Abstract
Elastin is the major extracellular matrix component synthesized, secreted and deposited by vascular smooth muscle cells (SMCs) in the arterial media and thus plays an important role in vascular homeostasis. Results of our previous studies showed that 1α,25-dihydroxycholecalciferol [1,25(OH)2D3-calcitriol] accelerates proliferation of SMCs and modulates their growth in vitro. The aim of this study was to find ultrastructural support for the idea that 1,25(OH)2D3-calcitriol affects elastic fibre formation due to accelerated proliferation of aortal SMCs in vitro. SMCs exposed 10 days to supraphysiological concentration (10 nM) of calcitriol in primary culture were examined by fluorescence and transmission electron microscopy. Morphological studies revealed that calcitriol altered elastin maturation by favouring accumulation of immature rather than fully processed elastic fibres. A substantial decrease in the amorphous elastin deposition and abnormal accumulation of microfibrillar component, in thickened multilayer culture, were observed. These studies suggest that 1,25(OH)2D3 affect formation of elastic fibres due to enhanced proliferation of SMCs in culture.
Keywords: calcitriol, elastic fibres, smooth muscle cells, ultrastructure
Smooth muscle cells (SMCs) constitute the most common cell type in the tunica media of arterial wall. At homeostasis, SMCs of mature animals manifest a quiescent, differentiated and contractile phenotype aimed to control vascular tonus by contraction or relaxation. These cells also produce and deposit highly structured extracellular matrix (ECM) consisting primarily of fibrillar collagen (types I and III), elastin, some proteoglycans and glycoproteins (Thyberg et al. 1990). Under pathological conditions, many external factors alter homeostatic balance, causing SMC to loose contractile properties and express an immature synthetic phenotype. Such conversion (modulation) includes a prominent structural reorganization with loss of myofilaments and formation of a large secretory apparatus. Functionally, the cells become potent to proliferate, migrate and secrete ECM (Ross 1993; Thyberg 1998). Accelerated proliferation of SMCs in response to injury and also disruption of elastic fibres are known to be common pathologic features in vascular proliferative diseases such as atherosclerosis and coronary restenosis (Raines 2000; Karnik et al. 2003; Owens et al. 2004).
Elastin is the principal structural intracellular component of large arteries, and contributes up to 50% of the vessels dry weight. Simultaneously, highly hydrophobic, insoluble elastin constitutes 90% of elastic fibres, while the remaining 10% includes microfibrillar glycoproteins. During elastogenesis, the biosynthetic precursor of elastin (tropoelastin) is secreted and properly positioned on the microfibrillar scaffold before being cross-linked by lysyl oxidase into a functional polymer. Elastic fibre assembly requires pre-existing scaffold of microfibrillar bundles that bind tropoelastin (Parks et al.1993; Debelle & Tamburro 1999). This process is well controlled during development and aging but remains responsive to external factors.
One of the nutritional factors that might alter the aortic elastin content and aortic function is an active metabolite of vitamin D (1,25(OH)2D3-calcitriol) (Norman et al. 2002). Calcitriol can exert a direct effect on vascular SMCs, which express vitamin D receptors (Merke et al. 1987). Although calcitriol affects proliferation of vascular SMCs, its inhibitory or stimulatory effect is poorly elucidated and controversial (Koh et al. 1988; Mitsuhashi et al. 1991; Tukaj & Wrzołkowa 1997; Tukaj et al. 2000, 2007; Somjen et al. 2005). In the present study, morphological techniques were used to elucidate the role of calcitriol in proliferation, migration and formation of elastic fibres in the primary culture of aortal SMCs. A major purpose was to find ultrastructural support for the idea that enhanced proliferation of aortal SMCs after calcitriol treatment indeed coincides with impaired elastogenesis.
