Abstract
Skin window procedures in humans have shown rapid accumulation of neutrophils into the exuded fluids above abraded skin. The present study was undertaken to determine if similar epicutaneous neutrophil accumulation might explain the extreme resistance of HRS/J mice, both hairless (hr/hr) and haired (hr/+), to experimental cutaneous Bacillus anthracis Sterne infections on abraded skin. In this study, very early (6 h) biopsies demonstrated a lack of bacilli in skin from the HRS/J hr/hr mice, indicating that the organisms never did invade in these animals as opposed to early skin entry and then efficient clearance by host responses in the tissues. Touch preparations of either the inoculation filter or the skin surface revealed more inflammatory cells, fewer bacilli, and a higher percentage of cell-associated bacilli in the HRS/J hr/hr mice than in comparator strains. In the HRS/J mice, cyclophosphamide treatment or separation of inoculated spores from the inflammatory infiltrates by a second filter below both produced marked increases in the number of bacilli observed. Examination of inoculation filter specimens demonstrated ingestion of spores and bacilli by neutrophils inside the filter at 6 h after inoculation. These findings suggest that an early and vigorous inflammatory cell infiltrate in HRS/J mice attacks the inoculated organisms above the skin surface and does not allow them to invade the tissues below.
Keywords: Bacillus anthracis, cutaneous anthrax, neutrophils
The present study was undertaken to investigate how the skin acts to prevent invasion by microbial pathogens, particularly Bacillus anthracis. Defenses of the skin against microbial invaders are complex, but they would appear to begin with effective physical barriers. The stratum corneum at the skin's outer surface is an insoluble layer composed of lipids and a variety of proteins that include the keratins, a group of mechanically hard and chemically unreactive proteins that protect animals and allow them to live in dry environments (Kimyai-Asadi et al. 2003; Tsuruta et al. 2002). For most organisms, the stratum corneum barrier must be injured in some way before they can effectively enter the skin; examples of cutaneous damage that appear necessary to permit infection include tape stripping or skin incision for Staphylococcus aureus (Akiyama et al. 2002; Kugelberg et al. 2005), microinjection for Hemophilus ducreyi (Spinola et al. 1994, 1996), and skin abrasion for Bacillus anthracis (Dixon et al. 1999; Hahn et al. 2005). Bacillus anthracis differs from most other bacterial pathogens of the skin in that its pathogenicity requires germination of spores, which are the infecting microbial form for this organism (Hanna & Ireland 1999). Studies showing differences in spore germination between rats vs. mice have indicated that reduced germination in rat tissues may be a reason for the relative resistance of this species (Hachisuka 1969).
In addition to protecting against infection, the stratum corneum also helps to maintain water homeostasis for the body (Tsuruta et al. 2002). Mild damage to this layer produces a serous exudate that contains a host inflammatory response consisting primarily of neutrophils (Wandall 1980; Yee et al. 1994; Koivuranta-Vaara 1985). For most cutaneous infections, it is not clear if these cells are directly involved in host defense, or are merely a reflection of an inflammatory response within the damaged skin where the host is directly attacking the invading microorganisms. In the former case, the neutrophilic infiltrates might represent a component of the skin's barrier function. We have developed a model system in mice using experimental inoculations of B. anthracis (Sterne) spores onto intact or abraded skin to investigate cutaneous defenses against this organism (Hahn et al. 2005). Recent work in this system has demonstrated that HRS/J mice, either hairless (hr/hr mutants) or haired (hr/+ heterozygotes), are particularly resistant to these inoculations and that this resistance appears to be related to an enhanced inflammatory response in the skin rather than to the abnormal hair follicles in the hr/hr mutant animals (Bischof et al. 2007a).
The previous studies in our experimental infection model demonstrated that the HRS/J hr/hr hairless mice had extremely few B. anthracis bacilli found histologically in the inoculated skin at 24 h as compared with mouse strains such as Balb/c that are known from other studies to be resistant to this organism. The present work was undertaken to determine why the host defense was so effective in these highly resistant animals, in particular to answer the following questions: (a) Are bacilli that invade the inoculated skin of HRS/J hr/hr mice killed and degraded rapidly in the tissues, resulting in a lack of organisms seen at later times (24 h)? (b) Are the spores unable to germinate into bacilli in this mouse strain? (c) Does the epicutaneous inflammatory response attack the organisms above the skin surface such that they never enter the skin at all?
