicle (n = 68) or AZA (n = 68; 100 mg/kg) by gavage daily for 10 days. On days 1, 3, 9, 22, 29, 43 and 57 postdosing, animals (n = 5–11 per group) were killed by an i.p. injection of pentobarbitone sodium (Sagatal) for the examination of blood, bone marrow and other tissues and organs.
Analysis of blood and bone marrow suspensions
Blood was removed from the right ventricle following a thoracotomy incision, and a 0.5 ml aliquot anti-coagulated with 1.5 mg/ml dipotassium EDTA (Teklab, Sacriston, Durham, UK) and any remaining blood was collected into serum separator tubes (Microtainer; Becton Dickinson and Co., Franklin Lakes, NJ, USA). The contents of the left femur were aspirated into 5 ml Iscove’s modified Dulbecco’s medium (IMDM; Life Technologies, Paisley, UK) supplemented with 10% foetal calf serum (FCS; PAA Laboratories GmbH, Linz, Austria) to give a marrow cell suspension; a marrow smear was prepared from the contents of the left tibia.
Blood samples and bone marrow suspensions in IMDM were analysed with the Advia 120 haematology analyser (Bayer Diagnostics UK Ltd, Newbury, Berks, UK) with mouse-specific software. The femoral marrow cell suspension in IMDM was used to obtain the total nucleated cell count [femoral nucleated cell count (FNCC)] using the basophil channel of the Advia 120. Tibial marrow smears were stained with May–Grünwald–Geimsa and differential counts performed by eye on 200 cells.
Bone marrow clonogenic assays
The right femur was removed with surrounding muscle and placed in 5 ml sterile IMDM with 10% FCS (PAA Laboratories GmbH); under sterile conditions the muscle and epiphyses were removed and the marrow flushed into 5 ml sterile IMDM supplemented with 10% FCS. Using trypan blue exclusion, the white blood cells (WBC) were counted and cultured at 105 WBC in 1 ml IMDM supplemented with 30% FCS, 1% de-ionized bovine serum albumin (BSA; Sigma), 10−4 M β-mercaptoethanol (Sigma), 0.05% NaHCO3, 2.1 mM l-glutamine (Sigma) and 0.9% methylcellulose (Stem Cell Technologies Inc., London, UK). Cultures were set up in duplicate in 35 mm dishes (Nunclon, Loughborough, Leicestershire, UK) with the following growth factors added to each dish: 4 IU human erythropoietin (hEPO; Janssen-Cilag Ltd, High Wycombe, Bucks, UK), 50 ng murine interleukin-3 (mIL-3; R&D Systems Europe Ltd, Abingdon, Berks, UK), 50 ng murine stem cell factor (mSCF; R&D Systems), 50 ng human interleukin-6 (hIL-6; Novartis Pharmaceuticals Ltd, Langley, Herts, UK) and 50 ng human granulocyte colony-stimulating factor (hG-CSF; Amgen UK Ltd, Cambridge, UK). The cultures were incubated at 37 °C in 5% CO2 in air for 14 days. On day 14, granulocyte-monocyte colony-forming units (CFU-GM), erythroid burst-forming units (BFU-E), and colonies containing both granulocyte-monocyte and erythroid elements (CFU-GEM) were counted. Results are expressed as CFU-GM or the total number of erythroid colonies (BFU-E + CFU-GEM) per femur, or as a percentage of the mean control value at the concurrent time point.
Apoptosis in bone marrow cells
Apoptosis in femoral bone marrow nucleated cells was assessed according to the method described by Philpott et al. (1995, 1996). Two hundred microlitres (0.5–1.0 × 106cells) of the femoral marrow flush was washed twice in phosphate-buffered saline (PBS) supplemented with 1% FCS and 0.05% Na azide. Cells were resuspended in 500 μl or 450 μl PBS for 7-amino-actinomycin D (7-AAD; Calbiochem, Nottingham, UK) unstained, and 7-AAD stained cells respectively. 7-AAD was dissolved in acetone, diluted in PBS to a concentration of 0.2 mg/ml, kept at −20 °C and protected from light until use. Cells were stained with 50 μl of 7-AAD for 20 min at 4 °C and protected from light. Cells were fixed in 2% paraformaldehyde solution (Sigma) and analysed within 30 min of fixation on a FACScan (Becton Dickinson, Mountain View, CA, USA). Data on 50,000 cells were acquired and processed using Cell Quest software™ (Becton Dickinson). Scattergrams of forward scatter (FSC) vs. 7-AAD fluorescence were generated. The FSC height threshold was set at 108 to exclude all events too small to be whole cells. Regions were drawn around populations showing negative, dim and bright 7-AAD fluorescence corresponding to live, apoptotic and dead cells respectively. A region was also drawn around cell debris and RBC to exclude these data.
Analysis of cytokines
The blood/serum in separator tubes was allowed to stand (75–90 min), centrifuged (room temperature, 400 g, 5 min), the serum harvested and stored at −80 °C. The presence of the cytokines interleukin (IL)-2, tumour necrosis factor (TNF)-α and interferon (IFN)-γ in serum were detected using a fluorokine® MAP kit (R&D Systems) with sensitivity of typically less than 1.99, 0.42 and 5.25 pg/ml for each cytokine respectively. The presence of the cytokine fms-like tyrosine kinase 3 (FLT-3) ligand (FL) was measured in serum samples using an enzyme-linked immunosorbent assay (ELISA, R&D Systems) with a sensitivity of typically less than 5 pg/ml, according to the manufacturer’s instructions.
Tissues
At autopsy the spleen, liver and kidneys were removed, weighed, and placed in 10.5% phosphate-buffered formalin fixative; the sternum and thymus were also removed and placed in fixative. Tissues were embedded in paraffin and sectioned; sections were stained with haematoxylin and eosin (H&E) for morphological examination by light microscopy.
Statistical analysis
Results from Experiment 2 (dose–response study) were analysed using a one-way analysis of variance (anova) followed by Tukey’s highest significance test for post hoc pairwise multiple comparison. In cases of violation of the assumptions for parametric testing, the Kruskal–Wallis test was used in combination with the Dunn’s post-test. Statistical analysis was performed using GraphPad Prism version 4.00 for windows (GraphPad software, San Diego, CA, USA).
In Experiment 3 (Main study), AZA-treated and control (vehicle-treated) groups at concurrent time points were compared using an unpaired one-tailed Student’s t-test (Microsoft excel; Microsoft Ltd, Microsoft UK, Reading, UK).
Results
Experiment 1. Preliminary dose-ranging study
Clinical signs and body weight changes
Mice were gavaged with AZA at dose levels from 25 to 400 mg/kg, daily for 10 days and studied for clinical evidence of drug toxicity for 19 days postdosing. At 25 and 50 mg/kg AZA, mean body weight increases during the 10-day dosing period were similar to the controls (an increase of 12.7%, controls; 13.8% at 25 mg/kg; 12.5% at 50 mg/kg). At 75 and 100 mg/kg AZA, the mean body weight increases during the dosing period were slightly reduced in comparison with the control animals, being 8.9% and 8.8% respectively. At 125, 150 and 200 mg/kg AZA, the mean body weight changes during the dosing period were 7.8%, −4.9% and −11.0% respectively. At the 250, 300, 350 and 400 mg/kg dose levels, mice did not survive through the 10-day period of AZA treatment; the animals were either killed in extremis (KIE), or on occasion were ‘found dead’ (FD); KIE and FD mice were grouped together and categorized as inter-current death (ICD) animals. The mean ICD day, for mice in the 250 and 300 mg/kg AZA groups, was day 7 of dosing and for mice in the 350 and 400 mg/kg AZA groups the mean ICD day was day 6 of dosing. The clinical signs of AZA toxicity at these higher dose levels were, in general, loss of condition, piloerection, stained fur in the urinary/genital/anal region, reduced movement, hunched posture, recumbancy and closed eyes.
In the 19 days of the postdosing period, animals in the control (vehicle-treated) group increased in body weight by 16.9%; animals in the 25, 50, 75 and 100 mg/kg AZA groups also increased in weight, by 28.6%, 12.2%, 24.4% and 23.0% respectively; there was no clear evidence of AZA toxicity in these groups during or after the dosing period. At 125, 150 and 200 mg/kg AZA, mice did not survive through the 19-day postdosing period; the mean ICD day for mice in these three groups was day 10, 6 and 1 postdosing respectively. All mice in the 125, 150 and 200 mg/kg groups showed the clinical signs of AZA toxicity, as described above.
It was decided, as a result of this dose-ranging pilot study, to treat mice with AZA at 40, 60, 80, 100 and 120 mg/kg in Experiment 2 (Dose–response study), with the autopsy at day 1 postdosing, and in Experiment 3 (Main study) to dose with AZA at 100 mg/kg.
Experiment 2. Dose–response study
Clinical signs and body weight changes
During the 10-day dosing period with AZA administered at 0 (vehicle-treated controls), and at 40, 60, 80, 100 and 120 mg/kg, both control and AZA-treated mice maintained a good state of health with no clinical evidence of toxic effects. The mean body weight of control (vehicle-treated) animals increased from 23.9 to 28.0 g (a 17.2% increase). Body weight changes in mice treated with AZA were of a much lower magnitude, the mean body weight of animals treated with 40 and 60 mg/kg AZA increased by 2.1% and 2.3% respectively. The mean body weights of animals treated with 100 and 120 mg/kg AZA increased by 4.5% and 4.1%, respectively, over the 10-day dosing period. However, animals treated at 80 mg/kg AZA remained at approximately the same mean weight throughout the dosing period.
Haematology results
At all dose levels, AZA induced some changes in peripheral blood parameters (Table 1). Significant reductions were seen in all mean erythrocyte parameters (RBC, Hb, HCT and reticulocytes) at 80 mg/kg AZA and above, leucocyte parameters (WBC, neutrophils, lymphocytes, monocytes and eosinophils) at 80 mg/kg AZA and above, and in the mean platelet counts at 80, 100 and 120 mg/kg AZA. A statistically significant increase (P < 0.05) in MCV was also seen in animals treated with 80 mg/kg AZA. There appeared to be a general trend for AZA to induce a dose-related effect in the changes for RBC, reticulocytes, platelets and WBC.
Table 1.
Haematological results from female CD-1 mice treated daily with 10 doses of azathioprine (AZA) and sampled 1 day after the final dose
| Treatment (mg/kg) | ||||||
|---|---|---|---|---|---|---|
| 0 (Control) | 40 | 60 | 80 | 100 | 120 | |
| RBC | 8.50 (0.28) | 7.14 (0.37) | 6.98 (0.66) | 6.83 (0.44)** | 6.32 (0.77)*** | 6.42 (0.86)*** |
| Hb | 136.7 (8.3) | 116.1 (5.97)*** | 111.3 (8.27)*** | 109.0 (6.98)*** | 101.1 (13.0)*** | 103.6 (14.2)*** |
| HCT | 0.45 (0.01) | 0.37 (0.02) | 0.37 (0.03)** | 0.38 (0.03)* | 0.34 (0.04)*** | 0.35 (0.05)*** |
| MCV | 52.7 (1.0) | 52.3 (1.7) | 52.9 (1.7) | 55.1 (1.9)* | 52.9 (1.3) | 53.8 (1.4) |
| MCH | 16.1 (0.9) | 16.2 (0.4) | 16.0 (0.6) | 16.0 (0.4) | 16.0 (0.3) | 16.1 (0.3) |
| MCHC | 305.2 (15.9) | 310.3 (6.4) | 301.9 (7.6) | 290.2 (6.6)*** | 302.4 (6.5) | 300.1 (10.3) |
| Retic | 334.46 (145.92) | 14.05 (14.75) | 2.44 (3.08)** | 1.96 (2.30)*** | 0.87 (0.40)*** | 1.25 (0.82)*** |
| Plt | 1305 (185) | 1123 (190) | 685 (160) | 389 (241)** | 238 (159)*** | 141 (60)*** |
| WBC | 1.69 (0.58) | 0.82 (0.36) | 0.66 (0.27) | 0.57 (0.29)** | 0.30 (0.13)*** | 0.46 (0.15)*** |
| Neut | 0.32 (0.14) | 0.16 (0.10) | 0.10 (0.05) | 0.10 (0.12)* | 0.01 (0.01)*** | 0.02 (0.01)*** |
| Lymph | 1.22 (0.49) | 0.61 (0.31) | 0.50 (0.25)* | 0.43 (0.16)* | 0.26 (0.12)*** | 0.41 (0.15)** |
| Mono | 0.03 (0.01) | 0.01 (0.01)** | 0.00 (0.00)*** | 0.00 (0.00)*** | 0.00 (0.00)*** | 0.00 (0.00)*** |
| Eo | 0.12 (0.04) | 0.04 (0.01) | 0.04 (0.01)* | 0.03 (0.03)*** | 0.03 (0.00)*** | 0.03 (0.01)*** |
| Baso | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.01) | 0.00 (0.00) | 0.00 (0.00) |
| FNCC | 5.58 (0.63) | 2.93 (1.07) | 1.97 (1.04)* | 1.00 (0.65)*** | 0.90 (0.33)*** | 0.85 (0.35)*** |
Values are means, SD in parentheses; n = 10 animals per group, except n = 9 in the 100 mg/kg AZA-treated group. Data analysed using a one-way analysis of variance (anova).