Material and methods
Cell culture
Smooth muscle cells were obtained from the media of neonatal Wistar rat’s aorta by enzymatic digestion method as previously described (Tukaj & Wrzołkowa 1996). The cells were routinely maintained in Eagle’s minimal essential medium (MEM; Biomed, Lublin, Poland) enriched with 10% foetal bovine serum (FBS; Sigma, St. Louis, MO, USA) with 100 U penicillin/ml. Medium changes were made every second day, and each time 10 nM of 1,25(OH)2D3 (D1530; Sigma) in 95% ethanol was added. The same amount of ethanol was added to one of the two control cultures and the final concentration of ethanol both in the test and the control culture medium was 0.1%. SMCs were examined after 10 days, when they grew logarithmically and formed multilayer. The purity and identity of the SMC culture was confirmed by staining for α-smooth muscle actin and was higher than 95% (Figure 1a). An analysis of SMC viability was performed by light microscopy after trypan-blue dye staining. Primary cultures of SMCs were used for experiments. Four separate experiments were made with similar findings. The procedures were provided in accordance with institutional requirements of the Ethics Committee for Animal Care of Poland.
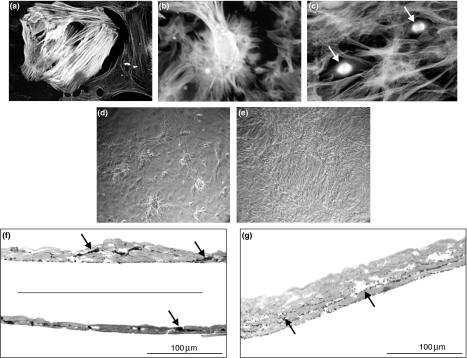
Figure 1.
(a) Positive reaction with anti-α-actin identifies SMCs in primary culture. Highly expressed network of actin stress fibres is visible in more mature contractile SMCs. (b,c) α-tubulin distribution in control (b) and calcitriol-treated (c) primary culture of SMCs. Note decreased cell adhesion and altered cell migration in culture exposed to calcitriol. Arrows show mitotically dividing cells. (d,e) SMC culture viewed under phase-contrast light microscope. Differences between both examined cultures in growth fashion are illustrated. (f,g) Semi-thin sections of SMCs in primary culture (1.5 μm, toluidine blue staining). (f) ‘Hills and valleys’ appearance in control SMC culture is visible. Characteristic close contact between the cells is also evident. Elastic fibres in extracellular spaces are visualized as black aggregates (arrows). (g) Calcitriol-treated SMCs exhibit uniform, highly abundant multilayer. Note altered deposition of elastic fibres (arrows).
Transmission electron microscopy
The cells were fixed directly in Petri dishes in 2.5% glutaraldehyde (GA) with 0.15% picric acid, and with 15% OsO4 in 0.1 M Na-cacodylate buffer at pH 7.4. Fixation was carried out at 4 °C for 2 h and then cell culture was rinsed three times in the same buffer. Following fixation, the cells were treated with 1.5% tannic acid, dehydrated with graded series of ethanol, immersed in propylene oxide, embedded in Epon 812 and polymerized. Semi-thin sections (1.5 μm) were stained with toluidine-blue and examined by light microscopy. The ultra-thin sections were collected on formvar coated cooper grids, double-stained with lead citrate and uranyl acetate and examined with the aid of a JEM 1200EX II electron microscope at an accelerating voltage of 80 kV.
Immunofluorescence
Demonstration of the presence and distribution of elastin was based on immunofluorescence detection performed by two-step reactions. Cells grown on glass cover slips were washed twice with ice-cold PBS to remove all non-cell layer-associated proteins. Cells were then fixed with ice-cold 100% methanol at −20 °C for 10 min. Non-specific immunoreactivity was blocked with 1% BSA in PBS for 1 h at room temperature. The cell layers were incubated with primary antibody: polyclonal rabbit anti-rat elastin (CL55041AP; Cedarlane, Burlington, Canada) diluted at 1:250 for 40 min at room temperature. Next, the cultures were washed in PBS and labelled for 60 min in the dark using a 1:200 dilution of a chicken anti-rabbit antibody conjugated with Alexa Fluor 488 fluorochrome (A-21441; Molecular Probes, Eugene, OR, USA). Secondary antibody alone was used as a control.
The cytoskeletal protein expression of SMCs in primary culture was performed employing the direct immunofluorescence method. The cells were incubated for 30 min at room temperature with the anti-α-tubulin monoclonal FITC-conjugated antibody (Sigma, diluted at 1:200) or anti-α-actin monoclonal FITC-conjugated antibody (Sigma, diluted at 1:500).