Methods
Organism
The Sterne strain of B. anthracis was obtained from the Colorado Serum Company (Denver, CO, USA) and cultured on brain heart infusion agar plates. Bacillus anthracis Sterne is a toxigenic non-encapsulated strain that is non-infectious in humans, but retains significant pathogenicity for certain inbred strains of mice (Hahn et al. 2005; Welkos & Friedlander 1988). Sporulation was induced by keeping the plates at room temperature for 4–7 days after confluent growth at 37 °C. Then the organisms were removed from the plates, washed with distilled water and treated by heating to 60 °C for 30 min to kill remaining vegetative forms. The preparations were then layered onto 58% renographin (Bracco Diagnostics, Princeton, NJ, USA), centrifuged at 3000 g for 30 min to remove remaining vegetative forms, and washed three times in saline. The spores were quantitated by both microscopic counts and colony counts. Spores were stored in saline with 10% glycerin at −20 °C.
Animals
The mouse strains that were used in these studies were HRS/J (hairless homozygous hr/hr and haired heterozygous hr/+), Balb/c, C57BL/6 or DBA/2. Previous studies have shown DBA/2 mice to be sensitive, Balb/c to be resistant, and C57BL/6 mice to be relatively resistant to B. anthracis infection (Welkos et al. 1986). The HRS/J mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA), and the other strains from Charles Rivers Laboratories (Wilmington, MA, USA); they were either male or female, and 8–14 weeks old. The mice were housed in a separate BSL 2 section of the Veterinary Medical Unit at the Milwaukee VA Medical Center.
Epicutaneous inoculations
On the day prior to the inoculations, the animals were carefully shaved over their flanks with an electric razor; the sites were examined for cutaneous defects the following day and only animals with clear skin were used. The sites to be inoculated were first washed with 10% povidone iodine solution and then with alcohol. The epidermis was prepared for epicutaneous inoculation under anaesthesia by scraping with a surgical scalpel blade until a non-bloody glistening skin layer resulted, representing damage to the stratum corneum water barrier. An inoculum of 107B. anthracis spores was added in 0.025 ml of saline to 4 mm filter paper discs (GB002, Schleicher & Schuell, Keene, NH, USA) placed onto an abraded area on the left flank of the animal, with a similar quantity of saline alone added to the disc on the opposite side. Both sites were covered with a 1.0 cm2 piece of plastic sheet (Handi-Wrap, Dow Chemical Co., Indianapolis, IN, USA), which was then taped with Transpore tape and overwrapped with Nexcare waterproof tape (both from 3M, St Paul, MN, USA). In some cases, a 1.2 cm piece of 0.45 μm filter (Millipore, Bedford, MA, USA) was put on top of the abraded skin before placement of the 4 mm spore-containing filter above it to separate the spore inoculum from the host's phagocytic cells entering the inoculation site from below. Some mice were treated with cyclophosphamide (150 mg/kg of the drug intraperitoneally 3 days before and 100 mg/kg 1 day before the inoculations) to render them leucopenic, as previously described (Sohnle & Hahn 1989). The animal studies were approved by the appropriate institutional review committees.
Monitoring of infections
After 6 or 24 h, the occlusive dressings were removed and filter touch preparations made by touching a glass slide with the skin side (underside) of the 4 mm filter disc; skin surface touch preparations were also made by touching a glass slide to the inoculated, abraded skin surface. The slides were then stained with the LeucoStat method (Fisher, Inc., Hanover Park, IL, USA). In other cases, the animals were killed at 6 h after removal of the dressings and skin samples taken for routine histology as discussed below.
Histology
Skin from inoculated areas was taken for histology with paraffin sections being prepared and stained with tissue gram stains. Each of 20 fields on each section was examined in a blind fashion for presence of organisms. In addition, the outlet of each hair follicle infundibulum seen on the entire section was examined for the presence of vegetative bacilli, with the data expressed as percent of hair follicle outlets infected.
Examination of touch preparations
After staining, the slides for the filter touch preparations were examined at 400× by light microscopy using a 20 × 20 square ocular micrometer to enumerate vegetative bacilli, host inflammatory cells and the percentage of host cell-associated bacilli. The latter represent the number of bacilli in contact with host cells divided by the total of bacilli counted. A total of 10 random fields was examined for each slide. Each bacillus in a chain was counted as a single organism. The data were expressed as total numbers of bacilli per mm2, total numbers of host cells per mm2, and percent of bacilli associated with host cells. Slides for the skin surface touch preparations were examined similarly, except that host cells and cell-associated bacilli were not enumerated because the host cells were sometimes clumped in these preparations.
