Abstract
The characterization of mice models of portal hypertension (PHT) is lacking in the literature. Therefore, the aim of the present study was to make a histological approach during development of PHT in two models of cirrhosis with PHT compared with one model of isolated PHT. The model of isolated PHT was developed by partial portal vein ligation (PPVL). Two portal hypertensive cirrhotic mice models were developed either by common bile duct ligation (CBDL) or administration of carbon tetrachloride (CCl4) subcutaneously (twice weekly, 1 ml/kg). These models represent, respectively, a secondary biliary cirrhosis and alcoholic cirrhosis. Mice were killed at several time points to evaluate liver changes by histological and ultrastructural methods. A correlation was made with portal pressure measurements. Histology revealed the absence of fibrosis or cirrhosis in PPVL mice. They developed an isolated portal hypertension. After CBDL induction, the mice developed the characteristics of cirrhosis after 6 weeks, with simultaneous increase in portal pressures. Fifty percent of the mice had ascites at that time point. Sixteen weeks after administration of CCl4, a micronodular cirrhotic aspect of the liver was seen associated with signs of portal hypertension. This is the first descriptive study of three widely used animal models in mice, allowing the study of pathophysiological changes in cirrhosis and portal hypertension. The PPVL in mice leads to a model of isolated portal hypertension. Secondary biliary cirrhosis developed after 6 weeks of common bile duct ligation in 50% of the mice that developed ascites. Subcutaneous injection of CCl4 for 16 weeks induces cirrhosis and poral hypertension, without ascites. Moreover, the present study is the first description of a cirrhotic model in mice developed by subcutaneous injections of CCl4. Well-described mice models will facilitate use of knock-out or transgenic mice and lead to a better understanding of the underlying molecular pathways in the field of portal hypertension and cirrhosis.
Keywords: bile duct ligation, carbon tetrachloride, cirrhosis, mice models, partial portal vein ligation, portal hypertension
Cirrhosis is currently ranked as one of the 10 most common causes of death in the Western world and is therefore a major health problem (Stewart & Day 2003). Portal hypertension (PHT) is the most frequent complication of cirrhosis. Taking into account the high prevalence of hepatitis C worldwide, excessive alcohol consumption and the emergence of non-alcoholic fatty liver disease, the incidence of cirrhosis and PHT is expected to increase significantly in the next decade. The mortality rates are still unacceptably high, therefore the search for new therapies is emergent and linked to the growing interest in a better knowledge of the pathophysiology. The use of animal models is of enormous importance for the study of pathophysiological disturbances of cirrhosis and PHT as they allow comprehensive study of questions, improve diagnosis and test potential therapies that cannot be addressed in human studies (Abraldes et al. 2006). Partial portal vein ligation (PPVL) (Groszmann et al. 1982; Vorobioff et al. 1983), common bile duct ligation (CBDL) (Kountouras et al. 1984; Lee et al. 1986; Colombato et al. 1992; Castaneda et al. 2000; Katsuta et al. 2005) and administration of carbon tetrachloride (CCl4) (Proctor & Chatamra 1982; Perez Tamayo 1983; Vorobioff et al. 1994) are the three most commonly used animal models for PTH. The major difference between these three models is that the first characterizes a prehepatic PTH without hepatic dysfunction, whereas the others represent PTH resulting from cirrhosis. The choice of the model that will be used will depend largely on the specific alteration of the pathophysiology of PTH to be studied. Models of prehepatic PHT are used to study alterations in the splanchnic circulation and the pathophysiology of the hyperdynamic circulation in PHT, while models of cirrhosis allow the study of the alterations in the intrahepatic microcirculation (Van de Casteele et al. 2001; Abraldes et al. 2006). So far, the most common animal used for studying PHT and cirrhosis is the rat. However, because of advances in molecular biology and the use of genetically modified animals (e.g. transgenic or knock-out mice), there is a need for well-described models in mice. In the current literature, the characterization of mice models for PHT remains rare (Chang et al. 2005). Therefore, the aim of the present study was to describe hepatic alterations and changes in portal pressures (PP) after CBDL, administration of CCl4 and PPVL in mice.
Most CCl4 cirrhotic rat models are induced by inhalation or intraperitoneal injection to achieve a high yield of cirrhosis (Benson et al. 2001; Janakat & Al-Merie 2002). These two methods have side effects. Inhalation of CCl4 might cause potential health hazards for its investigator. Peritoneal injection can lead to damage and adherences between the mesentery and bowel and subsequently limits the possibilities to perform in vivo experiments in the abdominal cavity (e.g. flow measurements and intravital microscopy). Therefore, the CCl4 model in the present study will also be the first description of a subcutaneously induced CCl4 model in mice.
Materials and methods
The experiments were performed in male Swiss albino mice (strain HsdWin: CFW-1) purchased from Harlan Laboratories (Horst, the Netherlands). The mice were kept under constant temperature and humidity in a 12 h controlled dark/light cycle. The Ethical Committee of experimental animals at the Faculty of Medicine and Health Sciences, Ghent University, Belgium, approved the protocols.
Mice model of isolated PTH
Induction of prehepatic PTH without cirrhosis was performed by PPVL. The surgical procedure was performed under sterile conditions. Mice were anaesthetized under isoflurane inhalation (Forene®; Abbott NV, Brussels, Belgium). A midline abdominal incision was performed and the portal vein was separated from the surrounding tissue. A ligature (silk cut 5-0) was tied around both portal vein and adjacent 27-gauge blunt-tipped needle (Fernandez et al. 2004). Subsequent removal of the needle yielded a calibrated stenosis of the portal vein. Mice were killed 2, 5, 7 and 14 days after PPVL (n = 6 in each group).
Mice models of cirrhosis and PHT
Common bile duct ligation
The surgical procedure was performed under sterile conditions. Under isoflurane inhalation anaesthesia, a midline abdominal incision was made and the common bile duct was isolated. The common bile duct was occluded with a double ligature of a non-resorbable suture (silk cut 5-0). The first ligature was made below the junction of the hepatic ducts and the second was made above the entrance of the pancreatic duct. The common bile duct was sectioned between the two ligatures. Mice were killed 1, 3, 4, 5 and 6 weeks after CBDL (n = 6 in each group).