After a subsequent double wash with PBS to remove excess probe, cover slips were placed on slides with a drop of anti-fade mounting medium (Citifluor; Agar Scientific Limited, Stansted, UK). Material was examined with a Nikon Eclipse 800 microscope equipped for epifluorescence using the appropriate filter set.
Results
Enzymatically isolated SMCs became attached and completely spread out within 2 days after seeding. Thereafter, cells began to proliferate and grew logarithmically up to the 10–11 day of the culture. 95% of the cells were positively identified as SMCs by their reaction with antibodies against α-smooth muscle actin, which is specific to both contractile and synthetic phenotypes (Figure 1a). Characteristic growth pattern with ‘hills and valleys’ was observed by contrast-phase and fluorescence microscopy in control culture (Figure 1b,d). Focal thickening of control culture and close contact between the cells was evident in toluidine-blue-stained semi-thin sections (Figure 1f). Semi-thin sections also visualized dark conglomerates of maturing elastic fibres (Figure 1f). As shown in Figure 1c, e and g, treatment with 10 nM of calcitriol dramatically changed growth pattern of SMCs in culture. The cells formed highly abundant multilayer with flattened configuration. These data are in agreement with those reported in previous studies (Tukaj et al. 2000, 2007). Cellular multilayer after calcitriol treatment was significantly thicker than in the control culture, sometimes with two- to threefold increase in the number of SMC layers (Figure 1g).
Electron microscopy observations
A few days after seeding, most of the cells in both culture systems modulated from the contractile to synthetic phenotype. Such a transition between phenotypes resulted in a high rate of proliferation, migration and production of EMC. On the other hand, the phenotypic reversion of SMCs to the contractile state in primary culture has also been occurred. SMCs exhibiting synthetic phenotypes were entirely devoid of basement membranes.
Control culture
An ultrastructural analysis of SMCs in primary culture confirmed morphological features revealed under light microscopy. In a multilayered nodular region, the closely arranged cells became elongated and spindle shaped, with elevation of myofilament density and required contraction (Figures 2c and 3c). Basement membrane was partially rebuilt after maturation of cells. Contractile-type and synthetic-type of cells were intermingled throughout the observed nodular regions (Figure 3c). Regular arrangement of maturated elastic fibres between the cells was seen (Figure 2c). Elastic fibres composed of clearly distinguishable conglomerates of highly stained amorphous material were distributed among the bundles of microfibrillar component (Figure 3c). Microfibrils were associated with elastin during assembly period, but gradually disappeared as the fibres reached maturity.
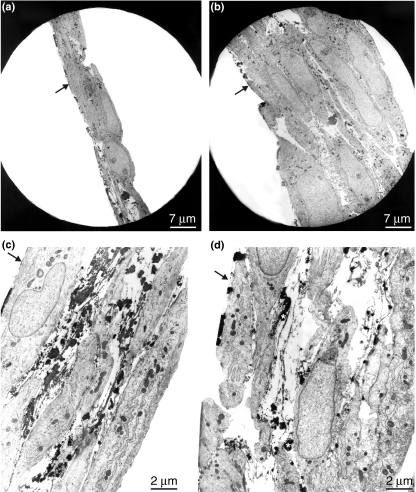
Figure 2.
Electron microscopic examination of the SMCs in primary culture. Arrows show the regions of adhesion to Petri dish. (a) Low-power magnification visualize cellular layer of aortal SMCs under control conditions. Elastic fibres are asymmetrically distributed between the cells. (b) Intensely abundant multilayer of calcitriol-treated SMC culture. (c) Nascent elastic fibres are present in the nodular region of SMC control culture. Most of the cells exhibit differentiated, myofilament-rich contractile phenotype. (d) Calcitriol-treated culture of SMCs. Oddly shaped cells with long processes probably indicating ongoing migrations are visible. Elastic fibres built of microfibrills in irregular array interspersed with small clumps of amorphous material (asterisk). Note wide extracellular spaces typical of calcitriol-treated culture.