Significantly different from controls, P < 0.05;
P < 0.01;
P < 0.001.
Abbreviations and units: RBC, red blood cells, ×106/μl; Hb, haemoglobin, g/dl; HCT, haematocrit, %; MCV, mean cell volume, fl; MCH, mean cell haemoglobin, pg; MCHC, mean cell haemoglobin concentration, g/dl; Retic, absolute reticulocyte count, ×103/μl; Plt, platelets, ×103/μl; WBC, white blood cells, ×103/μl; Neut, neutrophils, ×103/μl; Lymph, lymphocytes, ×103/μl; Mono, monocytes, ×103/μl; Eo, eosinophils, ×103/μl; Baso, basophils, ×103/μl; FNCC, femoral nucleated cell count, ×107.
Femoral nucleated cell count
Administration of AZA at all dose levels induced a reduction in the cellularity of the bone marrow (Table 1). The mean FNCC of mice treated with 40 and 60 mg/kg of AZA was reduced, to 52.4% and 35.4% of the control mean respectively. In mice treated with higher levels of AZA (80, 100 and 120 mg/kg), the reduction in marrow cellularity was much more pronounced being to 17.9%, 16.1% and 15.2% of the control mean respectively. There was an overall trend for a dose-related response in FNCC to the dose level of AZA administered.
Bone marrow differential counts
Estimated counts of myeloid, erythroid and lymphoid cells in the femoral marrow demonstrated that the cells of the myeloid and lymphoid lineages were reduced in all groups treated with AZA, with the reductions reaching statistical significance in the 80, 100 and 120 mg/kg AZA-treated groups (Table 2). Cells of the erythroid lineage were also profoundly reduced at all dose levels following treatment with AZA, with the average percentage reduction at all dose levels being to 24.0% of the control mean (P < 0.001 at all AZA dose levels).
Table 2.
Estimated counts (×107) of myeloid, erythroid and lymphoid cells, and the myeloid:erythroid (M:E) ratio in the femoral marrow of control mice, and animals treated with azathioprine (AZA) at 40–120 mg/kg daily, for 10 days and sampled at 1 day after the final dose
| AZA treatment (mg/kg) | ||||||
|---|---|---|---|---|---|---|
| 0 (Control) | 40 | 60 | 80 | 100 | 120 | |
| Myeloid | 2.63 (0.24) | 1.74 (0.85) | 0.82 (0.38) | 0.38* (0.36) | 0.13*** (0.10) | 0.16** (0.18) |
| Erythroid | 1.71 (0.15) | 0.50*** (0.24) | 0.30*** (0.13) | 0.33*** (0.13) | 0.50*** (0.20) | 0.42*** (0.18) |
| Lymphoid | 1.34 (0.27) | 0.57 (0.29) | 0.33 (0.16) | 0.17* (0.03) | 0.08*** (0.03) | 0.13** (0.11) |
| Other | 0.21 (0.05) | 0.16 (0.11) | 0.09 (0.03) | 0.07 (0.03) | 0.05** (0.03) | 0.05* (0.02) |
| M:E ratio | 1.54 (0.15) | 3.88 (2.17) | 2.87 (1.43) | 1.24 (0.98) | 0.24 (0.13) | 0.42 (0.50) |
Two hundred cells in the tibial marrow smears were differentially counted by eye and the absolute number of cells of each lineage was estimated for the nucleated marrow cell count of the femoral marrow flush. Values are means, SD in parenthesis; n = 6 randomly selected animals per group. Cells categorized as ‘other’ include monocytes and monocyte precursors, mast cells, megakaryocyes, plasma cells and unidentifiable cells. Data analysed using a one-way analysis of variance (anova).
Significantly different from controls, P < 0.05;
P < 0.01;
P < 0.001.
Cytokine analysis
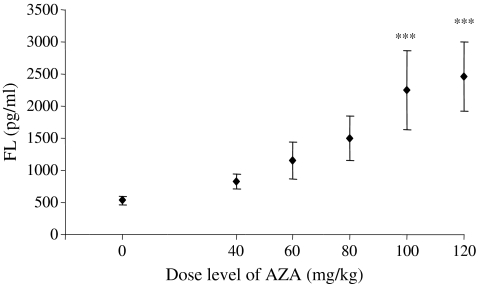
The concentration of serum fms-like tyrosine kinase-3 (FLT-3) ligand (FL) was measured in mice treated with AZA at 0 (vehicle control), and 40, 60, 80, 100 and 120 mg/kg. A dose-related increase in serum FL levels was evident (Figure 1). Mean levels of FL were increased to 2253.4 and 2455.7 pg/ml in mice treated with AZA at 100 and 120 mg/kg, respectively (P < 0.001 at both levels), compared with a mean control value of 529.4 pg/ml. Animals treated with lower doses of AZA (40, 60 and 80 mg/kg) also had elevated levels of serum FL with mean values being 828.2, 1153.4 and 1502.8 pg/ml respectively (NS at all three dose levels).
Figure 1.
Serum concentrations of fms-like tyrosine kinase-3 (FLT-3) ligand (FL) in mice treated with azathioprine (AZA) at 0 (vehicle control) and at 40, 60, 80, 100 and 120 mg/kg for 10 days and autopsied on day 1 postdosing. Values are means ± SD. There were 10 animals per group, except n = 9 in the 100 mg/kg AZA-treated group. ***Significantly different from control animals, P < 0.001.
Bone marrow clonogenic assays
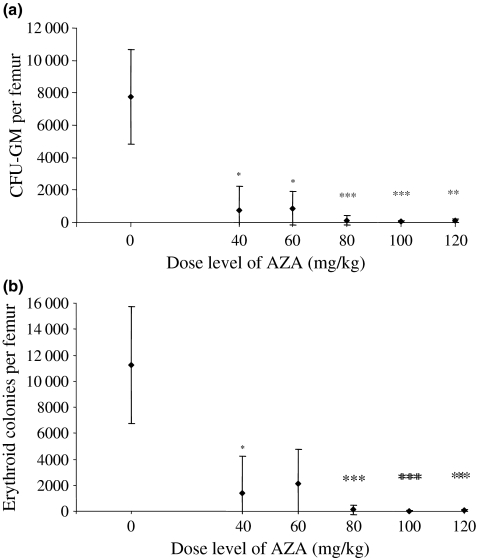
At day 1 after the final dose of AZA, the number of granulocyte-monocyte colony-forming units (CFU-GM) in the femur of AZA-treated mice was significantly reduced at all dose levels of the drug (Figure 2a); at 40, 60, 80, 100 and 120 mg/kg AZA the mean percentage reductions were to 9.7% (P < 0.05), 11.3% (P < 0.05), 1.4% (P < 0.001), 0.7% (P < 0.001) and 1.2% (P < 0.01) of the counts in the control animals respectively. There was some evidence of a dose-related trend for the effect of AZA on the number of CFU-GM colonies per femur.
Figure 2.
Committed progenitor cell content of femoral bone marrow in mice treated with azathioprine (AZA) at 0 (vehicle control) and at 40, 60, 80, 100 and 120 mg/kg for 10 days and autopsied on day 1 postdosing: (a) number of granulocyte-monocyte colony-forming units (CFU-GM) per femur; (b) number of erythroid colony-forming units per femur. Values are expressed as means ± SD. There were n = 10 animals in each group, except n = 9 in the 100 mg/kg AZA-treated group. *Significantly different from control animals, P < 0.05; **P < 0.01; ***P < 0.001.
A similar result was evident in the number of erythroid colonies per femur in AZA-treated mice (Figure 2b). At day 1 postdosing the number of erythroid colonies per femur were significantly reduced to 9.2% (P < 0.05), 23.5% (NS), 3.2% (P < 0.001), 0.3% (P < 0.001) and 0.7% (P < 0.01) of the mean count in the control animals at 40, 60, 80, 100 and 120 mg/kg AZA respectively. Again, as with the CFU-GM results, there was some evidence of a dose-related trend for the effect of AZA on the number of erythroid colonies per femur.
Apoptosis in bone marrow cells
Levels of apoptosis in nucleated femoral bone marrow cells at day 1 postdosing were assessed (Table 3). Values are expressed as the proportion (percentages) of live, apoptotic and dead cells, excluding cell debris. It is seen (Table 3) that the proportion of apoptotic cells is increased at 100 and 120 mg/kg AZA, and the proportion of dead cells, and apoptotic plus dead cells generally increases as the dose level of AZA increases; correspondingly, the proportion of live cells decreases with elevation of the AZA dose levels.
Table 3.
Apoptosis in the femoral bone marrow of female CD-1 mice treated with 10 daily doses of azathioprine (AZA) at 0 (vehicle control), 40, 60, 80, 100 and 120 mg/kg and sampled at 1 day after the final dose; the percentages of live, apoptotic, and dead cells, and apoptotic plus dead cells is given, but excluding cell debris
| AZA dose level (mg/kg) | Live cells (%) | Apoptotic cells (%) | Dead cells (%) | Apoptotic + dead cells (%) |
|---|---|---|---|---|
| 0 | 80.75 (4.22) | 10.22 (2.08) | 9.02 (2.47) | 19.25 (4.22) |
| 40 | 76.26 (4.52)** | 9.28 (2.09) | 14.46 (2.93)*** | 23.74 (4.52)** |
| 60 | 76.18 (7.21)* | 7.94 (2.72)* | 15.88 (5.02)*** | 23.82 (7.21)* |
| 80 | 63.15 (14.39)*** | 10.43 (4.59) | 26.43 (11.05)*** | 36.85 (14.39)*** |
| 100 | 56.22 (9.40)*** | 13.38 (2.44)*** | 30.40 (7.75)*** | 43.78 (9.40)*** |
| 120 | 57.73 (3.68)*** | 13.13 (2.11)*** | 29.13 (4.31)*** | 42.27 (3.68)*** |
Values are means, SD in parenthesis; n = 5 per dose level group;
significantly different from controls, P < 0.05;
P < 0.01;
P < 0.001.
Histopathological assessment of tissues
Sections of thymus, kidney, liver, spleen and sternum were assessed from five randomly selected control mice and mice treated with AZA at 40, 80 and 120 mg/kg (five mice per dose level group). Changes in the histology of the thymus were minimal, with the majority of animals assessed showing signs of mild atrophy, and there was also some evidence of apoptosis occurring in animals treated with AZA at 120 mg/kg.
The mean relative weights of the kidneys from mice treated with AZA at all dose levels were similar to those of control (vehicle-treated) animals. The histology of the kidney was unchanged in animals treated with 120 mg/kg AZA and therefore tissues from animals treated with lower doses of AZA were not assessed.
The mean relative weights of the livers from animals treated with AZA at 80, 100 and 120 mg/kg were increased by 13.8% (P < 0.01), 7.3% (P < 0.05) and 9.3% (P < 0.01), respectively, over the relative weights of the livers from control mice. The relative weight of the livers from mice treated at 40 and 60 mg/kg AZA were similar to those from control animals. Histological examination of the liver demonstrated centrilobular hepatocyte hypertrophy in all mice treated with 80 and 120 mg/kg AZA, and in the majority of mice treated with 40 mg/kg AZA; livers from mice treated at 60 and 100 mg/kg were not examined.