Administration of CCl4
Carbon tetrachloride (Merck, Darmstadt, Germany) was administered by a dorsal subcutaneous (SC) injection (1:1 dissolved in olive oil; 1 ml/kg) twice weekly. Five per cent alcohol was added to drinking water. Preliminary work showed the need for adding alcohol to the drinking water for intensifying the development of fibrotic tissue (data not shown). Hepatic necrosis is induced when cytochrome P450 2E1 enzyme is induced by administration of ethanol.
Experiments were performed 3 days after the last CCl4 administration. Mice were killed after 4, 8, 10, 12 and 16 weeks of CCl4 administration (n = 6 in each group).
Control mice
As control group for the PPVL and CBDL models, mice were sham-operated. The abdominal cavity was opened and the portal vein or common bile duct was isolated, but no ligature was placed. Control mice for the CCl4 group received pure olive oil (1 mg/kg) SC without CCl4. Alcohol was not added to the drinking water. The control mice were killed at the same time points as their experimental counterparts as described above (n = 4 in each group).
Portal pressure measurements
Mice were fasted overnight and anaesthetized with a mixture of ketamine (Ketalar®, 50 mg/ml; Pfizer, Brussels, Belgium) and xylazine (Rompun® 2%, 20 mg/ml; Bayer, Brussels, Belgium). To evaluate PTH, the PP was measured in each mouse. The portal vein was cannulated through an ileocolic vein with a 24-gauge catheter (Becton Dickinson, Erembodegem-Aalst, Belgium), which was advanced into the portal vein and connected to a highly sensitive pressure transducer. The external zero reference point was placed at the midportion of the animal. The measurements were recorded on a multichannel computer-based recorder (Powerlab; ADInstruments, Spechbach, Germany).
Histopathology of the liver
A liver section of about 1 cm in diameter of the left, right and middle lobe was taken and processed for histological examination. Livers were fixed in 4% phosphate-buffered formaldehyde solution (Klinipath, Geel, Belgium) and embedded in paraffin. From all tissue samples, 2 μm tissue sections were cut with a Leica RM 2145 sliding microtome (Leica Microsystems, Nussloch, Germany) for histology. The liver tissue sections were stained with Mayer haematoxylin–eosin (Klinipath), 0.1% picrosirius red (Klinipath) and reticulin.
The reticulin and Sirius Red staining provide information about the increased amounts of reticulin fibres and collagen deposition and permit evaluation of the stage of fibrosis/cirrhosis. Microscopic evaluation was carried out by two independent investigators blinded to the status of the animals. The liver sections were scored according to the semi-quantitative Metavir score (Bedossa & Poynard 1996). The Metavir fibrosis stage of the portal tract was scored as follows: F0, no fibrosis; F1, fibrotic changes confined to the portal tracts (portal fibrosis), with only mild portal expansion; F2, portal fibrosis present with formation of few septa; F3, formation of portal-to-portal fibrous septa (septal fibrosis); F4, cirrhosis.
The liver sections were further scored for inflammation (0 = absent, 1 = focal alteration of the periportal or central zone in some tracts, 2 = diffuse alteration in some portal or central tracts, 3 = diffuse alteration of the periportal or central zone in all tracts). Proliferation of bile ducts was scored (0 = absent, 1 = mild, 2 = present in all portal tract areas).
Alpha-smooth muscle actin (α-SMA) expression was used to identify activated hepatic stellate cells (HSCs) that undergo a myofibroblastic phenotype. Immunohistochemical demonstration of the HSCs was carried out applying the primary antibody of α-SMA (Dako Cytomation, Carpinteria, CA, USA) at a dilution of 1/100 for 30 min at room temperature. The second layer consisted of labelled polymer-horseradish peroxidase (HRP)-anti-mouse for 30 min at room temperature. 3,3′diaminobenzidine (DAB kit; Dako) was used as a chromogenic substrate to visualize immunolabelling, resulting in a brown precipitate.
Electron microscopy
Additional experiments were set up for performing an ultrastructural characterization of the livers in the different animal models. Liver samples were taken 1, 4 and 6 weeks after CBDL and 4, 8 and 12 weeks after CCl4 administration (n = 3 in each group). In the PPVL group, livers were analysed at 14 days after induction (n = 3). Livers were fixed by perfusion through a catheter in the portal vein with 4% formaldehyde in 0.1 m Na-cacodylate buffer under stable pressure. After perfusion, small pieces of the liver were washed in buffer, postfixed with 1% osmium tetroxide at 4 °C, dehydrated through a graded series of ethanol solutions, briefly rinsed in propylene oxide and embedded in epoxy resin. Liver samples with a thickness of 2 mm were processed for electron microscopy. Thin sections were cut on a Reichert Ultracut microtome (Leica); the sections were counterstained with uranylacetate and lead and photographed at 50 kV under a Zeiss transmission electron microscope (EM 900; Carl Zeiss, Brussels, Belgium). The portal, midzonal and centrolobular areas were separately investigated with description of the alterations.
Blood samples
Blood samples were taken by puncture of the aortic bifurcation after PP measurements and were assayed by automated procedures. They were evaluated for alanine aminotransferase (ALT, U/l), aspartate aminotransferase (AST, U/l) and total bilirubin (mg/dl).
Statistical analysis
One-way analysis (anova) was used to compare the different groups as appropriate. P < 0.05 was considered statistically significant.
Results
Macroscopic findings
Macroscopic features of all experimental groups are shown in Table 1. In sham-operated mice, there were no changes in liver weight and spleen volume at the different time points studied (data only shown at 14 days and 6 weeks after sham operation). After PPVL, liver weights were not influenced and remained comparable to that in sham-operated mice for all time points studied. In contrast, spleen weight increased significantly after 5 days PPVL. Spleen weights were significantly higher after 5, 7 and 14 days PPVL compared to that in sham-operated mice (Table 1).
Table 1.