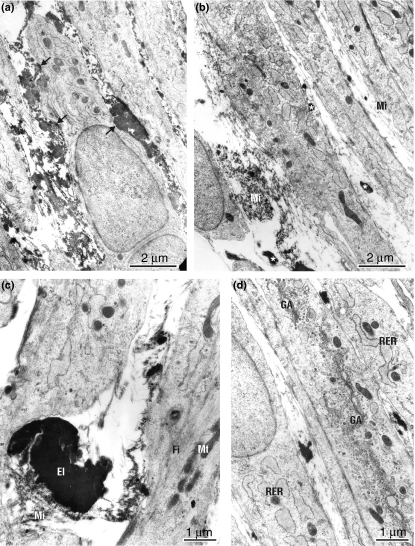
Figure 3.
(a) Smooth muscle cells in the media-like layer control culture are mostly of an ER-rich and Golgi-rich synthetic phenotype, but cells that exhibited contractile phenotypes are also present. Nascent matrix elements including amorphous elastin are located generally in cytoplasmic bays (arrows) between adjacent cells. (b) Impaired elastic fibre assembly, as small conglomerates of elastin (asterisk), among the bundles of microfibrills (Mi) predominate in extracellular space of calcitriol-treated SMC culture. (c) Newly produced elastic fibres, in control culture of SMCs, composed of amorphous elastin core (El) surrounded by a peripherally located microfibrills (Mi). Note actin filament-rich (Fi) mature, contractile SMC phenotype. Mitochondrium (Mt). (d) SMCs cultured in the presence of calcitriol demonstrate typical proliferative phenotype with dilated rough endoplasmic reticulum (RER), highly abundant Golgi apparatus (GA) and reduced contractile filaments. Impaired elastic fibre assembly is evident.
Calcitriol-treated culture
At the ultrastructural level, marked differences between SMCs in control culture and SMCs exposed to supraphysiological concentration (10 nM) of 1,25(OH)2D3 have been shown. As indicated in Figure 2b, calcitriol-treated culture revealed a significant increase in cell number and a decrease in cell adhesion. Intensively proliferating and migrating cells lost their contacts with neighbouring cells and surrounding matrix; so, they become disoriented (Figure 2d). The cells exhibited evident synthetic phenotype characterized by a prominent Golgi apparatus, rough endoplasmic reticulum and sparse intracytoplasmic filaments (Figure 3d). These marked changes in morphology observed under electron microscopy correlated with more intensive migration of cells in the culture. Addition of calcitriol to the culture markedly altered elastogenesis especially elastic fibres maturation. A comparison of the matrix within calcitriol-treated (Figures 2b,d and 3b,d) and control (Figures 2a,c and 3a,c) culture revealed differences in the amount and nature of elastin fibres. A substantial decrease in the insoluble elastin content and an abnormal accumulation of microfibrillar component was evident. Impaired elastic fibres consisted of small spotted patches of elastin embedded in a prominent microfibrillar sheet (Figures 2d and 3b,d). Frequently, microfibrils arranged into the three-dimensional meshwork within the matrix were covered with coarse, electron-dense granular material forming fibrillogranular bundles between SMCs (Figure 3b,d). Large amounts of cross-linked fine microfibrillar meshwork lacking elastin were also observed (Figure 3b).
Immunofluorescence observations
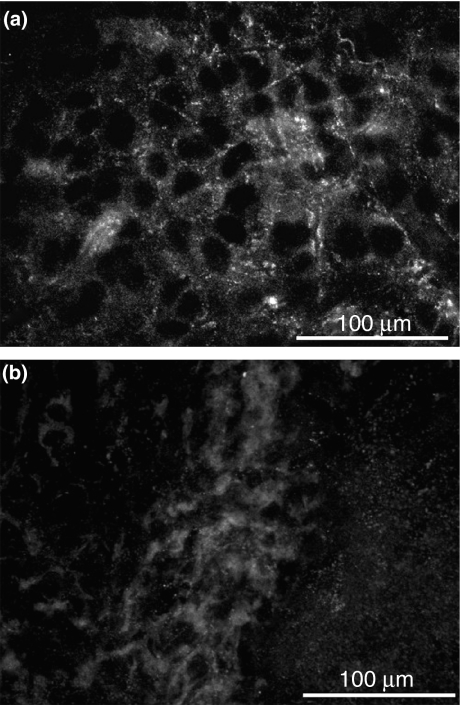
Extracellular matrix was assessed by immunofluorescence microscopy using specific elastin antibodies. Expression of elastic fibres network in control culture was evident (Figure 4a), whereas in calcitriol-treated culture was only slightly visualized (Figure 4b).