Preparations of the inoculation filter
The filter onto which spores were placed during an HRS/J hr/hr mouse inoculation was removed at 6 h, fixed in 2% glutaraldehyde, post-fixed in 1% osmium, dehydrated with a series of graded alcohols, and then imbedded into epon. Thick sections were prepared for light microscopy and stained with toluidine blue. Thin sections were prepared for electron microscopy and stained with uranyl acetate and lead citrate. The thin sections were examined with a Hitachi H-600 transmission electron microscope at 75 kV.
Statistics
Bacilli seen on 6 h skin biopsy specimens were expressed as the number of fields or sections with bacilli over the total number examined. Hair follicles were examined for infection and the data expressed as the number of follicles with bacilli present per number examined or mean ± SE of the percent of follicles infected. Numbers of bacilli or host inflammatory cells seen on either filter touch preparations or skin surface touch preparations were expressed as mean ± SE of each per mm2. Cell-associated bacilli seen on filter touch preparations were expressed as a percentage. Comparisons between these values were made using the non-parametric Kruskal–Wallis test and Dunn's multiple comparison test, with significance taken as P < 0.05. The graphpad prism 4.0c statistical package was used to make these determinations.
Results
Presence of B. anthracis bacilli on skin biopsies
Studies were done on inoculated skin taken at an early time point (6 h after inoculation) to determine if organisms might be invading early into the skin of HRS/J mice, only to be killed and degraded by inflammatory cells such that they would not be seen at a later time point such as 24 h. However, very few B. anthracis bacilli were found early in the HRS/J hr/hr mice, as compared to samples from resistant Balb/c and relatively resistant C57BL/6 mice (Table 1). In fact, only 2 of 120 total fields and 0 of 139 hair follicle outlets in HRS/J hr/hr mice were found to contain bacilli, as compared to much higher percentages in the other strains. On the two sections with bacilli in the HRS/J hr/hr mice, the organisms were found right at the skin surface. Therefore, it did not appear as if the organisms ever did enter the skin of these mice.
Table 1.
Enumeration of Bacillus anthracis organisms in skin biopsies at 6 h after epicutaneous inoculation of spores onto abraded skin
| Fields with organisms | Sections with organisms | |
|---|---|---|
| Mouse strain | No. of positive/counted | No. of positive/total |
| Organisms in skin | ||
| HRS/J hr/hr | 2/120 | 1/6 |
| C57BL/6 | 38/120 | 6/6 |
| C57BL/6-CY* | 72/120 | 6/6 |
| Balb/c | 52/120 | 6/6 |
| No. of infected/total counted | Mean % ± SE | ||
|---|---|---|---|
| Mouse strain | Organisms in hair follicle outlets | ||
| HRS/J hr/hr | 0/139 | 0.0 ± 0.0 | |
| C57BL/6 | 43/125 | 34.7 ± 5.2 | |
| C57BL/6-CY | 42/81 | 53.2 ± 8.6 | |
| Balb/c | 30/105 | 29.5 ± 10.8 | |
Data from 20 fields per section, or percent of hair follicle infected over total on the section. Results are from six mice per point tested in three experiments.
Note that at 6 h inoculated skin from HRS/J hr/hr mice generally did not show organisms in skin or hair follicle outlets (P < 0.05 for percent infected hair follicles for HRS/J hr/hr mice as compared with C57BL/6 mice by the Kruskal–Wallis test and Dunn's multiple comparison test).
CY, cyclophosphamide treated.
Enumeration of organisms and host cells in touch preparations
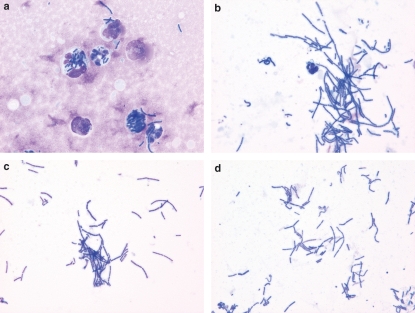
Slides made from the undersides of the filters onto which spores were placed during the inoculation process were examined to determine events occurring above the surface of the inoculated skin. Upon viewing the filter touch preparations in HRS/J hr/hr mice, large numbers of host inflammatory cells and significant percentages of cell-associated bacilli were found (Figure 1a) as compared to similar preparations from C57Bl/6 mice (Figure 1b). Comparing numerical values for HRS/J hr/hr mice to those from the C57BL/6 animals, numbers of bacilli were lower and host cells were higher (Table 2). Cell-associated bacilli were found to be higher in the HRS/J mice on filter touch preparations from 24 h, reaching 67.6% in these animals. At this time, ratios of bacilli to host cells in non-leucopenic HRS/J hr/hr mice were approximately 1 organism for 4 host inflammatory cells, compared to approximately 23 organisms per host cell at 24 h in C57BL/6 animals. Values for bacilli and host cells in the more resistant Balb/c mice and for the heterozygous HRS/J hr/+ mice were generally in-between those for the HRS/J hr/hr and C57BL/6 animals. It should also be noted that host cells were enumerated in the 6 h filter touch preparations of abraded skin inoculated with saline only and found to show 19.7 ± 5.9 cells/mm2 in HRS/J hr/hr mice and 4.2 ± 1.8 cells in C57BL/6 mice (six of each were tested). These numbers are much reduced from the host cells counted in B. anthracis inoculated skin (234.2 ± 87.3 host cells in HRS/J hr/hr and 16.9 ± 5.5 in C57BL/6 mice).