Macroscopic and laboratory features of all experimental groups: partial portal vein ligation
| Sham-operated 14 days (n = 4) | PPVL 2 days (n = 6) | PPVL 5 days (n = 6) | PPVL 7 days (n = 6) | PPVL 14 days (n = 6) | |
|---|---|---|---|---|---|
| Liver weight (g/10 g BW) | 0.37 ± 0.04 | 0.43 ± 0.02 | 0.48 ± 0.02 | 0.47 ± 0.03 | 0.41 ± 0.01 |
| Spleen weight (g/10 g BW) | 0.030 ± 0.002 | 0.028 ± 0.002 | 0.050 ± 0.005*† | 0.065 ± 0.009*† | 0.066 ± 0.005*† |
| AST (IU/l) | 43 ± 10 | 36 ± 9 | 29 ± 6 | 34 ± 5 | 38 ± 4 |
| ALT (IU/l) | 4 ± 1 | 3 ± 1 | 4 ± 0.1 | 3 ± 1 | 4 ± 0.1 |
| Bilirubin (mg/dl) | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 |
Values given are mean ± SEM. PPVL, partial portal vein ligation; BW, body weight; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
P < 0.05 vs. sham-operated.
P < 0.05 vs. PPVL 2d.
In common bile duct ligated mice, liver and spleen weights increased significantly after 1-week induction compared with sham-operated mice and remained about the same levels during the following 6 weeks. (Table 2).
Table 2.
Macroscopic and laboratory features of all experimental groups: common bile duct ligation
| Sham-operated 6 weeks (n = 4) | CBDL 1 week (n = 6) | CBDL 3 weeks (n = 6) | CBDL 4 weeks (n = 6) | CBDL 5 weeks (n = 6) | CBDL 6 weeks (n = 6) | |
|---|---|---|---|---|---|---|
| Liver weight (g/10 g BW) | 0.40 ± 0.05 | 0.77 ± 0.07* | 0.68 ± 0.12* | 0.78 ± 0.06* | 0.76 ± 0.06* | 0.70 ± 0.81* |
| Spleen weight (g/10 g BW) | 0.035 ± 0.003 | 0.054 ± 0.004* | 0.085 ± 0.013* | 0.109 ± 0.007* | 0.094 ± 0.006* | 0.085 ± 0.011* |
| AST (IU/l) | 35 ± 7 | 285 ± 58* | 730 ± 179*† | 655 ± 135*† | 518 ± 122*† | 647 ± 151*† |
| ALT (IU/l) | 4. ± 0.2 | 206 ± 14* | 332 ± 90* | 370 ± 57* | 430 ± 68* | 241 ± 68* |
| Bilirubin (mg/dl) | 0.3 ± 0.1 | 12 ± 2* | 12 ± 2* | 9 ± 3* | 16 ± 4* | 13 ± 1* |
Values given are mean ± SEM. CBDL, common bile duct ligation; BW, body weight; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
P < 0.05 vs. sham-operated.
P < 0.05 vs. CBDL 1 week.
Sham-operated and PPVL mice had no ascites. In contrast, 50% of the mice developed ascites after 6 weeks CBDL. In the CBDL mice, there were severe adhesions of the liver and bowel. Adhesions were not observed in the sham-operated mice, and were only mild present in the PPVL mice.
There was a significant difference in liver and spleen weight between the CCl4 group and control group (Table 3). Between the different CCl4 groups, liver and spleen weight were significantly higher in relation to the duration of CCl4 administration. At all time points, CCl4 mice did not develop ascites. There was no sticking of the liver and bowel and there were no lesions in the peritoneum.
Table 3.
Macroscopic and laboratory features of all experimental groups: CCl4
| Control 16 weeks (n = 4) | CCl4 4 weeks (n = 6) | CCl4 8 weeks (n = 6) | CCl4 10 weeks (n = 6) | CCl4 12 weeks (n = 6) | CCl4 16 weeks (n = 6) | |
|---|---|---|---|---|---|---|
| Liver weight (g/10 g BW) | 0.45 ± 0.01 | 0.60 ± 0.02 | 0.56 ± 0.01 | 0.55 ± 0.06 | 0.66 ± 0.02* | 0.70 ± 0.02* |
| Spleen weight (g/10 g BW) | 0.027 ± 0.005 | 0.036 ± 0.002 | 0.037 ± 0.003 | 0.042 ± 0.004* | 0.043 ± 0.004* | 0.055 ± 0.004* |
| AST (U/l) | 37 ± 5 | 77 ± 15* | 89 ± 8* | 104 ± 26* | 61 ± 3* | 105 ± 9* |
| ALT (U/l) | 20 ± 6 | 23 ± 1 | 42 ± 10 | 16 ± 5 | 27 ± 3 | 43 ± 10 |
| Bilirubin (mg/dl) | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 |
Values given are mean ± SEM. CCl4, carbon tetrachloride; BW, body weight; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
P < 0.05 vs. control.
Between the three portal hypertensive groups, liver volume was significantly larger in the CBDL and CCl4 group compared with the PPVL group. Spleen weights increased in the three experimental groups after induction simultaneously with increases in PP.
Mortality rate
No mortality was observed in the sham-operated mice or control mice for CCl4. Two of thirty mice (5%) died after CCl4 induction due to technical failure during injection. A mortality rate of 10% (3 of 30 mice) was seen in the CBDL mice model.
All deaths occurred in the first week after induction and the mortality resulted from bile leakage and subsequently sepsis. Preliminary experiments showed no further survival of all CBDL mice within 7 to 10 weeks after induction. During development of fibrosis/cirrhosis, macroscopic signs of cholestasis were observed by the yellow colouring of the ears and urine. The physical activity level of the CBDL mice decreased progressively, with less response to external stimuli. The abdomen was swollen because of ascite production. All CBDL mice died within 7 to 10 weeks after induction.
A mortality of 10% (3 of 30 mice) was also seen in the PPVL group because of technical failure of too tightened ligation of the portal vein with subsequent ischaemia and necrosis of the bowel. These deaths were observed within 24 h after portal vein ligation. Afterwards, the PPVL mice had a normal activity level during the 14 days after induction.
Laboratory tests
Aspartate aminotransferase (IU/l), ALT (IU/l) and bilirubin (mg/dl) levels are shown in Table 1 for all experimental groups. There were no changes in AST, ALT and bilirubin after PPVL induction and sham operation (Table 1). In contrast, there was a significant increase in AST, ALT and bilirubin after 1-week bile duct ligation. CBDL mice showed significantly higher AST, ALT and bilirubin values compared with sham-operated mice at 1, 3, 4, 5 and 6 weeks after induction (Table 2).