For the spleen, the mean relative weight was significantly reduced at all dose levels of AZA, to an average of 66.5% of the control spleen relative weight (P < 0.01 or P < 0.001 at each dose level); there was no evidence of a dose-related effect in the weight decreases. The histology of the spleen showed a significant dose-related reduction in extramedullary haemopoiesis, with erythropoiesis being virtually absent in the majority of animals examined. Granulopoiesis and megakaryopoiesis however did persist.
In the sternal marrow, a significant reduction in cellularity was seen; all cell lines were depleted in all animals treated with 80 and 120 mg/kg AZA and in the majority of mice treated with 40 mg/kg AZA.
Experiment 3. Main study
Clinical signs and body weight changes
Sixty-eight control mice were dosed by gavage with vegetable oil (vehicle), and 68 with AZA at 100 mg/kg for 10 daily doses. The mean body weight of mice on day 1 of dosing was 20.7 and 22.6 g, for control and AZA-treated mice respectively. At day 1 after dosing, the mean body weight of control mice had increased by 7.2% to 22.2 g. However, at day 1 postdosing, the mean body weight of AZA-treated mice had decreased by 1.0 g to a mean of 21.6 g (a 4.4% reduction).
During the 10-day dosing period, all control and AZA-treated animals maintained a good state of health (as in Experiment 2: Dose–response study), and no evidence of toxicity was recorded. In the first 10 days of the postdosing period, control animals continued to gain weight normally, but there was no increase in the body weight of the AZA-treated mice, and some of these animals began to demonstrate a loss of condition. This deterioration in the condition of the AZA-treated mice during this early postdosing period (day 1–10) was unexpected and gave cause for concern and a total of 25 AZA mice were either killed in extremis (KIE) or on occasion were found dead (FD); such animals are categorized as inter-current death (ICD) animals. There were a further two ICD AZA-treated mice on day 15 postdosing, bringing the total to 27 ICD animals. After this time point however, the condition of the AZA-treated mice began to improve and from this time (day 15 post AZA dosing), animals began to gain weight. Indeed, after day 18 postdosing, the mean body weight of the AZA-treated mice was greater than the controls, and this increased weight (above control animals) was maintained throughout the remainder of the experiment.
Haematology results
As in Experiment 2 (Dose–response study), 10 daily doses of 100 mg/kg AZA induced anaemia and thrombocytopenia immediately postdosing (Table 4). RBC, Hb and HCT were all significantly reduced at this time, being 81.7%, 80.9% and 78.7% of the mean control levels respectively. The mean WBC, neutrophil, monocyte and eosinophil counts were all significantly reduced, to 57.3%, 0.0%, 0.0% and 14.3% of the mean control level respectively. Furthermore, on day 1 postdosing, the mean reticulocyte and platelet counts were also reduced, to 0.3% and 9.2% of the control mean respectively (P < 0.001 for both parameters).
Table 4.
Haematological results from female CD-1 mice treated daily with 10 doses of azathioprine (AZA) at 100 mg/kg and sampled at 1–57 days after the final dose
| Day of sampling | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 9 | 22 | 29 | 43 | 57 | ||||||||
| Control | AZA | Control | AZA | Control | AZA | Control | AZA | Control | AZA | Control | AZA | Control | AZA | |
| RBC | 7.93 (0.35) | 6.48 (0.71)*** | 7.97 (0.32) | 5.25 (0.68)*** | 8.31 (0.45) | 1.71 (0.47)*** | 8.26 (0.22) | 7.17 (0.52)*** | 8.55 (0.42) | 7.41 (0.38)*** | 8.50 (0.34) | 8.08 (0.39)* | 8.73 (0.37) | 8.22 (0.39)* |
| Hb | 139.0 (4.7) | 112.4 (10.4)*** | 140.9 (5.0) | 91.0 (12.3)*** | 141.6 (4.7) | 29.2 (7.6)*** | 140.0 (3.9) | 136.2 (6.3) | 143.0 (6.6) | 134.8 (3.0)** | 141.3 (4.2) | 141.8 (6.1) | 142.7 (6.1) | 138.0 (5.3) |
| HCT | 0.47 (0.01) | 0.37 (0.04)*** | 0.47 (0.02) | 0.29 (0.04)*** | 0.47 (0.02) | 0.09 (0.02)*** | 0.46 (0.02) | 0.45 (0.02) | 0.47 (0.02) | 0.45 (0.01)* | 0.47 (0.02) | 0.46 (0.02) | 0.49 (0.02) | 0.47 (0.02) |
| MCV | 59.5 (1.7) | 57.0 (1.7)** | 58.6 (1.7) | 54.8 (1.0)*** | 57.0 (1.7) | 51.5 (1.8)*** | 56.2 (1.6) | 62.3 (2.0)*** | 55.5 (2.0) | 60.6 (2.8)*** | 54.7 (1.3) | 56.4 (0.8)** | 55.6 (2.2) | 57.4 (1.8) |
| MCH | 17.5 (0.6) | 17.4 (0.6) | 17.7 (0.6) | 17.3 (0.4) | 17.1 (0.5) | 17.0 (0.2) | 16.9 (0.2) | 19.0 (1.0)*** | 16.8 (0.6) | 18.2 (0.7)*** | 16.7 (0.4) | 17.5 (0.2)*** | 16.4 (0.6) | 16.8 (0.8) |
| MCHC | 295.3 (5.8) | 304.6 (5.8)** | 301.6 (9.4) | 315.4 (6.5)** | 299.8 (10.4) | 329.6 (7.5)** | 301.1 (6.4) | 305.0 (6.6) | 302.0 (6.3) | 300.4 (3.7) | 304.5 (5.9) | 311.2 (4.9)* | 294.3 (6.9) | 292.4 (7.2) |
| Retic | 292 (80) | 0.98 (0.4)*** | 249 (67) | 0.91 (0.6)*** | 354 (91) | 1.26 (0.4)*** | 319 (138) | 166 (39)** | 282 (95) | 275 (98) | 234 (70) | 315 (171) | 274 (98) | 249 (95) |
| Plt | 1440 (242) | 133 (103)*** | 1496 (268) | 57 (61)*** | 1477 (175) | 9 (2)*** | 1338 (156) | 930 (220)*** | 1407 (134) | 1171 (161)** | 1439 (139) | 1220 (155)** | 1445 (171) | 1366 (190) |
| WBC | 0.82 (0.29) | 0.47 (0.16)** | 1.49 (0.54) | 0.32 (0.14)*** | 1.09 (0.57) | 0.24 (0.11)*** | 1.20 (0.34) | 1.37 (0.39) | 1.07 (0.34) | 1.29 (0.29) | 0.93 (0.34) | 2.20 (1.29)** | 1.47 (0.56) | 1.54 (0.68) |
| Neut | 0.13 (0.06) | 0.00 (0.01)*** | 0.34 (0.12) | 0.00 (0.01)*** | 0.19 (0.10) | 0.00 (0.01)*** | 0.18 (0.07) | 0.31 (0.10)** | 0.19 (0.06) | 0.34 (0.19)* | 0.23 (0.13) | 0.49 (1.84)** | 0.24 (0.09) | 0.28 (0.13) |
| Lymph | 0.59 (0.19) | 0.45 (0.15) | 1.04 (0.43) | 0.30 (0.13)*** | 0.82 (0.44) | 0.22 (0.10)** | 0.91 (0.30) | 0.94 (0.29) | 0.82 (0.28) | 0.85 (0.13) | 0.62 (0.20) | 1.57 (1.17)* | 1.10 (0.48) | 1.09 (0.54) |
| Mono | 0.01 (0.01) | 0.00 (0.00)*** | 0.03 (0.01) | 0.00 (0.00)*** | 0.02 (0.02) | 0.00 (0.00)* | 0.02 (0.01) | 0.03 (0.02) | 0.02 (0.01) | 0.03 (0.01)* | 0.01 (0.01) | 0.03 (0.02)* | 0.02 (0.01) | 0.02 (0.01) |
| Eo | 0.07 (0.06) | 0.01 (0.00)** | 0.07 (0.03) | 0.01 (0.01)*** | 0.05 (0.02) | 0.00 (0.01)*** | 0.06 (0.02) | 0.07 (0.04) | 0.07 (0.02) | 0.06 (0.01) | 0.04 (0.01) | 0.04 (0.02) | 0.09 (0.02) | 0.13 (0.09) |
| Baso | 0.01 (0.00) | 0.00 (0.00) | 0.01 (0.01) | 0.00 (0.00)* | 0.00 (0.01) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.01) | 0.00 (0.00) | 0.00 (0.00)* | 0.00 (0.01) | 0.00 (0.00) | 0.01 (0.01) | 0.00 (0.00) |
| FNCC | 4.27 (0.52) | 0.67 (0.21)*** | 3.99 (0.51) | 0.58 (0.21)*** | 3.96 (0.75) | 1.56 (1.86)** | 4.07 (0.58) | 3.18 (0.60)** | 4.11 (0.73) | 3.86 (0.33) | 3.75 (0.50) | 3.11 (0.49)* | 4.12 (0.63) | 4.15 (1.04) |
Values are means, SD in parentheses; n = 8 control and AZA-treated mice on days 1 and 3, n = 10 (control) and 5 (AZA) at all other time points, except at day 9, where n = 8 (control) and 5 (AZA), and day 57 where n = 11 (control) and 5 (AZA). Data analysed using Student’s t-test.
Significantly different from controls, P < 0.05;
P < 0.01;
P < 0.001.
Abbreviations and units: see Table 1.
The peripheral blood changes seen on day 1 postdosing appeared to develop further on day 3 postdosing (Table 4), with significant reductions remaining evident in the mean RBC, Hb, HCT, WBC and platelet values. In addition, the reticulocyte counts also remained significantly decreased, together with the counts of neutrophils, lymphocytes, monocytes, eosinophils and basophils.
At day 9 postdosing, the RBC, Hb and HCT values, and all individual leucocyte counts remained significantly reduced compared with the concurrent control values. Reticulocyte and platelet counts were also profoundly decreased to 0.4% and 0.6% of the mean control values respectively (P < 0.001, for both counts).
However, on day 22 postdosing, a return of many haematological parameters towards normal (control) values was evident, and this return towards normal continued on day 29 (Table 4). However, the reductions in RBC, Hb, HCT and platelets also continued to be statistically significant on either one, or both, of the day 22 and 29 time points. Also, the MCV and MCH were both significantly increased in AZA-treated mice on days 22 and 29 (P < 0.001 for both parameters on both days). The WBC count was comparable to the concurrent control values on days 22 and 29. The neutrophil count was significantly elevated above the mean control value on day 22 and 29.
On day 43 postdosing, the mean RBC and platelet counts continued to maintain a significant reduction in comparison with the mean control value, while the MCV and MCH continued to be increased above the mean control values. The reticulocyte count was normal at day 43. The total leucocyte (WBC) count of AZA-treated mice was significantly increased on day 43; this elevation was due to increases in neutrophil, lymphocyte and monocyte counts in AZA-treated animals (Table 4).
A return of all leucocytes to normal (control) values was seen on day 57 postdosing, and indeed most of the haematological parameters under investigation were comparable to the controls at this time; however, a residual effect remained evident in the RBC, which continued to be significantly reduced at this late-stage; however, Hb and HCT were comparable to concurrent control values (Table 4).
Femoral nucleated cell count
The bone marrow of AZA-treated mice was significantly hypocellular on day 1 postdosing, with the FNCC being reduced to 15.7% of the mean concurrent control value (P < 0.001) (Table 4). This statistically significant bone marrow hypoplasia continued in mice treated with AZA on day 3 postdosing, with the FNCC being reduced to 14.5% of the control mean at this time point. On day 9 and 22 postdosing, the reduction in marrow cellularity was still statistically significant, however, at these time points evidence of recovery, and a return towards normal was seen; the reductions in FNCC were to 39.4% and 78.1% of the mean control values on day 9 and 22 respectively. On days 29 and 43 postdosing, the FNCC of AZA-treated mice was slightly reduced compared with the concurrent control values, but the decrease was only significant (P < 0.05) on day 43, before returning to the control range on day 57 postdosing.