Figure 1.
Bacillus anthracis organisms at 6 h after inoculation of spores onto abraded skin of mice. Photomicrographs were taken of touch preparations from the undersides of filters onto which the spores were inoculated. (a) Inflammatory cells and cell-associated bacilli in a non-leucopenic HRS/J hr/hr mouse. (b) Bacilli with fewer inflammatory cells in a non-leucopenic C57BL/6 mouse. (c) Bacilli without inflammatory cells in a leucopenic HRS/J hr/hr mouse. (d) Bacilli without inflammatory cells in a sample from a non-leucopenic HRS/J hr/hr mouse inoculated over a second (0.45 μm) filter separating the organisms from the skin below (LeukoStat stains with photomicrographs taken at an original magnification of 1000×).
Table 2.
Accumulation of Bacillus anthracis bacilli and inflammatory cells in epicutaneous exudates after inoculation of spores onto abraded skin
| Bacilli/mm2 SSTP* | Bacilli/mm2 FTP | Cells/mm2 FTP | C-A Bacilli FTP | |
|---|---|---|---|---|
| At 6 h | ||||
| HRS/J hr/hr | 16.0 ± 3.8 (8) | 177.9 ± 61.4 (8) | 234.2 ± 87.3 (8) | 46.2 ± 10.2 (8) |
| HRS/J hr/+ | 30.0 ± 14.6 (4) | 206.0 ± 47.3 (4) | 42.2 ± 15.3 (4) | 19.3 ± 4.8 (4) |
| HRS/J hr/hr-CY† | 3518.4 ± 1023.9 (6) | 226.5 ± 110.7 (4) | 8. 5 ± 7.1 (4) | 6.9 ± 6.9 (4) |
| C57BL/6 | 407.1 ± 98.2 (11) | 865.9 ± 245.5 (11) | 16.9 ± 5.5 (11) | 7.6 ± 2.5 (11) |
| C57BL/6-CY | 2696.6 ± 947.6 (4) | 849. 9 ± 185.8 (9) | 5.3 ± 1.7 (9) | 1.6 ± 0.8 (9) |
| DBA/2 | 1308.2 ± 193.5 (6) | 219.3 ± 129.6 (6) | 3.3 ± 4.9 (6) | 4.1 ± 6.6 (6) |
| Balb/c | 500.8 ± 203.5 (12) | 719.9 ± 179.8 (13) | 37.6 ± 8.4 (13) | 6.5 ± 1.3 (13) |
| Balb/c-CY | NT‡ | 1358.4 ± 326.2 (3) | 19.7 ± 9.4 (3) | 3.4 ± 1.6 (3) |
| At 24 h (n) | ||||
| HRS/J hr/hr | NT | 118.9 ± 43.9 (4) | 513.3 ± 143.3 (4) | 67.6 ± 5.9 (4) |
| HRS/J F on F¶ | NT | 5613.5 ± 832.5 (6) | 0.3 ± 0.3 (6) | – |
| C57BL/6 | NT | 1628.4 ± 598.4 (8) | 72.0 ± 24.5 (8) | 22.6 ± 8.1 (8) |
SSTP or FTP means values for B. anthracis bacilli/mm2 in skin surface touch preparations (SSTP), or for bacilli/mm2, host cells/mm2, or cell-associated bacilli (C-A Bacilli) in filter touch preparations (FTP), with numbers of animals given in parentheses. FTP were made from the underside of the filter discs onto which spores were placed during inoculation onto abraded skin; SSTP were made by touching a slide onto the inoculated abraded skin after removal of the dressings. Data represent mean ± SE.
CY, cyclophosphamide treated.
NT, not tested.
F on F = Bacilli/mm2 or host cells/mm2 in filter touch preparations made from the top filter placed over a larger 0.45 μm underlying filter on the skin surface; the data are combined from studies in two HRS/J hr/hr and four HRS/J hr/+ mice.