Carbon tetrachloride administration caused a significant increase in AST in the CCl4 groups compared with controls. There was no increase in bilirubin and a minor increase in ALT in the CCl4 groups compared with controls. These results were not significant between the different CCl4 groups (Table 3).
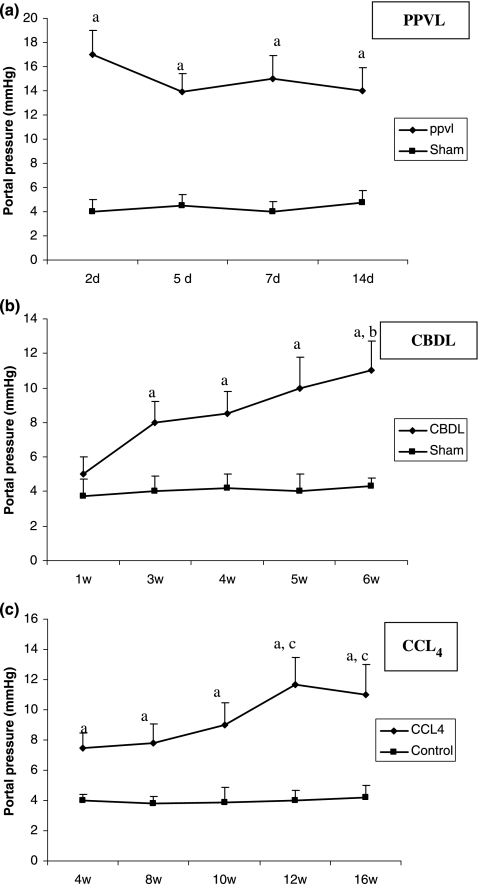
Portal pressure measurements
Portal pressures from all experimental groups are shown in Figure 1. PPVL caused a marked elevation of the PP as early as the second day after induction. Five days after induction, the PP was slowly decreased compared to 2 days and reached a plateau that was maintained for 7 and 14 days after PPVL induction. In sham-operated mice, the portal venous pressure was significantly lower than in PPVL mice at all time points studied (Figure 1a).
Figure 1.
Portal pressures (mmHg) of sham-operated, PPVL (a), CBDL (b) and CCl4 (c) mice. Data are expressed as mean ± SEM. aP < 0.05 vs. sham-operated or control, bP < 0.05 vs. 1 week CBDL, cP < 0.05 vs. 4 week CCl4. PPVL, partial portal vein ligation; CBDL, common bile duct ligation; CCl4, carbon tetrachloride.
After CBDL, the PP became significantly higher at 3 weeks after induction compared to sham-operated mice. A further slow increase was seen and the highest value was reached at 6 weeks after CBDL. The PP were significantly lower in sham-operated mice after 3, 4, 5 and 6 weeks compared with CBDL mice (Figure 1b).
Portal pressure was significantly higher in the mice after 4, 8, 10, 12 and 16 weeks of CCl4 administration compared with the control group. There was an increase in PP related to the duration of CCl4 administration and the highest value was reached after 12 weeks (Figure 1c). The increase in PP in CBDL and CCl4 correlated with the increase in fibrotic tissue formation.
Histopathology
Light microscopy
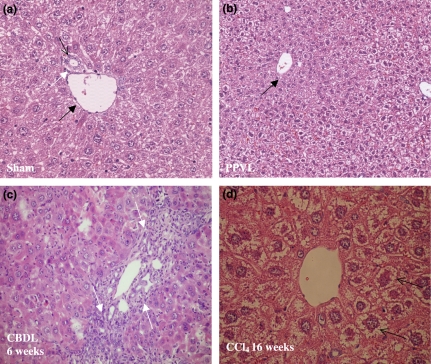
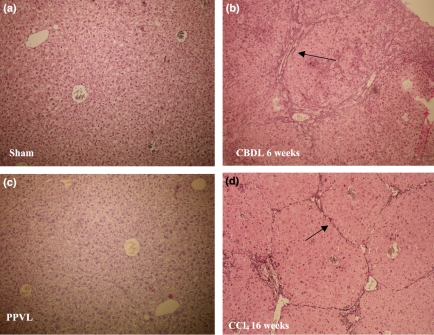
(i) Sham-operated and PPVL mice: A normal liver histology without evidence of nodular regenerative hyperplasia was seen in sham-operated and PPVL mice at haematoxylin–eosin staining (Figure 2a,b). A normal portal tract consists of a branch of the portal vein, a bile ductule and a branch of the hepatic artery. The bile ductule has approximately the same size as the accompanying branch of the hepatic artery. On Sirius Red and reticulin staining no fibrosis was observed in both sham and PPVL mice (Table 4, Figure 3a). No areas of portal inflammation and proliferation of bile ducts were seen in sham and PPVL mice.
Figure 2.
Haematoxylin–eosin staining. Normal liver histology is seen in sham-operated (a) (magnification ×20) and PPVL (b) (magnification ×10) mice. A portal tract is shown consisting of a portal vein (black arrow), bile duct (white arrow) and hepatic arteriole (open arrow). Marked proliferation of bile ductules is seen in CBDL mice after 6 weeks of induction (white arrows, c) (magnification ×20). Hepatocytes are swollen (arrows) and surrounded by fibrotic tissue with a tendency of dissection of groups of hepatocytes after a longer period of CCl4 induction (d) (magnification ×20). PPVL, partial portal vein ligation; CBDL, common bile duct ligation; CCl4, carbon tetrachloride.
Table 4.
Sirius Red staining. All mice developed full micronodular cirrhosis (F4) after 16 weeks of CCl4 administration. Six weeks after CBDL induction, marked portal-to portal fibrotic septa and nodule formation was clearly present (F4) in all mice
| CCl4 | F-score | CBDL | F-score | Sham-operated | F-score |
|---|---|---|---|---|---|
| 4 weeks | F1–F2 | 1 week | F1 | 6 weeks | F0 |
| 8 weeks | F3 | 3 weeks | F2 | ||
| 12 weeks | F3–F4 | 6 weeks | F4 | Control CCl4 16 weeks | F0 |
| 16 weeks | F4 |
CCl4, carbon tetrachloride; CBDL, common bile duct ligation.
Figure 3.