Bone marrow differential counts
The tibial marrow smears from control and AZA-treated mice (n = 5 or 6) were randomly selected at each time point and differential counts performed by eye on 200 cells. On day 1 postdosing, myeloid, erythroid and lymphoid cell lineages were significantly reduced in AZA-treated animals (Table 5), with counts being 5.7%, 31.3% and 9.8% of the mean concurrent control values respectively (P < 0.001). On day 3 postdosing, the number of myeloid, erythroid and lymphoid cells were also reduced in AZA-treated mice to 3.9%, 16.9% and 15.6% of the control mean respectively (P < 0.001). At days 9 and 22 postdosing, myeloid, erythroid and lymphoid cells continued to be reduced, however, a return towards normal values was clearly evident at these time points. From day 29 to 57 postdosing, mean myeloid and erythroid cell counts were comparable to mean concurrent control values. Lymphoid cell counts were however, slightly reduced in comparison with the controls on day 29 (NS) and 43 (P < 0.05) postdosing. Cells categorized as ‘other’ (monocytes and monocyte precursors, mast cells, megakaryocytes, plasma cells and unidentifiable cells), were reduced in AZA-treated animals on days 1, 3 and 9 postdosing (P < 0.001, P < 0.001 and P < 0.05 respectively) before returning to values comparable to the controls from day 22 postdosing onwards. In the immediate postdosing period (days 1, 3 and 9), the M:E ratio was significantly reduced (P < 0.001) before returning to values comparable to the controls on day 22 postdosing.
Table 5.
Estimated counts (×107) of myeloid, erythroid and lymphoid cells, and the myeloid:erythroid (M:E) ratio in the femoral marrow of control mice, and animals treated with azathioprine (AZA) at 100 mg/kg daily, for 10 days and sampled at 1–57 days after the final dose
| Day of sampling | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 9 | 22 | 29 | 43 | 57 | ||||||||
| Control | AZA | Control | AZA | Control | AZA | Control | AZA | Control | AZA | Control | AZA | Control | AZA | |
| Myeloid | 1.59 (0.14) | 0.09*** (0.06) | 1.52 (0.32) | 0.06*** (0.03) | 1.47 (0.26) | 0.31*** (0.46) | 1.67 (0.30) | 1.30* (0.32) | 1.79 (0.29) | 1.59 (0.22) | 1.42 (0.29) | 1.23 (0.26) | 1.58 (0.43) | 1.55 (0.33) |
| Erythroid | 1.31 (0.27) | 0.41*** (0.19) | 0.89 (0.35) | 0.15*** (0.09) | 1.12 (0.25) | 0.62 (0.80) | 1.14 (0.18) | 0.96 (0.29) | 1.28 (0.36) | 1.34 (0.10) | 1.37 (0.14) | 1.23 (0.18) | 1.60 (0.37) | 1.44 (0.37) |
| Lymphoid | 1.12 (0.29) | 0.11*** (0.07) | 1.54 (0.37) | 0.24*** (0.10) | 1.36 (0.39) | 0.60* (0.64) | 1.20 (0.29) | 0.81* (0.20) | 0.90 (0.26) | 0.84 (0.16) | 0.77 (0.11) | 0.60* (0.19) | 0.79 (0.28) | 1.06 (0.31) |
| Other | 0.14 (0.04) | 0.05*** (0.03) | 0.13 (0.02) | 0.03*** (0.01) | 0.09 (0.01) | 0.04* (0.05) | 0.13 (0.04) | 0.11 (0.03) | 0.11 (0.04) | 0.09 (0.02) | 0.07 (0.03) | 0.06 (0.02) | 0.09 (0.03) | 0.10 (0.05) |
| M:E ratio | 1.27 (0.36) | 0.28*** (0.22) | 1.92 (0.71) | 0.55*** (0.39) | 1.34 (0.21) | 0.33*** (0.27) | 1.52 (0.45) | 1.39 (0.35) | 1.46 (0.33) | 1.20 (0.24) | 1.06 (0.29) | 1.01 (0.20) | 0.98 (0.13) | 1.08 (0.08) |
200 cells in the tibial marrow smears were differentially counted by eye and the absolute number of cells of each lineage estimated for the nucleated marrow cell count of the femoral marrow flush. Values are means, SD in parenthesis; n = 6 per group. Cells categorized as ‘other’ include monocytes and monocyte precursors, mast cells, megakaryocytes, plasma cells and unidentifiable cells. Data analysed using a Student’s t-test.
Significantly different from controls, P < 0.05;
**P < 0.01;
P < 0.001.
Cytokine analysis
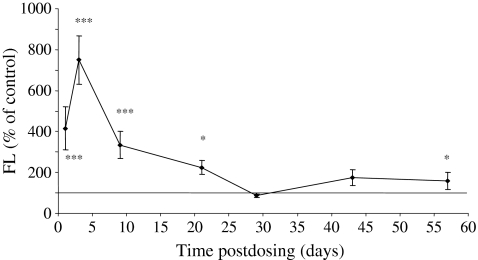
On day 1 postdosing, the mean serum FL level was significantly increased in AZA-treated mice to a mean of 1759.1 pg/ml compared with the control mean value of 423.2 pg/ml (P < 0.001) (Figure 3). On days 3 and 9 postdosing, mean FL levels continued to be significantly elevated in AZA-treated mice to 2556.0 and 1436.1 pg/ml compared with mean control values of 337.3 and 429.5 pg/ml respectively (P < 0.001). On day 21 postdosing, serum FL continued to be significantly elevated in AZA-treated mice (P < 0.05) before returning to a value comparable to the controls on day 29 postdosing. On days 43 and 57 postdosing, FL was slightly increased in AZA-treated mice.
Figure 3.
Serum fms-like tyrosine kinase-3 (FLT-3) ligand (FL) results from mice treated with azathioprine (AZA) (100 mg/kg) and autopsied on days 1, 3, 9, 22, 29, 43 and 57 postdosing. Values are means ± SD expressed as a percentage of the concurrent control mean value. There were three to seven animals in the control groups and four to eight animals in the AZA-treated groups. *Significantly different from control animals, P < 0.05; ***P < 0.001.
Serum from control and AZA-treated mice was also assayed for IL-2, TNF-α and IFN-γ. The concentrations of these three cytokines in the serum of both control and AZA-treated mice were however, below detectable levels. The reasons for this are unclear.
Bone marrow clonogenic assays
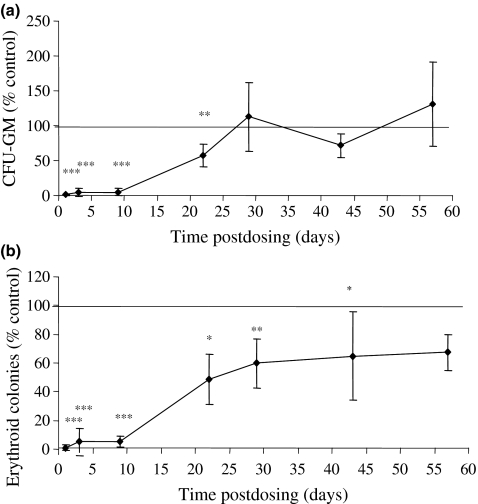
On day 1, 3 and 9 postdosing, the number of CFU-GM colonies in the femur of AZA-treated mice was significantly reduced to 1.0%, 3.9% and 5.1% of the mean concurrent control value respectively (P < 0.001) (Figure 4a). On day 22, the number of CFU-GM colonies in AZA-treated animals was showing signs of recovery; on day 29 postdosing, the mean number of CFU-GM colonies per femur in the bone marrow of AZA-treated mice was comparable to the control value. A slight reduction in CFU-GM colonies was evident on day 43 but on day 57 postdosing, the number of CFU-GM colonies in the femur was similar to the control mice.
Figure 4.
Committed progenitor cell content of femoral marrow from mice treated with azathioprine (AZA) (100 mg/kg) and autopsied on days 1, 3, 9, 22, 29, 43 and 57 postdosing. (a) The number of granulocyte-monocyte colony-forming units (CFU-GM) per femur; (b) the number of erythroid colonies per femur. Values are expressed as means ± SD, expressed as a percentage of the concurrent control mean value. The number of animals in the control and AZA-treated groups are set out in Table 4; *significantly different from control animals, P < 0.05; **P < 0.01; ***P < 0.001.
Similar changes were seen in the number of erythroid colonies per femur after AZA treatment (Figure 4b). In the immediate postdosing period, the number of erythroid colonies per femur was reduced to 1.0%, 5.3% and 5.2% of the control mean values on days 1, 3 and 9 respectively. On days 22 and 29 postdosing, signs of recovery were evident in the number of erythroid colonies but the numbers continued to remain significantly reduced. Indeed, at the later stages of the experiment (days 43 and 57 postdosing), erythroid colonies remained reduced compared with the mean control value, this decrease was however only statistically significant on day 43.
Apoptosis in bone marrow cells
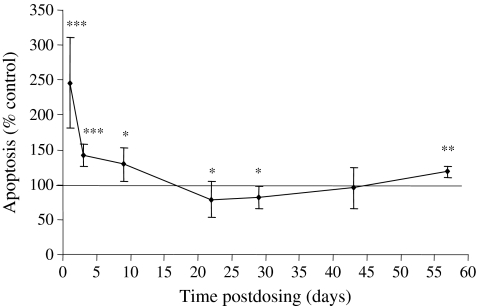
Immediately after dosing (day 1), the level of apoptosis in the femoral bone marrow cells of AZA-treated mice was significantly elevated to 246.1% of the mean control value (Figure 5). On days 3 and 9 postdosing, the levels of apoptosis in bone marrow cells continued to be significantly elevated compared with the control mice; however, a return towards normal control values was clearly evident at these times. However, on days 22 and 29, the levels of apoptosis were reduced, being 78.5% and 82.3% of the control mean value respectively. At the later stages of the experiment, on days 43 and 57 postdosing, levels of apoptosis were slightly decreased (day 43), or increased (day 57), in relation to the control; this effect was, however, only significant on day 57.
Figure 5.
Apoptosis in the femoral bone marrow cells of mice treated with azathioprine (AZA) (100 mg/kg) and autopsied on days 1, 3, 9, 22, 29, 43 and 57 postdosing. Values are means ± SD expressed as a percentage of the concurrent control mean value. The number of animals in the control and AZA-treated groups are set out in Table 4; *significantly different from control animals, P < 0.05; **P < 0.01; ***P < 0.001.
Organ weight changes and histopathological assessment of tissues
On day 1 and 3 postdosing, a significant reduction in the mean relative spleen weight was evident (P < 0.001 at each time point); the decreases in weight were to 59.4% and 72.9% of the concurrent control mean relative weights respectively. However, relative spleen weights returned to values comparable to the control animals on day 9 postdosing. On days 22, 29 and 43 postdosing, increases in relative spleen weights were present in AZA-treated mice; the increases were to 125.5% (P < 0.01), 118.4% (NS), and 128.0% (P < 0.01) of the control values respectively. On day 57 postdosing the relative spleen weight in AZA-treated animals was similar to the control values.
The mean relative liver weight of mice treated with AZA were comparable to the control relative liver weights on day 1 postdosing. However, on day 3 postdosing, relative liver weights were increased significantly in AZA-treated mice to 111.0% of control (P < 0.01). On days 9, 22 and 29 postdosing, the mean relative liver weights of AZA-treated mice was similar to the control animals. On day 43 and 57 postdosing, relative liver weights in AZA-treated mice were significantly increased (to 111.3% of control, P < 0.01, day 43; and to 115.6% of control, P < 0.01, day 57).
The relative weights of the kidneys of animals treated with AZA were increased on day 9 postdosing (to 114.5% of the controls, P < 0.01). However, this change was related to the decreased body weight of the AZA-treated animals at this time point (the absolute kidney weights were not increased) and therefore the increase in relative weight is not considered to have a biological significance. Increased relative kidney weights were not evident at other time points.
Sections of sternum, spleen, liver and thymus were assessed histologically in control mice autopsied on days 1 and 57 postdosing (n = 5 randomly selected mice on each occasion) and in AZA-treated mice autopsied on days 1, 9, 22 and 57 postdosing (n = 5 randomly selected mice at each time point).