Note that for 6 h values as compared to C57BL/6 mice, HRS/J hr/hr mice show fewer bacilli in SSTPs (P < 0.05) and fewer host cells in FTPs (P < 0.05); for comparison, host cells numbers in saline-inoculated abraded skin were 19.7 ± 5.9 per mm2 in HRS/J hr/hr vs. 4.3 ± 1.8 per mm2 in C56BL/6 mice. Cyclophosphamide treatment of HRS/J hr/hr mice increased bacilli numbers in SSTPs (P < 0.001) and decreased host cell numbers in FTPs (P < 0.01). Cell-associated bacilli were found to be higher on 24 h FTPs in HRS/J hr/hr mice compared with C57BL/6 mice (P < 0.02). F on F preparations for HRS/J mice revealed markedly increased bacilli and reduced cells at 24 h compared with standard FTPs for these animals (P < 0.01 for both). Statistical comparisons were made using the non-parametric Kruskal–Wallis test and Dunn's multiple comparison test.
Treatment with cyclophosphamide reversed the findings in the HRS/J hr/hr mice, with fewer host inflammatory cells being seen on the samples (Figure 1c). Numerical values in the leucopenic HRS/J hr/hr mice demonstrated marked change, with increased numbers of bacilli seen on the skin surface touch preparations and reduced numbers of host cells seen on the filter touch preparations (Table 2).
Results after inoculation above a second filter
One possibility to explain the relative lack of bacilli in HRS/J hr/hr mice is that the spores may not germinate or the vegetative bacilli may not proliferate in the epicutaneous fluids of this strain. Therefore, some inoculations were performed with the inoculation filter separated from the abraded skin below by a larger (1.2 cm) 0.45 μm filter underneath. Examination of inoculation filter (the top filter) touch preparations from HRS/J hr/hr mice handled in this way did indeed show numerous bacilli at 24 h after inoculation (Figure 1d). Many of these organisms were seen to be in chains, indicating proliferation from the initial germinated spore. Such inoculations were carried out in 2 HRS/J hr/hr and 4 HRS/J hr/+ mice; values for bacilli found per mm2 on preparations from the undersides of the top filter were as follows: 6114 and 4363 in the hr/hr animals, and 2516, 6386, 5360 and 8669 in the hr/+ ones. Therefore, the organisms do germinate and proliferate readily in the epicutaneous fluid exudates of the HRS/J mice when inflammatory cells are excluded.
Examination of the inoculation filter
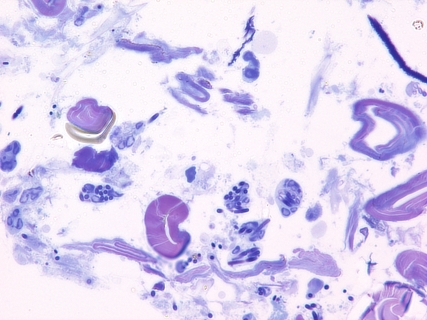
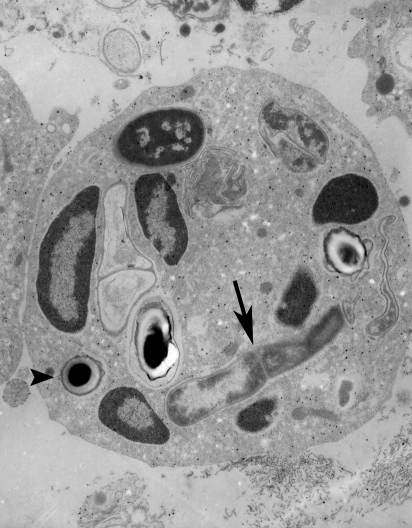
Light microscopy of epon sections made from the 6 h inoculation filter on an HRS/J hr/hr mouse demonstrated that host inflammatory cells, mostly neutrophils, had infiltrated into the filter and contacted the B. anthracis spores and bacilli there (Figure 2). Inflammatory cells that had contacted or ingested the organisms were readily seen within the filter interstices. Electron microscopy of a like preparation demonstrated that internalization of ungerminated spores and vegetative bacilli had occurred (Figure 3). Therefore, it would appear that the inflammatory infiltrate of the HRS/J hr/hr mouse strain is very active in these inoculations and is capable of attacking the organisms in regions above the surface of the abraded skin.
Figure 2.
Bacillus anthracis organisms at 6 h after inoculation of spores onto abraded skin of an HRS/J hr/hr mouse. Photomicrograph was taken of an epon section made from the filter onto which the spores were placed during inoculation. Note that many of the spores and bacilli within the inoculation filter itself have been ingested by inflammatory cells (Toluidine blue stain with photomicrograph taken at an original magnification of 1000×).