Reticulin staining (magnification ×10). No fibrosis is observed in sham-operated mice (a) and PPVL mice (c). Marked portal-to-portal fibrotic septa and nodule formation (black arrows) are observed in CBDL mice after 6 weeks of induction (b). Sixteen weeks of CCl4 administration induces central-portal fibrotic alterations (black arrow) (d). PPVL, partial portal vein ligation; CBDL, common bile duct ligation; CCl4, carbon tetrachloride.
(ii) CBDL mice: Enlargement of the portal tracts appeared as soon as 1 week after CBDL. This was accompanied by dilatation of bile canaliculi and proliferation of the smaller bile ducts. Polymorphonuclear leucocytes and macrophages infiltrated and surrounded the periportal ductular proliferations with signs of cholangitis. An increasing number of single mononuclear cells appeared at the peripheral edge of the portal tract. These cells were considered to be progenitor cells. These alterations persisted and worsened with increasing numbers of proliferating ductules as the experiment progressed. After 3 weeks, the periportal alterations were accompanied by fibrotic changes to be described as F2 and evolving into F3 after 5 weeks of CBDL. Proliferative changes at the level of the interlobular ducts persisted and were accompanied with the infiltration of inflammatory cells (Table 5a, Figure 2c). After 6 weeks, secondary biliary cirrhosis (F4) developed with nodular changes in the liver parenchyma (Figure 3b).
Table 5.
Presence of inflammation and proliferation of bile ducts scored on haematoxylin–eosin in CBDL (a) and CCl4 (b)
| 1 week | 3 weeks | 6 weeks | |
|---|---|---|---|
| (a) | |||
| Portal inflammation | 2 ± 0 | 3 ± 1 | 2 ± 0 |
| Proliferation of small bile ducts | 1 ± 0 | 2 ± 0 | 2 ± 0 |
| 4 weeks | 8 weeks | 12 weeks | 16 weeks | |
|---|---|---|---|---|
| (b) | ||||
| Inflammation centrolobular area | 1 ± 0 | 2 ± 0 | 3 ± 1 | 3 ± 1 |
| Proliferation of small bile ducts | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
CBDL, common bile duct ligation; CCl4, carbon tetrachloride. The liver sections were scored for inflammation (0 = absent, 1 = focal alteration of the periportal or centrolobular zone in some tracts, 2 = diffuse alteration in some portal or centrolobular tracts, 3 = diffuse alteration of the periportal or centrolobular zone in all tracts). Proliferation of small bile ducts was scored (0 = absent, 1 = mild, 2 = present in all portal tract areas).
(iii) CCl4mice (Table 5b): Control mice revealed a normal histology with preservation of the lobular architecture. No cellular damage was present.
After 4 weeks of CCl4 administration, the experimental animals demonstrated changes in the centrolobular area. They were characterized by swelling of the hepatocytes and infiltration of ceroid pigment-laden macrophages. The hepatocytes were swollen with enlarged nuclei (Figure 2d). Increased numbers of sinusoidal cells appeared around the central vein. Single hepatocytic cell necrosis was found. The reticulin and Sirius Red stains demonstrated fibrotic changes in the centrolobular area. No inflammatory or fibrotic changes appeared in the portal area.
After 8 weeks of CCl4 administration the liver architecture demonstrated a reversed lobulation due to the development of centro-central fibrotic linkages accompanied by degenerating hepatocytes surrounded by macrophages. Increasing numbers of sinusoidal cells were found consisting of cells with oval nuclei and barely visible cytoplasm. These cells were found along the sinusoidal space. The portal tracts did not develop major alterations.
After 12 weeks the reversed lobulation was accentuated with the development of centro-portal thin fibrotic septa apart from the centro-central fibrotic linkages. At the central area, the fibrotic changes became severe with separation of groups of hepatocytes appearing swollen with cellular degeneration and infiltration of numerous macrophages. At this time, already a part of the group reached the cirrhotic stage. However, after 16 weeks, all mice had homogeneous characteristics of cirrhosis (Figure 3c).
α-SMA immunohistochemistry