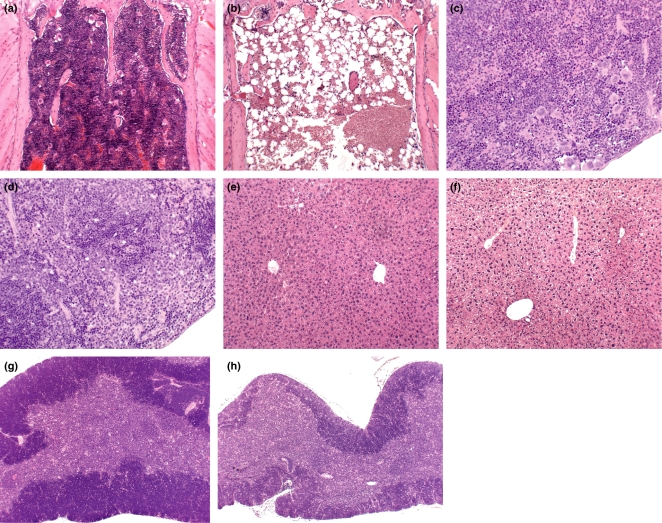
The histological assessment of sternal sections of the bone marrow showed a significant reduction in cellularity on days 1 and 9 postdosing (Figure 6a,b). However, the cellularity of the sternum was comparable to control mice on days 22 and 57 postdosing. Histological assessment of the spleen (Figure 6c,d) showed a marked reduction in extramedullary haemopoiesis on days 1 and 9 postdosing; this reduction in extramedullary haemopoiesis then decreased in significance after day 9, with only mild changes being evident on day 22 postdosing. Centrilobular hypertrophy was evident in the livers of AZA-treated mice autopsied on day 1 postdosing (Figure 6e,f). However, this change had resolved by day 9 postdosing. Hepatocyte cytoplasmic vacuolation consistent with glycogen vacuolation/rarefaction was evident in some AZA-treated and control animals. Mild atrophy of the thymus was evident on day 1 postdosing in AZA-treated mice (Figure 6g,h). This relatively mild change on day 1 became more pronounced on day 9, before returning to normality on day 57 postdosing. As Experiment 2 (Dose–response study) did not show evidence of AZA-induced kidney toxicity, the kidneys from animals in Experiment 3 were not examined histologically.
Figure 6.
Haematoxylin and eosin stained sections of sternum, spleen, liver and thymus from control and azathioprine- (AZA-) treated mice (100 mg/kg daily for 10 days). (a) Sternum from a control (vehicle-treated) mouse at day 1 postdosing showing normal marrow cellularity. [Original magnification (OM) × 100.] (b) Sternum from an AZA-treated mouse at day 1 after the final dose showing significant depletion in the cellularity of the marrow, with increased numbers of adipocytes. (OM × 100.) (c) Spleen from a control (vehicle-treated) mouse at day 9 postdosing, showing the normal appearance of the spleen. (OM × 200.) (d) Spleen from an AZA-treated mouse at day 9 postdosing showing the absence of extramedullary haemopoiesis. (OM × 200.) (e) Liver from a control (vehicle-treated) mouse at day 1 postdosing, showing the normal appearance of the hepatic lobules. (OM × 100.) (f) Liver from an AZA-treated mouse at day 1 postdosing showing centrilobular hypertrophy; hepatocytes show cytoplasmic vacuolation consistent with glycogen vacuolation/rarefaction. (OM × 100.) (g) Thymus from a control (vehicle-treated) mouse at day 9 postdosing. (OM × 50.) (h) Thymus from an AZA-treated mouse on day 9 postdosing showing marked atrophy. (OM × 50.)
Discussion
AZA, a derivative of 6-MP, was initially developed as a slow-release form of 6-MP (Elion 1989). After administration in man, AZA rapidly undergoes glutathione-dependent non-enzymatic cleavage, principally within erythrocytes (Marino & Doyle 1994), to yield 6-MP and an imidazole side chain (Elion 1989). 6-MP then undergoes further metabolism via three competing pathways (Dollery 1999; Sweetman 2005). Firstly, in the intestinal mucosa and liver, 6-MP is converted into 6-thiouric acid by xanthine oxidases; secondly, the enzyme thiopurine methyl-transferase (TPMT) converts 6-MP into the inactive metabolite 6-methyl-mercaptopurine (6-MMP), and thirdly, hypoxanthine phosphoribosyltransferase (HPRT) converts 6-MP into 6-thioguanine nucleotides (6-TGNs). The generation of 6-TGNs is essential for the therapeutic success of AZA therapy in man. Indeed, an increased rate of relapse is seen in children with leukaemia who, following treatment with 6-MP, produce lower than average concentrations of 6-TGNs as a result of higher TPMT activity (Lennard 1992; Lennard et al. 1993). 6-TGNs are however, also responsible for the toxic side effects resulting from AZA treatment (Lennard 1992). 6-TGNs disrupt purine synthesis thus reducing the availability of ribonucleotides, and in turn, reducing the synthesis of RNA and DNA (Dollery 1999). In addition, 6-TGNs become incorporated into DNA, and replace guanine residues, resulting in the formation of single-strand breaks and the kinking and damage of chromosomes (Jensen & Huttel 1976; Fairchild et al. 1986; Lennard et al. 1989, 1992).
The conversion of 6-MP into 6-MMP by TPMT is of great importance, as it is the activity of this enzyme which dictates the level of resulting toxicity (Lennard et al. 1989; Ben Ari et al. 1995; Aarbakke et al. 1997; Pandya et al. 2002; Schwab et al. 2002). The activity of TPMT is subject to significant inter-individual variation owing to a genetic polymorphism. The majority of human populations (89%) are homozygous for the wild-type TPMT allele, and therefore, activity of the enzyme is high. However, 11% of the population are heterozygous, and therefore, these individuals have one wild-type and one mutant allele, and as a consequence have an intermediate level of TPMT activity. But, a further 0.3% of the population are homozygous for the mutant TPMT allele, and therefore the activity of TPMT is very low or negligible in these individuals (Lennard 1992; Aarbakke et al. 1997; Leipold et al. 1997; Colombel et al. 2000). Individuals with low TPMT activity have been shown to have an increased risk of myelotoxicity to AZA due to a shifting of drug metabolism from the TPMT pathway to the HPRT pathway which yields 6-TGN (Lennard 1992; Kerstens et al. 1995; Black et al. 1998; Scerri 1999).
A number of papers describe the association between TPMT activity and myelotoxicity in renal transplant recipients and in patients with auto-immune disorders treated with AZA (Ben Ari et al. 1995; Leipold et al. 1997; Stolk et al. 1998; Colombel et al. 2000; Pandya et al. 2002; Schwab et al. 2002). Furthermore, the level of 6-TGNs in the erythrocytes of children with leukaemia is inversely proportional to the degree of neutropenia (Lennard et al. 1983). To reduce the risk of haematological side effects as a result of AZA treatment it has therefore been proposed that patients should first be screened to assess TPMT activity (Lennard et al. 1989; Kader et al. 2000). Prescreening would avoid treating susceptible patients with AZA, thus reducing the incidence of AZA-induced myelotoxicity (Kerstens et al. 1995; McGovern et al. 2002; McGovern & Travis 2003). Such a measure could prove cost-effective in the long run, taking into account the cost of monitoring patients receiving AZA therapy and the cost of supportive care in patients developing significant toxic effects (Black et al. 1998; Pandya et al. 2002; Schwab et al. 2002).
In the present studies (Experiments 2 and 3) CD-1 mice treated with AZA developed significant anaemia, leucopenia and thrombocytopenia at day 1, immediately postdosing (Tables 1 and 4). The peripheral blood counts (RBC, WBC, platelets) of mice treated with AZA further declined on days 3 and 9 postdosing (Table 4) with a significant increase in bone marrow cell apoptosis also occurring at these time points in comparison with the concurrent controls (Figure 5). This delay in the appearance of the most severe bone marrow depression (the ‘nadir’) until day 3/9 postdosing may be the result of 6-TGN incorporation into DNA. It is reported that the damaging effects of 6-TGN production may not be immediate, but that following incorporation into the DNA strand, and the subsequent replication of this faulty template, the production of DNA occurs which may contain unilateral chromatid damage and single-strand breaks (Fairchild et al. 1986; Lennard 1992).
Profound bone marrow aplasia has been found to occur in approximately 2% of patients treated with AZA with the majority of these cases presenting during the first 4 weeks of treatment. However, in man, bone marrow aplasia induced by AZA treatment is readily reversed following withdrawal of the drug (Present et al. 1989; Connell et al. 1993; Leipold et al. 1997). Nevertheless, in the present study (Experiment 3), mice treated with AZA continued to show decreases in bone marrow cellularity (FNCC) lasting until 22/29/43 days after dosing, and there was a persistent mild reduction (i.e. a ‘residual’ effect) continuing in the RBC count at day 57 postdosing (Table 4). Indeed, the RBC counts were significantly reduced at all time points following AZA treatment. Similarly, bone marrow culture demonstrated a reduction in the number of erythroid progenitor cells within the bone marrow of AZA-treated animals at all postdosing time points (Figure 4). Another effect on the erythroid line was seen in the early postdosing period, when the MCV was significantly reduced in AZA-treated mice (on day 1, 3 and 9 postdosing, Table 4). However, at later stages of the experiment (days 22, 29, 43 and 57), AZA-treated mice demonstrated macrocytosis. These two changes in erythropoiesis (anaemia and macrocytosis) are well-documented side effects of AZA treatment in man (Bottiger & Rausing 1972; McGrath et al. 1975; DeClerck et al. 1980; Creemers et al. 1993; Pruijt et al. 1996; Thompson & Gales 1996).
There is a paucity of information in the literature on the haemotoxicity of AZA in different laboratory animal species with which the present haematological findings in the mouse can be compared. In a study in the mouse, Van Furth et al. (1975) described a reversible decrease in the monocytes in the peripheral blood and bone marrow following AZA treatment. In a comprehensive investigation in the rat, De Waal et al. (1995) reported that in AZA-treated animals there was a reduction in RBC, Hb and HCT values, in conjunction with decreased MCV and MCH; the WBC count was also reduced, this being due to decreased lymphocyte and monocyte counts; in the femoral bone marrow there were decreases in the numbers of nucleated leucocytes in AZA-treated rats. In a recent study in the rat (Smith et al. 2003), AZA was gavage dosed for 30 days at 25 mg/kg (reduced to 17 mg/kg on day 10); in the AZA-treated animals the relative weight of the thymus was reduced, as was the cellularity of the spleen and the thymus; haematological studies demonstrated an AZA-induced anaemia (reduced RBC, Hb, HCT) but an increase in the reticulocyte counts; the WBC and lymphocyte counts were also reduced. Elion et al. (1961) reported that the bone marrow in the AZA-treated rat and in the dog was agranulocytic. In a report on AZA-induced bone marrow toxicity in the dog, Rinkardt and Kruth (1996) described the induction of peripheral blood pancytopenia, and the marrow was hypocellular with reductions in the myeloid, erythroid and megakaryocyte series. In a study in the AZA-treated cat, Beale et al. (1992) recorded peripheral blood leucopaenia, neutropaenia, anaemia and pancytopaenia, and there was also thrombocytopaenia and thrombocytosis; the bone marrow became hypocellular with decreases in the myeloid and erythroid series. Therefore, although the information in species other that the mouse on the haemotoxicity of AZA is sparse and rather disjointed, the overall picture (with one or two exceptions) is one which compares with the present haematological results in Experiments 2 and 3. The similarity of the haematological changes caused by AZA in other species also shows many correlations with the characteristic features of the haemotoxicity of the drug in man.