Figure 3.
Bacillus anthracis organisms at 6 h after inoculation of spores onto abraded skin of an HRS/J hr/hr mouse. Electron micrograph was taken of an epon section made from the filter onto which the spores were placed during inoculation. Note that ungerminated spores (arrowhead) and vegetative bacilli (arrow) have been ingested by a neutrophil (original magnification of 8000×).
Discussion
Previous studies using the present B. anthracis Sterne epicutaneous inoculation system demonstrated that mice of the HRS/J strain, either hairless hr/hr mutants or haired heterozygote hr/+ animals, were markedly resistant to the expected cutaneous infections and that this resistance appeared to be related to an enhanced inflammatory response rather than to the deformed hair follicles and lack of hair shafts in the hr/hr mice. The present experiments were undertaken to explain why the inoculated skin of the HRS/J mice showed minimal numbers of B. anthracis bacilli, whereas many more organisms were found in similar preparations from other mouse strains. One possibility is that the organisms in HRS/J hr/hr mice had already invaded the skin surface, but had been attacked and cleared by the time (24 h after inoculation) that the tissues were examined in the previous work (Bischof et al. 2007a). However, the 6 h time points of the present study again demonstrated an almost complete lack of organisms in the inoculated skin of HRS/J hr/hr mice as compared to samples from other mouse strains, suggesting that cutaneous invasion never does occur in the HRS/J mice. Specimens from an above-surface site, consisting of touch preparations from the undersides of the inoculation filters or skin surface touch preparations, demonstrated reduced numbers of bacilli, more cells, and more cell-associated bacilli in the HRS/J hr/hr mice than in comparator mice.
Cyclophosphamide treatment of the HRS/J hr/hr mice caused significant changes, including a decrease in host cells seen on the filter touch preparations and an increase in the numbers of bacilli seen on the skin surface touch preparations in the treated animals. In the previous study, cyclophosphamide treatment of the HRS/J hr/hr mice markedly increased tissue invasion observed on histological sections at 24 h after inoculation. As an alternative to induction of leucopenia in the present study, inoculations were performed over a second filter to separate the organisms from the inflammatory infiltrate of the HRS/J hr/hr mice; under these conditions, the spores were found to readily germinate and to produce significant numbers of vegetative bacilli. Thus, exclusion of inflammatory cells from the skin surface sites (by either induction of leucopenia or by use of a second filter) appears to markedly increase organism growth at this superficial site. Furthermore, inflammatory cells (predominantly neutrophils) were found to migrate into the inoculation filter at 6 h after inoculation in the HRS/J hr/hr mice and to attack spores and bacilli at that site. Therefore, several lines of evidence from the present study suggest that an early and vigorous inflammatory cell infiltrate in HRS/J mice is capable of preventing the inoculated organisms from invading the abraded skin under the inoculation site. The result may be to reduce the effective inoculum of viable organisms to a number below that needed to produce a sustained cutaneous infection in the animals.
These findings suggest neutrophil clearance of B. anthracis organisms in HRS/J mouse epicutaneous fluids at inoculation sites on abraded skin. When skin is abraded, as is readily evident from skin window studies in humans, there is a brisk exudation of host inflammatory cells that are primarily neutrophils (Wandall 1980; Yee et al. 1994; Koivuranta-Vaara 1985). We have previously found a similar exudation of inflammatory cells, predominantly neutrophils, in the abraded skin of mouse strains such as C57BL/6, DBA/2, and HRS/J hr/hr (Bischof et al. 2007a). Also, neutrophils from humans and mice have been found to be capable of killing B. anthracis spores and/or bacilli in vitro (Welkos et al. 1989; Mayer-Scholl et al. 2005); in fact, in one study encapsulated bacilli of the B. anthracis Vollum strain were phagocytosed and killed efficiently by human neutrophils through a mechanism dependent upon α-defensins (Mayer-Scholl et al. 2005). On the other hand, studies using specific neutrophil and macrophage depletion in mice have demonstrated that neutrophils appear to be unimportant in the defense against experimental B. anthracis Ames infections produced using intraperitoneal and inhalational inoculations (Cote et al. 2006). However, these inoculation routes would be expected to place the spores in contact initially with macrophages, rather than neutrophils. As discussed above, the latter appear to be the predominant inflammatory cells in early epicutaneous fluids generated by skin abrasion; these cells may be more important at this superficial cutaneous site than at deeper locations. Therefore, the neutrophils found in cutaneous exudate fluids, such as those seen in skin window studies, may actually have a direct antimicrobial function rather than merely reflecting the host's ability to mount an inflammatory response. They may constitute part of the skin's barrier function against infection.