Figure 4.
(a,b) Immunofluorescence localization of matrix-associated elastin using polyclonal anti-elastin antibody and Alexa-Fluor 488-conjugated secondary antibody. (a) 10-day-old control culture demonstrates the network of newly formed elastic fibres within the matrix. (b) In contrast to control conditions, there is no deposition of mature elastin in calcitriol-treated SMC culture.
Discussion
Phenotypical modulation of SMCs from differentiated, contractile state to immature synthetic phenotype, resulted by an increase in the proliferation, migration and synthesis of ECM proteins, have been recognized as central features in vascular diseases. Under certain circumstances, which are not well defined, SMCs migrate from media into the intima forming neointima (Schwartz et al. 1986; Thyberg et al. 1990; Ross 1993; Thyberg 1998; Owens et al. 2004). Over the past two decades, several external factors as cytokines, vitamins and hormones that influence vascular SMC behaviour, have been identified in experiments performed both in vivo and in vitro.
One of the factors that play an important role in vascular homeostasis is 1,25(OH)2D3. Experiments on animal models have demonstrated that the excess of 1,25-(OH)2D3 is highly toxic to vascular tissues. Massive doses of vitamin D caused arterial injury characterized by degeneration of SMCs, disruption of elastic lamellae and medial calcification (Weishaar et al. 1990; Fischer et al. 1991). Norman & Powell (2005) demonstrated that exposure to increased amounts of vitamin D in developing organisms results in a reduction in elastin content and, on the other hand in the increasing force generation in the aorta of chronically vitamin D-deficient rats. It has been also recognized that 1,25(OH)2D3 changes phenotype and modulates growth of aortal SMC culture, but the obtained results are limited and controversial (Koh et al. 1988; Mitsuhashi et al. 1991; Tukaj & Wrzołkowa 1997; Tukaj et al. 2000, 2007; Somjen et al. 2005). The investigations performed by Somjen et al. revealed the local production of 1,25(OH)2D3 by 1α-hydroxylase system in human vascular SMCs. In their experimental model, phytoestrogens induced synthesis of calcitriol, but in consequence caused reduction in SMC proliferation. We previously reported that calcitriol promotes the initial rate of phenotypic modulation, resulting in an increase in SMC proliferation in the log-phase of growth (Tukaj & Wrzołkowa 1997). The proliferative effect of low concentrations (1–100 nM) of calcitriol was evidenced by analysing the kinetics of aortal SMC divisions using flow cytometry method (Tukaj et al. 2007). None of the examined low concentrations of calcitriol caused apoptosis.