In a report by Hildner et al. (1998), changes in the levels of the inflammatory cytokines TNF-α and IFN-γ were measured in the serum of mice treated with AZA. The animals were treated with two i.p. injections of AZA at 1, 10 and 100 mg/kg on day 0 and day 2 of the study, with serum collected on day 3. TNF-α was reduced to 42%, 20% and 15% of control in the 1, 10 and 100 mg/kg treatment groups respectively. IFN-γ was also decreased, but not by the same magnitude, the reductions being to 90%, 89% and 80% of the controls at 1, 10 and 100 mg/kg AZA respectively. In man, peripheral blood mononuclear cells, separated from Crohn’s disease patients undergoing AZA treatment, were shown to produce lower concentrations of IFN-γ when stimulated in culture for 48 h (Cuffari et al. 2004). It was also shown in this report that the level of IFN-γ produced was related to the concentration of 6-TGN in the erythrocytes. Patients with high levels of 6-TGNs produced significantly lower levels of IFN-γ, compared with Crohn’s disease patients (who had low levels of 6-TGN), and compared with patients who were not treated with AZA. In the present study (Experiment 3) it was found that there was no change in the serum concentrations of TNF-α, IFN-γ or IL-2 in AZA-treated mice. Indeed, the concentrations of these cytokines in the serum of both the control and AZA-treated mice were below detectable limits. It is possible that the assay of these cytokines in mouse serum are not the optimal method of detection, and that measuring the production of the cytokines by lymphocytes in vitro may have been more productive, as in the study reported by Cuffari et al. (2004).
The concentration of FL in the serum was found to be raised in AZA-treated mice immediately postdosing (Figures 1 and 3); this increase in FL was expected, taking into account the immediate bone marrow hypoplasia induced by AZA treatment. Indeed, in a previous study (Molyneux et al. 2005) in the female CD-1 mouse treated with the anti-neoplastic drug mitomycin (MMC), a similar pattern of changes in serum FL concentrations was seen, in comparison with Experiment 3. In the MMC investigation, control mice at day 1, 7, 14 and 28 postdosing showed mean serum FL values of 467, 496, 476 and 487 pg/ml respectively. In MMC-treated mice the mean FL values were 2428, 2902 and 2006 pg/ml on days 1, 7 and 14 postdosing, respectively, falling to control levels (552 pg/ml) on day 28. Similar responses have also been identified in the busulphan-treated mouse (Molyneux et al. 2008). An increase in FL during periods of bone marrow aplasia has also been described in mice and primates following radiotherapy (Gratwohl et al. 1998; Bertho et al. 2001; Prat et al. 2005). In man, an increase in the concentration of FL in plasma has been reported in patients receiving chemotherapy and radiotherapy, and also in patients with haematological disorders associated with stem cell defects (e.g. aplastic anaemia and Fanconi’s anaemia) (Lyman et al. 1995; Wodnar- Filipowicz et al. 1996; Chklovskaia et al. 1999; Huchet et al. 2003).
In the present studies, atrophy of the thymus was observed on days 1 and 9 postdosing in AZA-treated mice. This change has been described previously following AZA treatment in both rodents and in the rhesus monkey (De Waal et al. 1995; Dollery 1999; Smith et al. 2003). In addition to thymic atrophy, a significant reduction in the lymphocyte count of AZA-treated mice was seen on days 3 and 9 postdosing (Table 4). Both atrophy of the thymus, and reduced blood lymphocyte counts, in AZA-treated mice may be a result of an increase in apoptotic cell death. In a recent report by Tiede et al. (2003), apoptosis was elevated in lymphocytes treated with AZA in vitro. This increase in lymphocyte apoptosis was found to be a result of 6-MP interaction with the guanosine triphosphate- (GTP-) binding protein, RAC-1. RAC-1 blocks the upregulation of mRNA coding for a dominant regulator of apoptosis, the protein Bcl-xL, which promotes cell survival by inhibiting apoptosis.
In Experiment 2 and 3 of the present studies, the histological examination of kidneys from AZA-treated mice revealed no evidence of toxicity. However, the liver showed centrilobular hepatocyte hypertrophy and in the spleen there was a reduction in extramedullary haemopoiesis; in the thymus there was mild atrophy. The sternal bone marrow demonstrated reduced cellularity in AZA-treated animals involving all cell lines. There are a small number of reports, in the mouse, rat, cat and dog with which these findings can be compared. In the kidney, Elion et al. (1961) recorded no histological changes in the AZA-treated dog and this was also the finding of Beale et al. (1992) in the cat; a similar negative result was reported by De Waal et al. (1995) and by Frankel et al. (1970) in the rat. However, histological changes in the liver of AZA-treated animals have been well described by several authors. Hess et al. (1976) reported that AZA was toxic for hepatocytes in the rat, but only when bile flow was impeded; also in the rat, De Waal et al. (1995) described vacuolar degeneration of hepatocytes and Frankel et al. (1970) reported scattered, enlarged, vacuolated hepatic parenchymal cells, hydropic swelling and centrilobular hepatocyte atrophy. Watanabe et al. (1979), also in the rat, reported in a 3/4-week study with AZA, the centrilobular necrosis of liver cells with the formation of scar tissue. Finally, in the cat, Beale et al. (1992) described AZA-induced multifocal to diffuse vacuolar degeneration of hepatocytes. Investigations by several authors involving hepatocytes treated with AZA in vitro have also reported the toxicity of the drug, relating the injury to glutathione depletion and mitochondrial injury (DeLeve et al. 1996; Lee & Farrell 2001; Tapner et al. 2004).
Histological changes in the spleen and thymus have been less well investigated in AZA-treated animals. Elion et al. (1961) reported that in the rat treated with AZA the spleens were agranulocytic, and De Waal et al. (1995) in the same species recorded that the spleen and thymus were significantly reduced in weight, with the spleen showing reduced cellularity of the periarteriolar lymphoid sheaths, and the cellularity of the red pulp was also reduced; the thymus showed reduced cellularity. In a study in the cat treated with AZA, however, Beale et al. (1992) recorded that the spleen was characterized by mild to moderate increase in size of the perivascular lymphoid aggregates in the white pulp, and this was a presumed reactive hyperplasia; there was also lymphoid depletion in the lymph nodes. Elion et al. (1961) reported that in the dog, AZA caused agranulocytic spleens. It can be concluded therefore that the histological changes apparent in AZA-treated mice in Experiment 2 and 3 of the present studies, in general terms, compare with the findings in the literature in laboratory animal species other than the mouse.
It has been shown in the present study (Experiment 3) that mice treated with AZA initially developed significant bone marrow depression and this was then followed by a period of recovery. However, AZA-treated mice showed some evidence of a sustained (i.e. late-stage) mild ‘residual’ injury to cells of the erythroid lineage. The effects of this disruption in erythropoiesis were seen both in the peripheral blood and in bone marrow cell cultures. The peripheral red blood cell counts of AZA-treated mice were statistically significantly reduced at all time points (Table 4) with evidence of macrocytosis on days 22, 29 and 43 postdosing. Similarly, bone marrow cultures showed a deficit in the number of erythroid colonies at all time points studied (Figure 4). Therefore, the reduction in erythroid colony number corresponded to a reduction in the number of committed progenitor cells of the erythroid lineage in the bone marrow of AZA-treated mice. However, differential cell counts performed on bone marrow smears from AZA-treated mice (Table 5) did not show a reduced number of erythroid cells. TPMT genetic polymorphisms which play a role in the development of bone marrow injury following AZA treatment in man also occur in some strains of mouse (Hernandez et al. 1990). It is possible that if the present studies had been conducted with a strain of mouse that is known to have a genetically low TMPT activity (for example, the C57BL/6J or AKR/J strains) a more acute bone marrow injury may have been observed involving all haemopoietic cell lineages.
In Experiment 1 (Preliminary dose-ranging pilot study), CD-1 mice (Charles River UK Ltd) were treated for 10 days with AZA by gavage at 0, 25, 50, 75, 100, 125, 150, 200, 250, 300, 350 and 400 mg/kg. Significant toxicity was evident in mice treated with AZA at 125 mg/kg and above. All of the higher dose level groups were either killed in extremis or were found dead during the dosing period or in the first 19 days postdosing. Mice treated with AZA at 25, 50, 75 and 100 mg/kg did not however show any evidence of AZA-induced toxicity during the postdosing period of study (19 days). This is in contrast to Experiment 3 (Main study); here the condition of the mice [ICR (CD-1) Harlan] treated with AZA daily by gavage at 100 mg/kg began to deteriorate in the immediate postdosing period. In the first 10 days postdosing, 25 AZA-treated mice were categorized as ICD animals; 2 further ICD mice were identified on day 15 postdosing. Therefore, from the 68 mice treated with in Experiment 3, a total of 27 ICD animals were recorded, resulting in a total mortality of 39.7%. The ICR (CD-1) mouse used in Experiment 3 was therefore found to be more susceptible to AZA toxicity than the CD-1 mouse used in Experiments 1 and 2. Although both strains of mouse were derived originally from the Swiss albino mouse (Charles River 2005; Harlan 2005) the possibility of strain differences in response to AZA toxicity should be borne in mind if it is necessary to purchase animals from more than one supplier.
It is of interest to compare the dose levels of AZA administered to mice in the present investigation with the rather limited information available from other published studies. In Experiment 1, 10 daily doses of AZA were given by gavage at dose levels up to 400 mg/kg, and in Experiment 2, doses from 40 to 120 mg/kg were administered; in the final study (Experiment 3), 100 mg/kg was given. Elion et al. (1961) reported the i.p., single dose, mouse LD50 of AZA to be 650 mg/kg and 2500 mg/kg p.o.; the maximum tolerated dose for the drug given on five consecutive days was 100 mg/kg i.p. and 200 mg/kg p.o. Elion et al. (1961) provided no information on the strain, sex or age of their animals. Van Furth et al. (1975) gave 200 mg/kg of AZA by s.c. injection to mice, daily for 9 days (Swiss mice, male, 25–30 g). In a carcinogenicity study by Imamura et al. (1973), AZA was given (100 mg/kg s.c.) to male and female 7-week-old C57BL mice twice a week for 2 weeks and then once a week for 7 months. Smith et al. (1999) used the transgenic lacZ mouse (males; age 4–6 weeks); animals were dosed with AZA at 10, 50 and 100 mg/kg p.o. daily for 5 days. Finally, Weisburger (1977) in a carcinogenicity study in male and female Swiss/Webster mice, administered 7.5–30 mg/kg AZA, i.p., three times weekly for 6 months. Therefore, against this background of AZA dose levels, the dose used in Experiment 3 did appear appropriate.
It is concluded therefore that the repeat dose administration (10 daily doses) of AZA to the female CD-1 mouse induces a significant bone marrow depression. In the immediate postdosing period, bone marrow cellularity is reduced and the peripheral blood shows a decrease in RBC, reticulocyte and platelet counts, and there is a leucopenia involving neutrophils, lymphocytes and monocytes; all these effects are dose-related. Over a period of 40/50 days postdosing, bone marrow and peripheral blood parameters return to normal, although there is some evidence of a mild ‘residual’ (i.e. late-stage) effect on the erythroid cell lineage. The general response in the mouse consequently appears to show similarities with the sometimes severe dose-related myelosuppression seen in the AZA-treated patient, which results in a significant leucopenia, anaemia and thrombocytopenia in the peripheral blood. The changes in the bone marrow and blood in the CD-1 mouse would therefore appear to merit further investigation as an acceptable animal model of the haemotoxicity of AZA in man.
Acknowledgments
This work was supported by grants from the Aplastic Anaemia Trust (GM, FG), The School of Pharmacy (GM) and the Triangle Trust (GM). We wish to acknowledge with thanks the assistance of the technical staff at The School of Pharmacy for their husbandry of the animals. We also gratefully acknowledge the assistance of staff in the Clinical Pathology Unit (GSK) for the analysis of blood and bone marrow samples and staff of the Pathology Department (HLS) for the preparation of tissue sections. Thanks are also due to Janssen-Cilag Ltd, Novartis Pharma Ltd and Amgen UK Ltd for their kind donation of human cytokines.