(i) Sham-operated and PPVL mice: α-SMA staining was observed around the blood vessels (like portal vein and hepatic arteries) in control mice (Figure 4a). No staining of HSC was observed.
Figure 4.
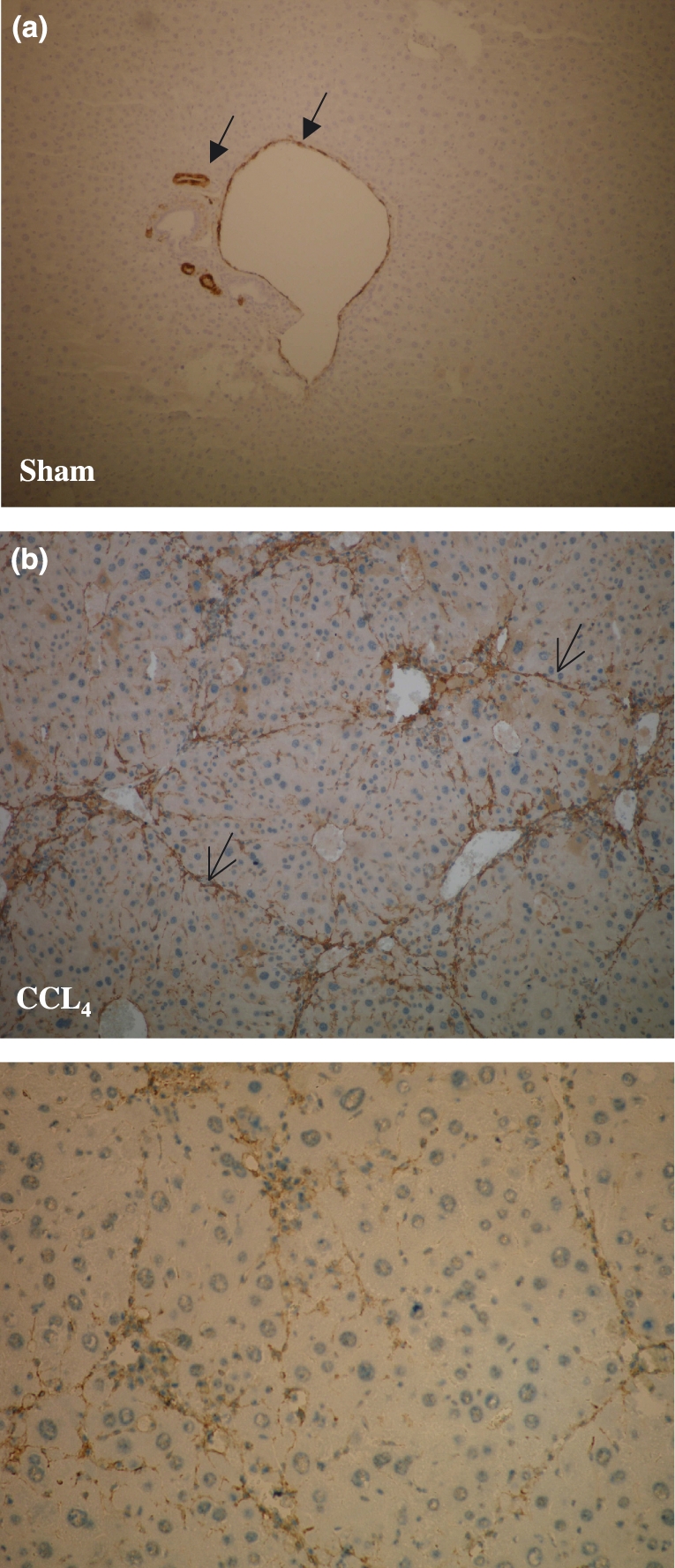
Alpha-smooth muscle actin (α-SMA) immunohistochemistry (magnification ×10). Alpha-SMA staining was observed around the blood vessels (portal vein and hepatic arteries) (closed arrows) in sham-operated mice (a). No staining of hepatic stellate cells was observed. Marked accumulation of α-SMA positive cells (brown colouring) was seen within the septal formation of fibrotic tissue in CCl4 mice after 16 weeks (open arrows; b) CCl4, carbon tetrachloride.
(ii) CBDL mice: As early as 3 weeks after CBDL, the extent of portal tract demonstrated an increased staining with spindle-shaped cells along the sinusoidal wall. The increase of positive α-SMA cells paralleled the development of fibrosis to cirrhosis in the CBDL mice model.
(iii) CCl4mice: After 1 week, no alterations were seen around the central vein. In the portal tract staining of the smooth muscle wall of the vessels was present. No staining was found along the sinusoidal wall.
After 4 weeks, increased sinusoidal wall staining was apparent and related to the occurrence of spindle cells along the sinusoidal wall. They were found in groups of two or three cells surrounded by stained fibres around the central vein.
After 16 weeks, the septa dissected the parenchyma with nodular transformation (Figure 4b).
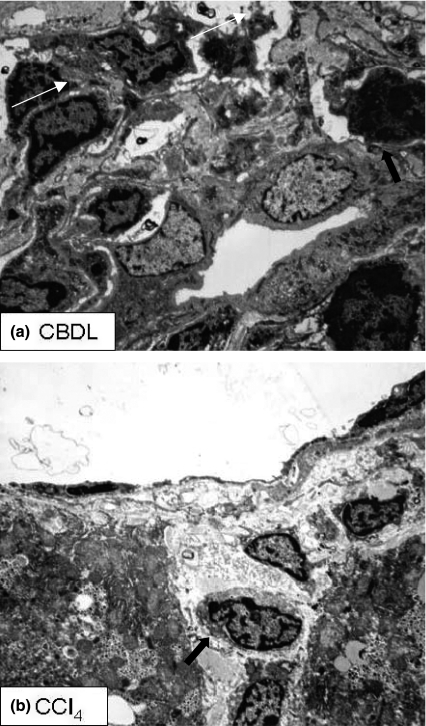
Electron microscopy
The portal, midzonal and centrilobular areas will be discussed separately with description of the alterations in each experimental model (Figure 5).
Figure 5.
Electron microscopy. (a) Portal tract of CBDL mice (4 weeks). Proliferating bile ducts are surrounded by inflammatory cells (arrows). (b) Central vein area of CCl4 mice (8 weeks). Degenerating hepatocytes undergo necrotic changes (arrows) and are surrounded by inflammatory cells. CBDL, common bile duct ligation; CCl4, carbon tetrachloride.
Sham-operated and PPVL mice
Centrilobular area: Endothelial cells whose cytoplasmic extensions with fenestrae rest on a discontinuous basement membrane lined the central vein. The surrounding hepatocytes displayed regular outlines with finely dispersed glycogen particles. The intercellular bile canaliculi were lined by fine cytoplasmic extensions of the lateral basement membrane of the hepatocytes.
Portal area: The portal tracts revealed bile canaliculi lined by two to three bile ductular cells surrounded by a basement membrane. Rarely single ‘progenitor’ cells were found. They were characterized by oval nuclei with coarse heterochromatin. No inflammatory cells were present.
CBDL mice
After 1 week of ligation:
Centrilobular area: The endothelial cells around the central vein did not undergo any major alterations. The basement membrane was not thickened. The surrounding hepatocytes did not undergo major alterations.
Midzonal area: The hepatocytes of the lobular area were shaped regularly without necrosis and without inclusion of fat droplets. The sinusoidal cells revealed regular HSC without changes. A few Kupffer cells contained amorphic debris.
Portal area: The portal tracts were enlarged and oedematous with infiltration by polymorphonuclear cells, deposition of collagen fibres and increased numbers of fibroblasts. The bile ductular cells were characterized by swollen nuclei with irregular cytoplasmic extensions. Some of the inflammatory cells were found in the lumen of the altered bile ductular structures. Isolated progenitor cells surrounded the inflamed bile ductules. These cells were not separated by a basement membrane. The hepatocytes in the surrounding parenchyma had swollen nuclei containing fat droplets.
After 4 and 6 weeks of ligation:
Centrilobular area: Around the central vein increased amounts of fibrous tissue were observed with activated fibroblasts. The hepatocytes were swollen with inclusion of fat droplets.
Midzonal area: No major alterations were observed in the hepatocytes.