However, it is well known that progression in atherosclerotic and restenotic lesion formation involves not only proliferative and migratory response of vascular SMCs to a variety of environmental agents but also alteration of synthesis and deposition of EMC (Katsuda et al. 1990; Raines 2000; Urban et al. 2002; Jacob 2003; Karnik et al. 2003; Krettek et al. 2003). Especially, disruption and destruction of elastin is an important contributor to the pathogenesis of occlusive vascular diseases (Karnik et al. 2003; Krettek et al. 2003). The present study provides the first demonstration that accelerated proliferation of aortal SMCs exposed to active metabolite of vitamin D-1,25 D3 indeed coincides with the impaired formation of elastic fibres in vitro. We used aortal SMCs derived from neonatal rats because it is known to produce much more elastin than that obtained from an adult one. The supra-physiological (10 nM) concentration of 1,25(OH)2D3 was chosen on the basis of results on before mentioned studies, in which the maximal proliferative effect was assessed (Tukaj et al. 2007). Results presented here visualize ongoing but ineffective elastogenesis. Defect in elastin maturation by favouring accumulation of immature rather than fully processed elastin fibres was observed. It is likely, that more aggressive proliferation of SMCs exposed to calcitriol was associated with their phenotypic alterations including the initiation of an abnormal synthetic programme. On the other hand, it has been demonstrated that 1,25(OH)2D3, an active metabolite of vitamin D3 inhibits elastin gene expression and synthesis in monolayer culture of SMCs (Hinek et al. 1991). The findings describing here are in agreement rather with those observed by Katsuda et al. (1990) showing that abnormal accumulation of microfibrils and deficiency of cell-elastic matrix connections contributes to excessive elastolysis of performed or newly formed elastic fibres during tissue remodelling. Similarly, Hinek and Wilson (2000) hypothesized that proteoglycans accumulation in atheroma may prevent integration into the functional elastic fibres. It has also been implicated that elastin is a negative regulator of SMC activity within the arterial wall and promotes their quiescent, contractile phenotype (Urban et al. 2002; Karnik et al. 2003). Therefore, it is interesting to speculate if observed here structural alterations, with loss of interaction between cells and ECM, may be the result of either inhibition of tropoelastin synthesis or defect in elastic fibres maturation or an enzymatic degradation of newly formed elastic fibres.
In summary, our findings support that calcitriol plays an important role in vascular biology due to its effects on elastic fibre formations. The in vitro alterations presented here in structure and distribution of elastic fibres suggest also that this hormone contributes to the development of atherosclerotic lesions in vivo.
Acknowledgments
This work was supported by grant BW-989 from the Medical University of Gdańsk. I thank Teresa Skałkowska, Sylwia Ścisłowska and Dr Jerzy Bohdanowicz for their technical assistance.
References
- Debelle L, Tamburro AM. Elastin: molecular description and function. Int. J. Biochem. Cell Biol. 1999;31:261–272. doi: 10.1016/s1357-2725(98)00098-3. [DOI] [PubMed] [Google Scholar]
- Fischer E, Armentano R, Levenson J, et al. Paradoxically decreased aortic wall stiffness in response to vitamin D3-induced calcinosis. Circ. Res. 1991;68:1549–1559. doi: 10.1161/01.res.68.6.1549. [DOI] [PubMed] [Google Scholar]
- Hinek A, Wilson SE. Impaired elastogenesis in Hurler disease: dermatan sulfate accumulation linked to deficiency in elastin-binding protein and elastic fiber assembly. Am. J. Pathol. 2000;156:925–938. doi: 10.1016/S0002-9440(10)64961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A, Botney MD, Mecham RP. Inhibition of tropoelastin expression by 1,25dihydroxyvitamin D3. Connect. Tissue Res. 1991;26:155–166. doi: 10.3109/03008209109152434. [DOI] [PubMed] [Google Scholar]
- Jacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed. Pharmacother. 2003;57:195–202. doi: 10.1016/s0753-3322(03)00065-9. [DOI] [PubMed] [Google Scholar]
- Karnik SK, Brooke BS, Bayes-Genis A, et al. A critical role for elastin signaling in vascular morphogenesis and disease. Development. 2003;130:411–423. doi: 10.1242/dev.00223. [DOI] [PubMed] [Google Scholar]