References
- Aarbakke J, Janka-Schaub G, Elion GB. Thiopurine biology and pharmacology. TiPS. 1997;18:3–7. doi: 10.1016/s0165-6147(96)01007-3. [DOI] [PubMed] [Google Scholar]
- Al-Uzri A, Yorgin PD, Kling PJ. Anemia in children after transplantation: etiology and the effect of immunosuppressive therapy on erythropoiesis. Pediatr. Transplant. 2003;7:253–264. doi: 10.1034/j.1399-3046.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- Bacon BR, Treuhaft WH, Goodman AM. Azathioprine-induced pancytopenia. Occurrence in two patients with connective-tissue diseases. Arch. Intern. Med. 1981;141:223–226. [PubMed] [Google Scholar]
- Beale KM, Altman D, Clemmons RR, Bolon B. Systemic toxicosis associated with azathioprine administration in domestic cats. Am. J. Vet. Res. 1992;53:1236–1240. [PubMed] [Google Scholar]
- Ben Ari Z, Mehta A, Lennard L, Burroughs AK. Azathioprine-induced myelosuppression due to thiopurine methyltransferase deficiency in a patient with autoimmune hepatitis. J. Hepatol. 1995;23:351–354. doi: 10.1016/0168-8278(95)80481-1. [DOI] [PubMed] [Google Scholar]
- Bertho JM, Demarquay C, Frick J, et al. Level of Flt3-ligand in plasma: a possible new bio-indicator for radiation-induced aplasia. Int. J. Radiat. Biol. 2001;77:703–712. doi: 10.1080/09553000110043711. [DOI] [PubMed] [Google Scholar]
- Black AJ, McLeod HL, Capell HA, et al. Thiopurine methyltransferase genotype predicts therapy-limiting severe toxicity from azathioprine. Ann. Intern. Med. 1998;129:716–718. doi: 10.7326/0003-4819-129-9-199811010-00007. [DOI] [PubMed] [Google Scholar]
- Blumenthal RD, Lew W, Juweid M. Plasma FLT3-L levels predict bone marrow recovery from myelosuppressive therapy. Cancer. 2000;88:333–343. [PubMed] [Google Scholar]
- BNF. British National Formulary. London: British Medical Association; 2004. [Google Scholar]
- Borel JF, Feurer C, Gubler HU, Stahelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976;6:468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- Bostrom B, Erdmann G. Cellular pharmacology of 6-mercaptopurine in acute lymphoblastic leukemia. Am. J. Pediatr. Hematol. Oncol. 1993;15:80–86. [PubMed] [Google Scholar]
- Bottiger LE, Rausing A. Pure red cell anaemia: immunosuppressive treatment. Ann. Intern. Med. 1972;76:593–597. doi: 10.7326/0003-4819-76-4-593. [DOI] [PubMed] [Google Scholar]
- Burke DA, Dixon MF, Axon AT. Ulcerative colitis: prolonged remission following azathioprine-induced pancytopenia. J. Clin. Gastroenterol. 1989;11:327–330. [PubMed] [Google Scholar]
- Calne RY. The rejection of renal homografts. Inhibition in dogs by 6-mercaptopurine. Lancet. 1960;1:417–418. doi: 10.1016/s0140-6736(60)90343-3. [DOI] [PubMed] [Google Scholar]
- Calne RY. Inhibition of the rejection of renal homografts in dogs by purine analogues. Transplant. Bull. 1961;28:65–81. [PubMed] [Google Scholar]
- Calne R. Cyclosporine as a milestone in immunosuppression. Transplant. Proc. 2004;36:13S–15S. doi: 10.1016/j.transproceed.2004.01.042. [DOI] [PubMed] [Google Scholar]
- Calne RY, Alexandre GP, Murray JE. A study of the effects of drugs in prolonging survival of homologous renal transplants in dogs. Ann. N. Y. Acad. Sci. 1962;99:743–761. doi: 10.1111/j.1749-6632.1962.tb45358.x. [DOI] [PubMed] [Google Scholar]
- Calne RY, White DJ, Rolles K, Smith DP, Herbertson BM. Prolonged survival of pig orthotopic heart grafts treated with cyclosporin A. Lancet. 1978;1:1183–1185. doi: 10.1016/s0140-6736(78)90971-6. [DOI] [PubMed] [Google Scholar]
- Calne RY, Rolles K, White DJ, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979;2:1033–1036. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- Charles River. Charles River UK Limited, Margste, Kent, UK. Product information (Technical support). http://www.criver.com.
- Chklovskaia E, Jansen W, Nissen C, et al. Mechanism of flt3 ligand expression in bone marrow failure: translocation from intracellular stores to the surface of T lymphocytes after chemotherapy-induced suppression of hematopoiesis. Blood. 1999;93:2595–2604. [PubMed] [Google Scholar]
- Colombel JF, Ferrari N, Debuysere H, et al. Genotypic analysis of thiopurine S-methyltransferase in patients with Crohn’s disease and severe myelosuppression during azathioprine therapy. Gastroenterology. 2000;118:1025–1030. doi: 10.1016/s0016-5085(00)70354-4. [DOI] [PubMed] [Google Scholar]
- Connell WR, Kamm MA, Ritchie JK, Lennard-Jones JE. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993;34:1081–1085. doi: 10.1136/gut.34.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creemers GJ, Van Boven WP, Lowenberg B, Van Der Heul C. Azathioprine-associated pure red cell aplasia. J. Intern. Med. 1993;233:85–87. doi: 10.1111/j.1365-2796.1993.tb00654.x. [DOI] [PubMed] [Google Scholar]
- Cuffari C, Li DY, Mahoney J, Barnes Y, Bayless TM. Peripheral blood mononuclear cell DNA 6-thioguanine metabolite levels correlate with decreased interferon-gamma production in patients with Crohn’s disease on AZA therapy. Dig. Dis. Sci. 2004;49:133–137. doi: 10.1023/b:ddas.0000011614.88494.ee. [DOI] [PubMed] [Google Scholar]
- De Waal EJ, Timmerman HH, Dortant PM, Kranjc MA, Van Loveren H. Investigation of a screening battery for immunotoxicity of pharmaceuticals within a 28-day oral toxicity study using azathioprine and cyclosporin A as model compounds. Regul. Toxicol. Pharmacol. 1995;21:327–338. doi: 10.1006/rtph.1995.1047. [DOI] [PubMed] [Google Scholar]
- DeClerck YA, Ettenger RB, Ortega JA, Pennisi AJ. Macrocytosis and pure RBC anemia caused by azathioprine. Am. J. Dis. Child. 1980;134:377–379. doi: 10.1001/archpedi.1980.04490010035012. [DOI] [PubMed] [Google Scholar]
- DeLeve LD, Wang X, Kuhlenkamp JF, Kaplowitz N. Toxicity of azathioprine and monocrotaline in murine sinusoidal lining cells and hepatocytes: the role of glutathione and relevance to hepatic venoocclusive disease. Hepatology. 1996;23:589–599. doi: 10.1002/hep.510230326. [DOI] [PubMed] [Google Scholar]
- Dollery C. Therapeutic Drugs. Edinburgh: Churchill Livingstone; 1999. [Google Scholar]
- Elion GB. The purine path to chemotherapy. Science. 1989;244:41–47. doi: 10.1126/science.2649979. [DOI] [PubMed] [Google Scholar]
- Elion GB, Bieber S, Hitchins GH. A summary of investigations with 2-amino-6-[(1-methyl-4-nitro-5-imidazolyl)thio]purine (B.W. 57-323) in animals. Cancer Chemother. Rep. 1960;8:36–43. [PubMed] [Google Scholar]
- Elion GB, Callahan S, Bieber S, Hitchings GM, Rundles RW. A summary of investigations with 6-[(1-methyl-4-nitro-5-imidazolyl)thio] purine (B.W. 57-322) Cancer Chemother. Rep. 1961;14:93–98. [Google Scholar]
- Fairchild CR, Maybaum J, Kennedy KA. Concurrent unilateral chromatid damage and DNA strand breakage in response to 6-thioguanine treatment. Biochem. Pharmacol. 1986;35:3533–3541. doi: 10.1016/0006-2952(86)90623-4. [DOI] [PubMed] [Google Scholar]
- Frankel HH, Yamamoto RS, Weisburger EK, Weisburger JH. Chronic toxicity of azathioprine and the effect of this immunosuppressant on liver tumor induction by the carcinogen N-hydroxy-N-2-fluorenylacetamide. Toxicol. Appl. Pharmacol. 1970;17:462–480. doi: 10.1016/0041-008x(70)90203-6. [DOI] [PubMed] [Google Scholar]
- Gibson FM, Andrews CM, Diamanti P, et al. A new model of busulphan-induced chronic bone marrow aplasia in the female BALB/c mouse. Int. J. Exp. Pathol. 2003;84:31–47. doi: 10.1046/j.1365-2613.2003.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin WE, Kaufman JJ, Mims MM, et al. Human renal transplantation. I. Clinical experiences with six cases of renal homotransplantation. J. Urol. 1963;89:13–24. doi: 10.1016/S0022-5347(17)64491-4. [DOI] [PubMed] [Google Scholar]
- Gratwohl A, John L, Baldomero H, et al. FLT-3 ligand provides hematopoietic protection from total body irradiation in rabbits. Blood. 1998;92:765–769. [PubMed] [Google Scholar]
- Harlan. Harlan UK Limited, Bicester, Oxon, UK. Product information (Technical support). http://www.harlan.com.