Portal area: The portal tracts demonstrated increased amounts of fibrous tissue. Swollen bile ductular cells infiltrated by polymorphonuclear cells and surrounded by lymphocytes and plasmocytes lined the bile ducts. The bile ductules also had irregular outlines with multilayered appearance. A high number of progenitor cells were found. The surrounding hepatocytes in the periportal area underwent necrotic changes. Activated HSCs were seen as demonstrated by the presence of rough endoplasmatic reticulum and loss of large fat droplets.
CCl4 mice
After 4 weeks of CCl4 administration:
Centrilobular area: The endothelial cells rested on a thick fibrous band composed of regularly woven collagen fibres and high numbers of fibroblasts. The surrounding hepatocytes contained fat droplets. Single appearing mononuclear cells and histiocytes were seen.
Midzonal area: The hepatocytes contained increased numbers of lysosomes. Some hepatocellular necrosis was found and was surrounded by histiocytes.
Portal area: In the portal tracts, regularly shaped bile ducts were found. No increased amounts of fibrous tissue were seen. A rare progenitor cell could be observed.
After 8 weeks of CCl4 administration:
Centrilobular area: Large amounts of collagen fibres were found and irregularly distributed among necrotizing hepatocytes containing amorphous bile pigment complexes. Increased numbers of HSC demonstrated stages of transformation into spindle-shaped cells with myofibroblastic features. Collagen fibres surrounded these cells. The surrounding hepatocytes had irregular nuclei with multiple lysosomes in the cytoplasm and fat droplets.
Portal area: The portal tracts displayed regular bile ducts amid increased amounts of fibrous tissue. Some progenitor cells were found.
After 12 weeks of CCl4 administration:
Centrolobular area: A high number of polymorphonuclear cells were found amid large amounts of collagen fibres. Remnants of necrotizing hepatocytes containing amorphous bile pigment complexes were present among the inflammatory cells. Increased numbers of Kupffer cells containing amorphous contents were situated in the sinusoids.
Midzonal area: The surrounding hepatocytes had irregular nuclei with multiple lysosomes in the cytoplasm and fat droplets.
Portal area: The portal tracts displayed regular bile ducts amid increased amounts of fibrous tissue. Some progenitor cells were found.
Discussion
The present study gives a description of three different mice models that can be used to study the pathogenesis of PTH and cirrhosis. Mice models of PTH and cirrhosis are currently used sporadically (Canbay et al. 2002; Iwakiri et al. 2002; Biecker et al. 2004; Fernandez et al. 2004) and a good characterization of histological changes in comparison with PP measurements is lacking in the current literature. We will discuss the results of the present study separately for each model.
Partial portal vein ligation model
Partial portal vein ligation has been widely used as an animal model to induce prehepatic PTH without cirrhosis. Haemodynamic measurements are described extensively in the rat and usually carried out 14 days after induction, a period when splanchnic vasodilation, hyperdynamic circulation and portal-systemic shunts are developed (Groszmann et al. 1982; Vorobioff et al. 1983; Abraldes et al. 2006). Recently, PPVL was also introduced in mice (Iwakiri et al. 2002; Fernandez et al. 2004). The PPVL model is induced by a fixed stenosis of the portal vein. The induction technique is comparable as in the rat model, only the needle has a smaller diameter (27 gauge) than in rats (20 gauge). There is low mortality when the operation is performed in experienced hands. An immediate high PP after 2 days induction is seen, which reaches a plateau of significantly higher PP compared to sham-operated mice after 7–14 days. This is associated with a parallel enlargement of the spleen weight, also an indication of the presence of PTH. These data confirm previous reported observations in PPVL mice (Fernandez et al. 2004). Fernandez et al. also showed that the mice model develops portal-systemic shunting and hyperdynamic circulatory changes, comparable to that of the rat PPVL model. The liver histology is not disturbed and no fibrosis is seen after PPVL induction in mice.
In the literature, the PPVL model is widely used to study alterations in the systemic and splanchnic circulation related to PHT. We may assume that PPVL in mice is a reliable and reproducible model to induce prehepatic PTH after 14 days, with low mortality rates in experienced hands.
Common bile duct ligation model
Common bile duct ligation in mice is most frequently used in the current literature as a model for acute cholestasis (Mennon et al. 2006; Georgiev et al. 2007). Data about the development of fibrosis and progression to cirrhosis and PHT in this mice model are rare.
The CBDL mice model is induced by a double ligation of the common bile duct followed by section between these two ligatures. This induction technique is also used in the rat (Colle et al. 2004a,b). Macroscopically, dilation of the gallbladder and formations of bilioma are seen in mice after CBDL, but these are mild and never lead to perforation or choleperitoneum. A mortality rate of 10% is observed in the first week after CBDL. An overall death is seen in the period of 7–10 weeks after induction because of liver failure. Fifty per cent of all CBDL mice develop ascites after 5 weeks of induction. These data are quite similar to that in the rat model.
Rapidly after bile duct ligation, mice develop obstructive jaundice and cholestasis, as demonstrated by markedly elevated serum transaminases and bilirubin level and macroscopic evidence of yellow ears and urine. Liver weights are increased after CBDL induction, as early as 1 week, and can be related due to the ductular proliferation.
Histopathological changes include proliferation of intralobular ductules, portal tract expansion and the appearance of mixed inflammatory infiltrates around the portal tracts, consisting of both mononuclear and polymorphonuclear leucocytes. Also typical of obstructive cholestasis is bile plugging in the intralobular ducts. Apart from the proliferation of bile ductules an increased number of progenitor cells is found. Periportal fibrosis develops after 1 to 3 weeks of CBDL induction and progresses to cirrhosis at 6 weeks. These alterations are also reflected in the ultrastructural study.
In normal liver, HSCs are non-parenchymal, quiescent cells that have three main physiological functions as vitamin A storage, production of extracellular matrix in the space of Disse and the regulation of sinusoidal microcirculatory flow. In response to liver injury HSCs undergo an activation process characterized by proliferation and myofibroblastic transformation. In response to their activation HSCs show an intense cytoplasmic α-SMA immuoreactivity. α-SMA is an actin isoform and a specific marker for smooth muscle cell differentiation. Marked accumulation of α-SMA-positive cells is seen within extended portal tracts 3 weeks after CBDL. The progression of increased α-SMA-positive cells parallels the development of fibrosis to cirrhosis in the CBDL mice model as shown after 5 weeks of ligation.