The findings of the present study may also relate to cutaneous infections of abraded skin with other bacterial pathogens such as S. aureus or Streptococcus pyogenes. An active neutrophilic response in epicutaneous fluids might be expected to readily kill these bacteria, at least as compared to virulent encapsulated B. anthracis strains. On the other hand, these organisms do not require an initial period of germination as does B. anthracis and therefore could possibly invade damaged skin before an initial neutrophilic response would clear them. Germination of B. anthracis spores is quite rapid in appropriate media and we have found that this process is well underway within 1–3 h after inoculation in this model system (Bischof et al. 2007b). However, it may be that even a short interval like this is enough to shift the kinetics of B. anthracis infection towards clearance by the host inflammatory cells, particularly for non-encapsulated strains. Another consideration is that B. anthracis lethal toxin can suppress actin-based neutrophil chemotaxis (During et al. 2005), and early growth of this organism could prevent accumulation of neutrophils in the epicutaneous fluids of more susceptible mouse strains. Regarding S. aureus cutaneous infections, leucocidins produced by this organism could damage the phagocytic cell infiltrates and enhance the organism's ability to invade abraded skin, particularly for the strains that are now producing community-acquired infections (Dufour et al. 2002; Gladstone 1957). Therefore, although clearance in epicutaneous fluids of abraded skin is a potential host defense mechanism, its relevance may vary greatly for different pathogens.
It also appears that enhanced superficial neutrophil exudates may not explain the resistance or sensitivity of all mouse strains to B. anthracis skin infections. We find Balb/c mice to be resistant to intradermal spore injections or epicutaneous application of B. anthracis spores to abraded skin, but these animals do not show the same degree of neutrophil accumulation or control of bacilli numbers in their superficial exudates as do the HRS/J hr/hr mice. It may be that they depend more on inflammatory cell attack on the bacilli once they invade into the inoculated skin. On the other hand, the extreme resistance of HRS/J mice does seem to relate to the early attack on inoculated organisms in the epicutaneous fluids of these animals.
In summary, the results of the present investigation indicate that in HRS/J hr/hr mice a rapid inflammatory cell exudation into the epicutaneous fluids of abraded skin attacks inoculated B. anthracis Sterne organisms before they can invade the skin surface below. This mechanism may be relevant for other types of microbial invasion into damaged skin as well.
Acknowledgments
This work was supported by the United States Department of Veterans Affairs. The authors wish to thank Clive Wells of the Facility for Electron Microscopy at the Medical College of Wisconsin for his excellent electron microscopy work.
References
- Akiyama H, Huh WK, Yamasaki O, Oono T, Iwatsuki K. Confocal laser scanning microscopic observation of glycocalyx production by Staphylococcus aureus in mouse skin – does S. aureus generally produce a biofilm on damaged skin? Br. J. Dermatol. 2002;147:879–885. doi: 10.1046/j.1365-2133.2002.04962.x. [DOI] [PubMed] [Google Scholar]
- Bischof TS, Hahn BL, Sohnle PG. Experimental cutaneous Bacillus anthracis infections in hairless HRS/J mice. Int. J. Exp. Pathol. 2007a;88:75–84. doi: 10.1111/j.1365-2613.2006.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof TS, Hahn BL, Sohnle PG. Characteristics of spore germination in a mouse model of cutaneous anthrax. J. Infect. Dis. 2007b;195:888–894. doi: 10.1086/511824. [DOI] [PubMed] [Google Scholar]
- Cote CK, Van Rooijen N, Welkos SL. Roles of macrophages and neutrophils in the early host response to Bacillus anthracis spores in a mouse model of infection. Infect. Immun. 2006;74:469–480. doi: 10.1128/IAI.74.1.469-480.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon TC, Meselson M, Guillemin J, Hanna PC. Anthrax. N. Engl. J. Med. 1999;341:815–826. doi: 10.1056/NEJM199909093411107. [DOI] [PubMed] [Google Scholar]