- Katsuda S, Okada Y, Nakanishi I. Abnormal accumulation of elastin-associated microfibrils during elastolysis in the arterial wall. Exp. Mol. Path. 1990;52:13–24. doi: 10.1016/0014-4800(90)90054-h. [DOI] [PubMed] [Google Scholar]
- Koh E, Morimoto S, Fukuo K, et al. 1,25 dihydroxyvitamin D3 binds specifically to rat vascular smooth muscle cells and stimulates their proliferation in vitro. Life Sci. 1988;42:215–223. doi: 10.1016/0024-3205(88)90685-6. [DOI] [PubMed] [Google Scholar]
- Krettek A, Sukhova GK, Libby P. Elastogenesis in human arterial disease. Arterioscler. Thromb. Vasc. Biol. 2003;23:582–587. doi: 10.1161/01.ATV.0000064372.78561.A5. [DOI] [PubMed] [Google Scholar]
- Merke J, Hofmann W, Goldschmidt D, Ritz E. Demonstration of 1,25(OH)2 vitamin D3 receptors and actions in vascular smooth muscle cells in vitro. Calcif. Tissue Int. 1987;17:135–1142. doi: 10.1007/BF02555253. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi T, Morris RC, Ives HE. 1,25-dihydroxyvitamin D3 modulates growth of vascular smooth muscle cells. J. Clin. Invest. 1991;87:1889–1895. doi: 10.1172/JCI115213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman PE, Powell JT. Vitamin D, shedding light on the development of disease in peripheral arteries. Arterioscler. Thromb. Vasc. Biol. 2005;25:9–46. doi: 10.1161/01.ATV.0000148450.56697.4a. [DOI] [PubMed] [Google Scholar]
- Norman P, Moss I, Sian M, Gosling M, Powell J. Maternal and postnatal vitamin D ingestion influences rat aortic structure, function and elastin content. Cardiovasc. Res. 2002;55:169–174. doi: 10.1016/s0008-6363(02)00444-3. [DOI] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Parks WC, Pierce RA, Lee KA, Mecham RP. The extracellular matrix. Adv. Mol. Cell. Biol. 1993;6:133–182. [Google Scholar]
- Raines EW. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int. J. Exp. Pathol. 2000;81:173–182. doi: 10.1046/j.1365-2613.2000.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Schwartz SM, Campbell GR, Campbell JH. Replication of smooth muscle cells in vascular disease. Circ. Res. 1986;58:427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Somjen D, Weisman Y, Kohen F, et al. 25-hydroxyvitamin D3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005;111:1666–1672. doi: 10.1161/01.CIR.0000160353.27927.70. [DOI] [PubMed] [Google Scholar]
- Thyberg J. Phenotypic modulation of smooth muscle cells during formation of neointimal thickenings following vascular injury. Histol. Histopathol. 1998;13:871–891. doi: 10.14670/HH-13.871. [DOI] [PubMed] [Google Scholar]
- Thyberg J, Hedin U, Sjölund M, Palmberg L, Bottger BA. Regulation of differentiated properties and proliferation of arterial smooth muscle cells. Arteriosclerosis. 1990;10:966–990. doi: 10.1161/01.atv.10.6.966. [DOI] [PubMed] [Google Scholar]
- Tukaj C, Wrzołkowa T. Effects of vitamin D on aortic smooth muscle cells in culture. Toxicol In Vitro. 1996;10:701–711. doi: 10.1016/s0887-2333(96)00057-4. [DOI] [PubMed] [Google Scholar]
- Tukaj C, Wrzołkowa T. 1,25(OH)2D3 stimulates phenotypical changes and proliferation of vascular smooth muscle cells in culture. In: Norman AW, editor. Vitamin D Chemistry, Biology and Clinical Application of the Steroid Hormone. Riverside, NJ: University of California; 1997. pp. 787–788. Proceedings of the Tenth Workshop on Vitamin D. [Google Scholar]
- Tukaj C, Kubasik- Juraniec J, Kraszpulski M. Morphological changes of aortal smooth muscle cells exposed to calcitriol in culture. Med. Sci. Monit. 2000;6:668–674. [PubMed] [Google Scholar]
- Tukaj C, Trzonkowski P, Kubasik Juraniec J, Myśliwski A. Quantifying division of aortal smooth muscle cell in culture stimulated by 1,25(OH)2D3. J. Steroid Biochem. Mol. Biol. 2007;103:525–528. doi: 10.1016/j.jsbmb.2006.12.100. [DOI] [PubMed] [Google Scholar]
- Urban Z, Riazi S, Seidl TL, et al. Connections between elastin haploinsufficiency and cell proliferation in patients with supravalvular aortic stenosis and Williams–Beuren syndrome. Am. J. Hum. Genet. 2002;71:30–44. doi: 10.1086/341035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weishaar R, Kim S, Saunders D, Simpson R. Involvement of vitamin D3 with cardiovascular function Effects on physical and morphological properties. Am. J. Physiol. 1990;258:E134–E142. doi: 10.1152/ajpendo.1990.258.1.E134. [DOI] [PubMed] [Google Scholar]