- Hernandez JS, Van Loon JA, Otterness DM, Weinshilboum RM. Mouse thiopurine methyltranserase pharmacogenetics: correlation of immunoreactive protein and enzymatic activity. J. Pharmacol. Exp. Ther. 1990;252:658–673. [PubMed] [Google Scholar]
- Hess F, Jerusalem C, Polak M. Azathioprine hepatotoxicity, direct complication or secondary effect in rat liver transplantation. Eur. Surg. Res. 1976;8:156–165. doi: 10.1159/000127857. [DOI] [PubMed] [Google Scholar]
- Hildner K, Marker-Hermann E, Schlaak JF, et al. Azathioprine, mycophenolate mofetil, and methotrexate specifically modulate cytokine production by T cells. Ann. N. Y. Acad. Sci. 1998;859:204–207. doi: 10.1111/j.1749-6632.1998.tb11129.x. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R, Michels M, Heininger K, Besinger U, Toyka KV. Azathioprine toxicity during long-term immunosuppression of generalized myasthenia gravis. Neurology. 1988;38:258–261. doi: 10.1212/wnl.38.2.258. [DOI] [PubMed] [Google Scholar]
- Home Office. Code of Practice for the Housing and Care of Animals Used in Scientific Procedures. London: Her Majesty’s Stationary Office; 1989. [Google Scholar]
- Huchet A, Belkacemi Y, Frick J, et al. Plasma Flt-3 ligand concentration correlated with radiation-induced bone marrow damage during local fractionated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2003;57:508–515. doi: 10.1016/s0360-3016(03)00584-4. [DOI] [PubMed] [Google Scholar]
- Imamura N, Nakano M, Kawase A, Kawamura Y, Yokoro K. Synergistic action of N-nitrosobutylurea and azathioprine in induction of leukemia in C57BL mice. Gann. 1973;64:493–498. [PubMed] [Google Scholar]
- Jensen MK, Huttel MS. Assessment of the effect of azathioprine on human bone marrow cells in vivo, combining chromosome studies and the micronucleus test. Dan. Med. Bull. 1976;23:152–154. [PubMed] [Google Scholar]
- Jeurissen ME, Boerbooms AM, Van De Putte LB. Pancytopenia related to azathioprine in rheumatoid arthritis. Ann. Rheum. Dis. 1988;47:503–505. doi: 10.1136/ard.47.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kader HA, Wenner WJ, Jr, Telega GW, Maller ES, Baldassano RN. Normal thiopurine methyltransferase levels do not eliminate 6-mercaptopurine or azathioprine toxicity in children with inflammatory bowel disease. J. Clin. Gastroenterol. 2000;30:409–413. doi: 10.1097/00004836-200006000-00011. [DOI] [PubMed] [Google Scholar]
- Kerstens PJ, Stolk JN, Hilbrands LB, van de Putte LB, De Abreu RA, Boerbooms AM. 5-nucleotidase and azathioprine-related bone-marrow toxicity. Lancet. 1993;342:1245–1246. [PubMed] [Google Scholar]
- Kerstens PJ, Stolk JN, De Abreu RA, Lambooy LH, van de Putte LB, Boerbooms AA. Azathioprine-related bone marrow toxicity and low activities of purine enzymes in patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:142–145. doi: 10.1002/art.1780380122. [DOI] [PubMed] [Google Scholar]
- Klippel JH, Decker JL. Relative macrocytosis in cyclophosphamide and azathioprine therapy. JAMA. 1974;229:180–181. [PubMed] [Google Scholar]
- Lawson DH, Lovatt GE, Gurton CS, Hennings RC. Adverse effects of azathioprine. Adverse Drug React. Acute Poisoning Rev. 1984;3:161–171. [PubMed] [Google Scholar]
- Lee AU, Farrell GC. Mechanisms of azathioprine-induced injury to hepatocytes: roles of glutathione depletion and mitochondrial injury. J. Hepatol. 2001;35:756–764. doi: 10.1016/s0168-8278(01)00196-9. [DOI] [PubMed] [Google Scholar]
- Leipold G, Schutz E, Haas PJ, Oellerich M. Azathioprine-induced severe pancytopenia due to a homozygous two-point mutation of the thiopurine methyltransferase gene in a patient with juvenile HLA-B27-associated spondylarthritis. Arthritis Rheum. 1997;40:1896–1898. doi: 10.1002/art.1780401026. [DOI] [PubMed] [Google Scholar]
- Lemley DE, Delacy LM, Seeff LB, Ishak KG, Nashel DJ. Azathioprine induced hepatic veno-occlusive disease in rheumatoid arthritis. Ann. Rheum. Dis. 1989;48:342–346. doi: 10.1136/ard.48.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard L. The clinical pharmacology of 6-mercaptopurine. Eur. J. Clin. Pharmacol. 1992;43:329–339. doi: 10.1007/BF02220605. [DOI] [PubMed] [Google Scholar]
- Lennard L, Rees CA, Lilleyman JS, Maddocks JL. Childhood leukaemia: a relationship between intracellular 6-mercaptopurine metabolites and neutropenia. Br. J. Clin. Pharmacol. 1983;16:359–363. doi: 10.1111/j.1365-2125.1983.tb02178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard L, Van Loon JA, Weinshilboum RM. Pharmacogenetics of acute azathioprine toxicity: relationship to thiopurine methyltransferase genetic polymorphism. Clin. Pharmacol. Ther. 1989;46:149–154. doi: 10.1038/clpt.1989.119. [DOI] [PubMed] [Google Scholar]
- Lennard L, Hale JP, Lilleyman JS. Red blood cell hypoxanthine phosphoribosyltransferase activity measured using 6-mercaptopurine as a substrate: a population study in children with acute lymphoblastic leukaemia. Br. J. Clin. Pharmacol. 1993;36:277–284. doi: 10.1111/j.1365-2125.1993.tb00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman SD, Seaberg M, Hanna R, et al. Plasma/serum levels of flt3 ligand are low in normal individuals and highly elevated in patients with Fanconi anemia and acquired aplastic anemia. Blood. 1995;86:4091–4096. [PubMed] [Google Scholar]
- Marino IR, Doyle HR. Conventional immunosuppressant drugs. In: Thomsom AW, Starzl TE, editors. Immunosuppressive Drugs. Developments in Anti-rejection Therapy. Boston: Little Brown; 1994. pp. 5–7. [Google Scholar]
- MC. Medicines Compendium. Epsom: Datapharm Communications; 2003. [Google Scholar]
- McGovern DP, Travis SP. Thiopurine therapy: when to start and when to stop. Eur. J. Gastroenterol. Hepatol. 2003;15:219–223. doi: 10.1097/01.meg.0000049993.68425.13. [DOI] [PubMed] [Google Scholar]
- McGovern DP, Travis SP, Duley J, Shobowale-Bakreel M, Dalton HR. Azathioprine intolerance in patients with IBD may be imidazole-related and is independent of TPMT activity. Gastroenterology. 2002;122:838–839. doi: 10.1053/gast.2002.32124. [DOI] [PubMed] [Google Scholar]
- McGrath BP, Ibels LS, Raik E, Hargrave MJ. Erythroid toxicity of azathioprin. Macrocytosis and selective marrow hypoplasis. Q. J. Med. 1975;44:57–63. [PubMed] [Google Scholar]
- Molyneux G, Gibson FM, Marway H, et al. Myelotoxic effects of the immunosuppressant drug azathioprine (AZA) in the CD-1 mouse after repeat dose administration. Toxicology. 2004;202:83. [Google Scholar]
- Molyneux G, Gibson FM, Gordon-Smith EC, et al. The haemotoxicity of mitomycin in a repeat dose study in the female CD-1 mouse. Int. J. Exp. Pathol. 2005;86:415–430. doi: 10.1111/j.0959-9673.2005.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneux G, Gibson FM, Whayman M, Turton JA. Serum FLT-3 ligand in a busulphan-induced model of chronic bone marrow hypoplasia in the female CD-1 mouse. Int. J. Exp. Pathol. 2008;89:159–170. doi: 10.1111/j.1365-2613.2008.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Merrill JP, Harrison JH, Wilson RE, Dammin GJ. Prolonged survival of human-kidney homografts by immunosuppressive drug therapy. N. Engl. J. Med. 1963;268:1315–1323. doi: 10.1056/NEJM196306132682401. [DOI] [PubMed] [Google Scholar]
- Nicholls AJ, Davidson RJ. Development of macrocytosis during azathioprine therapy after renal transplantation. A correlation of renal function with MCV. Transplantation. 1979;27:220–221. [PubMed] [Google Scholar]
- Old CW, Flannery EP, Grogan TM, Stone WH, San Antonio RP. Azathioprine-induced pure red blood cell aplasia. JAMA. 1978;240:552–554. [PubMed] [Google Scholar]
- Pandya B, Thomson W, Poulton K, Bruce I, Payne D, Qasim F. Azathioprine toxicity and thiopurine methyltransferase genotype in renal transplant patients. Transplant. Proc. 2002;34:1642–1645. doi: 10.1016/s0041-1345(02)02963-9. [DOI] [PubMed] [Google Scholar]
- Philpott NJ, Scopes J, Marsh JC, Gordon-Smith EC, Gibson FM. Increased apoptosis in aplastic anaemia bone marrow progenitor cells: possible pathophysiological significance. Exp. Hematol. 1995;23:1642–1648. [PubMed] [Google Scholar]
- Philpott NJ, Turner AJ, Scopes J, et al. The use of 7-amino-actinomycin D in identifying apoptosis: simplicity of use and broad spectrum of application compared with other techniques. Blood. 1996;87:2244–2251. [PubMed] [Google Scholar]
- Prat M, Demarquay C, Frick J, Thierry D, Gorin NC, Bertho JM. Radiation-induced increase in plasma Flt3 ligand concentration in mice: evidence for the implication of several cell types. Radiat. Res. 2005;163:408–417. doi: 10.1667/rr3340. [DOI] [PubMed] [Google Scholar]
- Present DH, Meltzer SJ, Krumholz MP, Wolke A, Korelitz BI. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann. Intern. Med. 1989;111:641–649. doi: 10.7326/0003-4819-111-8-641. [DOI] [PubMed] [Google Scholar]
- Pruijt JF, Haanen JB, Hollander AA, Den Ottolander GJ. Azathioprine-induced pure red-cell aplasia. Nephrol. Dial. Transplant. 1996;11:1371–1373. [PubMed] [Google Scholar]
- Rinkardt NE, Kruth SA. Azathioprine-induced bone marrow toxicity in four dogs. Can. Vet. J. 1996;37:612–613. [PMC free article] [PubMed] [Google Scholar]
- Rundles RW, Laszlo J, Itoga T, Hobson JB, Garrison FE., Jr Clinical and hematologic study of 6[(1-methyl-4-nitro-5-imidazolyl)thio]-purine (B.W.57-322) and related compounds. Cancer Chemother. Rep. 1961;14:99–115. [PubMed] [Google Scholar]
- Scerri L. Azathioprine in dermatology. An overview with special emphasis on its use in non-bullous inflammatory dermatoses. Rheuma. Derm. 1999;53:343–348. [PubMed] [Google Scholar]
- Schwab M, Schaffeler E, Marx C, et al. Azathioprine therapy and adverse drug reactions in patients with inflammatory bowel disease: impact of thiopurine S-methyltransferase polymorphism. Pharmacogenetics. 2002;12:429–436. doi: 10.1097/00008571-200208000-00003. [DOI] [PubMed] [Google Scholar]
- Schwartz R, Dameshek W. Drug-induced immunological tolerance. Nature. 1959;13:1682–1683. doi: 10.1038/1831682a0. [DOI] [PubMed] [Google Scholar]
- Schwartz R, Stack J, Dameshek W. Effect of 6-mercaptopurine on antibody production. Proc. Soc. Exp. Biol. Med. 1958;99:164–167. doi: 10.3181/00379727-99-24281. [DOI] [PubMed] [Google Scholar]
- Smith CC, Archer GE, Forster EJ, Lambert TR, Rees RW, Lynch AM. Analysis of gene mutations and clastogenicity following short-term treatment with azathioprine in MutaMouse. Environ. Mol. Mutagen. 1999;34:131–139. [PubMed] [Google Scholar]
- Smith HW, Winstead CJ, Stank KK, Halstead BW, Wierda D. A predictive F344 rat immunotoxicology model: cellular parameters combined with humoral responses to NP-CγG and KLH. Toxicology. 2003;194:129–145. doi: 10.1016/j.tox.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Stolk JN, Boerbooms AM, De Abreu RA, et al. Reduced thiopurine methyltransferase activity and development of side effects of azathioprine treatment in patients with rheumatoid arthritis. Arthritis Rheum. 1998;41:1858–1866. doi: 10.1002/1529-0131(199810)41:10<1858::AID-ART19>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Sudhir RR, Rao SK, Shanmugam MP, Padmanabhan P. Bilateral macular hemorrhage caused by azathioprine-induced aplastic anemia in a corneal graft recipient. Cornea. 2002;21:712–714. doi: 10.1097/00003226-200210000-00016. [DOI] [PubMed] [Google Scholar]
- Sweetman SC. Martindale: The Complete Drug Reference. 34. London: Pharmaceutical Press; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapner MJ, Jones BE, Wu MW, Farrell GC. Toxicity of low dose azathioprine and 6-mercaptopurine in rat hepatocytes. Role of xanthine oxidase and mitochondrial injury. J. Hepatol. 2004;40:454–463. doi: 10.1016/j.jhep.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Thompson DF, Gales MA. Drug-induced pure red cell aplasia. Pharmacotherapy. 1996;16:1002–1008. [PubMed] [Google Scholar]
- Tiede I, Fritz G, Strand S, et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J. Clin. Invest. 2003;111:1133–1145. doi: 10.1172/JCI16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton JA, Sones WR, Andrews CM, et al. Further development of a model of chronic bone marrow aplasia in the busulphan-treated mouse. Int. J. Exp. Pathol. 2006;87:49–63. doi: 10.1111/j.0959-9673.2006.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Furth R, Gassmann AE, Diesselhoff-Den Dulk MM. The effect of azathioprine (Imuran) on the cell cycle of promonocytes and the production of monocytes in the bone marrow. J. Exp. Med. 1975;141:531–546. doi: 10.1084/jem.141.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Hobara N, Tobe K, Endo H, Nagashima H. Biochemical and morphological study on hepatotoxicity of azathioprine in rat. Acta Med. Okayama. 1979;33:5–14. [PubMed] [Google Scholar]
- Weisburger EK. Bioassay program for carcinogenic hazards of cancer chemotherapeutic agents. Cancer. 1977;40:1935–1949. doi: 10.1002/1097-0142(197710)40:4+<1935::aid-cncr2820400827>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Whisnant JK, Pelkey J. Rheumatoid arthritis: treatment with azathioprine (IMURAN (R)). Clinical side-effects and laboratory abnormalities. Ann. Rheum. Dis. 1982;41:S44–S47. doi: 10.1136/ard.41.suppl_1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasinghe SN, Dodsworth H, Rault RM, Hulme B. Observations on the incidence and cause of macrocytosis in patients on azathioprine therapy following renal transplantation. Transplantation. 1974;18:443–446. doi: 10.1097/00007890-197411000-00009. [DOI] [PubMed] [Google Scholar]
- Wodnar-Filipowicz A, Lyman SD, Gratwohl A, Tichelli A, Speck B, Nissen C. Flt3 ligand level reflects hematopoietic progenitor cell function in aplastic anemia and chemotherapy-induced bone marrow aplasia. Blood. 1996;88:4493–4499. [PubMed] [Google Scholar]