- Dufour P, Gillet Y, Bes M, et al. Community-acquired methicillin-resistant Staphylococcus aureus infections in France – emergence of a single clone that produces Panton–Valentine leukocidin. Clin. Infect. Dis. 2002;35:819–824. doi: 10.1086/342576. [DOI] [PubMed] [Google Scholar]
- During RL, Li W, Hao B, et al. Anthrax lethal toxin paralyzes neutrophil actin-based motility. J. Infect. Dis. 2005;192:837–845. doi: 10.1086/432516. [DOI] [PubMed] [Google Scholar]
- Gladstone GP. Van Heyningen WE. Staphylococcal leucocidins. Br. J. Exp. Pathol. 1957;38:123–137. [PMC free article] [PubMed] [Google Scholar]
- Hachisuka Y. Germination of B. anthracis spores in the peritoneal cavity of rats and establishment of anthrax. Japan J. Microbiol. 1969;13:199–207. doi: 10.1111/j.1348-0421.1969.tb00454.x. [DOI] [PubMed] [Google Scholar]
- Hahn BL, Sharma S, Sohnle PG. Analysis of epidermal entry in experimental cutaneous Bacillus anthracis infections in mice. J. Lab. Clin. Med. 2005;146:95–102. doi: 10.1016/j.lab.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Hanna PC, Ireland JAW. Understanding Bacillus anthracis pathogenesis. Trends Microbiol. 1999;7:180–182. doi: 10.1016/s0966-842x(99)01507-3. [DOI] [PubMed] [Google Scholar]
- Kimyai-Asadi A, Jih MH, Freedberg IM. Epidermal cell kinetics, epidermial differentiation, and keratinization. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Fitzpatrick's Dermatology in Clinical Medicine. 6. New York: McGraw-Hill; 2003. pp. 89–98. [Google Scholar]
- Koivuranta-Vaara P. Neutrophil migration in vivo – analysis of a skin window technique. J. Immunol. Methods. 1985;79:71–78. doi: 10.1016/0022-1759(85)90393-x. [DOI] [PubMed] [Google Scholar]
- Kugelberg E, Norstrom T, Petersen TK, Duvold T, Andersson DI, Hughes D. Establishment of a superficial skin infection model in mice by using Staphylococcus aureus and Streptococcus pyogenes. Antimicrob. Agents Chemother. 2005;49:3435–341. doi: 10.1128/AAC.49.8.3435-3441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Scholl A, Hurwitz R, Brinkmann V, et al. Human neutrophils kill Bacillus anthracis. PLoS Pathogens. 2005;1:179–186. doi: 10.1371/journal.ppat.0010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohnle PG, Hahn BL. Effect of immunosuppression on epidermal defenses in a murine model of cutaneous candidiasis. J. Lab. Clin. Med. 1989;113:700–707. [PubMed] [Google Scholar]
- Spinola S, Wild MLM, Apicella MA, Gaspari AA, Campagnari AA. Experimental human infection with Haemophilus ducreyi. J. Infect. Dis. 1994;169:1146–1150. doi: 10.1093/infdis/169.5.1146. [DOI] [PubMed] [Google Scholar]
- Spinola SM, Orazi A, Arno JN, et al. Haemophilus ducreyi elicits a cutaneous infiltrate of CD4 cells during experimental human infection. J. Infect. Dis. 1996;173:394–402. doi: 10.1093/infdis/173.2.394. [DOI] [PubMed] [Google Scholar]
- Tsuruta D, Green KJ, Getsios S, Jones JCR. The barrier function of skin – how to keep a tight lid on water loss. Trends Cell Biol. 2002;12:355–357. doi: 10.1016/s0962-8924(02)02316-4. [DOI] [PubMed] [Google Scholar]
- Wandall JH. Leucocyte mobilization to skin lesions. Acta Pathol. Microbiol. Scand. 1980;88:255–261. doi: 10.1111/j.1699-0463.1980.tb00103.x. [DOI] [PubMed] [Google Scholar]
- Welkos SL, Friedlander AM. Pathogenesis and genetic control of resistance to the Sterne strain of Bacillus anthracis. Microb. Pathogen. 1988;4:53–69. doi: 10.1016/0882-4010(88)90048-4. [DOI] [PubMed] [Google Scholar]
- Welkos SL, Keener TJ, Gibbs PH. Differences in susceptibility of inbred mice to Bacillus anthracis. Infect. Immun. 1986;51:795–800. doi: 10.1128/iai.51.3.795-800.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welkos SL, Trotter RW, Becker DM, Nelson GO. Resistance to the Sterne strain of B. anthracis– phagocytic cell responses of resistant and susceptible mice. Microb. Pathogen. 1989;7:15–35. doi: 10.1016/0882-4010(89)90108-3. [DOI] [PubMed] [Google Scholar]
- Yee J, Giannias B, Kapadia B, Chartrand L, Christou NV. Exudative neutrophils – modulation of microbicidal function in the inflammatory microenvironment. Arch. Surg. 1994;129:99–105. doi: 10.1001/archsurg.1994.01420250111014. [DOI] [PubMed] [Google Scholar]