The progressive rebuilding of the liver due to fibrosis leads to an increase in intrahepatic resistance (sinusoidal component). This parallels the increase in PP measurements that we observed at the different time points after CBDL induction and can be classified as a sinusoidal PTH with maximal PP after 4 weeks. A presinusoidal component (because of bile obstruction) contributing to PHT has also been described in this model in rats (Franco et al. 1979; Sikuler et al. 1991). Furthermore, the observed increasing splenomegaly in the present study supports the evolutive portal hypertensive syndrome over time in this model.
Evaluation of the splanchnic circulation, hyperdynamic circulation and portal-systemic shunting was not performed in the present study. From the CBDL rat model, we knew that splanchnic vasodilation, hyperdynamic circulation and portal-systemic shunting of 30–60% are present 4 weeks after bile duct ligation (Franco et al. 1979; Lee et al. 1986). The presence of these changes needs to be well described in future studies in the CBDL mice model.
From the present data we suggest that the CBDL mice model can serve as a reliable, reproducible model for studying the underlying pathophysiological mechanisms related to secondary biliary cirrhosis.
CCl4 model
Within the different experimental animal models, the CCl4 model has the characteristics most closely resembling that of human alcoholic cirrhosis. The time required to develop cirrhosis depends on the route of administration, dose and time interval between each dose of CCl4. The time interval should not be too long to avoid recovery of the damaged cells. Most models are induced by intraperitoneal injection in rats (Perez Tamayo 1983; Janakat & Al-Merie 2002; Abraldes et al. 2006) and mice (Natsume et al. 1999; Niggemann et al. 2004; Chang et al. 2005). However, peritoneal injection may cause damage and sticking of peritoneum, liver and intestines. For this reason, we developed an experimental mouse model of micronodular cirrhosis by using the dorsal SC injection route. In this way, there are no adhesions in the abdominal cavity. SC injections yield low mortality rates, about 5%, but the duration of the development of cirrhosis (16 weeks) is longer than the other administration routes (Jimenez et al. 1992). Intraperitoneal or intramuscular injections, oral administration or inhalation results in high mortality rates (20–50%), but the period of CCl4 administration is reduced to 8–10 weeks.
The centrilobular region of the liver is the preferential zone of toxicity related to CCl4. Cytochrome P450 2E1 is the enzyme that produces CCl4 hepatotoxic metabolites, which cause liver damage (Lieber 1984; Koop & Coon 1986; Zimmerman 1986; Garro & Lieber 1990). Mice receive 5% ethanol in their drinking water to induce the cytochrome P450 2E1 enzyme and subsequently to enhance hepatic necrosis due to CCl4 (Garro & Lieber 1990). As early as 4 weeks after SC injection of CCl4, we can detect histopathological changes in the centrolobular area caused by CCl4 hepatotoxic metabolites. These changes are characterized by swollen hepatocytes and infiltration by macrophages. The portal tracts did not develop major alterations contradictory to the CBDL model. The mice become cirrhotic after 16 weeks due to the development of thin fibrous septa connecting the centro-central areas with the portal tracts. Ultrastructural studies at different time points of CCl4 administration illustrate toxic changes in the hepatocytes and the ongoing fibrosis in the centrolobular area.
As described in the CBDL model, α-SMA stains spindle-shaped cells, suggesting the appearance of myofibroblasts. It is suggested that these cells represent activated HSC.
We have observed a progressive increase in PP that parallels the fibrotic changes in the centrolobular area. PP reaches the highest value when the mice become cirrhotic at week 16. In addition, the liver weight and spleen weight increase significantly during genesis of cirrhosis. AST and ALT liver tests are increased, but bilirubin remains unchanged compared with controls. Janakat and Al-Merie (2002) demonstrated that elevations in ALT, AST and bilirubin in rats reached a peak after 3 days of CCl4 administration and declined gradually to normal within 3 days. We take blood samples beyond 3 days of CCl4 administration, which may explain why there is little increase in these liver tests.
This mouse model, by using the SC route of CCl4 administration, can serve as a model for micronodular cirrhosis and PHT, without lesions in the peritoneum and adherences between the liver and bowel. The period of induction of cirrhosis takes longer than the other administration routes, but the mortality rate is much lower.
In conclusion, the present study describes for the first time a histological analysis of the liver in consecutive stages of fibrosis and cirrhosis and PP measurements in three different mice models of PTH and cirrhosis. CBDL leads to secondary biliary cirrhosis after 6 weeks with PTH and in 50% ascites. CCl4 SC injection causes cirrhosis and PHT after 16 weeks. Prehepatic PTH occurs after 14 days of PPVL. The use of mouse models for cirrhosis and PHT will help to improve pathophysiological studies in this field.
References
- Abraldes JC, Marcos P, Garcia-Pagan JC. Animal models of portal hypertension. World J. Gastroenterol. 2006;12:6577–6584. doi: 10.3748/wjg.v12.i41.6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- Benson JM, Tibbetts BM, Thrall KD, Springer DL. Uptake, tissue distribution, and fate of inhaled carbon tetrachloride: comparison of rat, mouse, and hamster. Inhal. Toxicol. 2001;13:207–217. doi: 10.1080/08958370150502449. [DOI] [PubMed] [Google Scholar]
- Biecker E, Neef M, Sagesser H, Shaw S, Koshy A, Reichen J. Nitric oxide synthase 1 is partly compensating for nitric oxide synthase 3 deficiency in nitric oxide synthase knock-out mice and is elevated in murine and human cirrhosis. Liver Int. 2004;24:345–353. doi: 10.1111/j.1478-3231.2004.0933.x. [DOI] [PubMed] [Google Scholar]
- Canbay A, Higuchi H, Bronk SF, Taniai M, Sebo T, Gores G. Fas enhanced fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterology. 2002;123:1323–1330. doi: 10.1053/gast.2002.35953. [DOI] [PubMed] [Google Scholar]
- Castaneda B, Debernardi-Venon W, Bandi JC, et al. The role of portal pressure in the severity of bleeding in portal hypertensive rats. Hepatology. 2000;31:581–586. doi: 10.1002/hep.510310306. [DOI] [PubMed] [Google Scholar]
- Chang ML, Yeh CT, Chang PY, Chen JC. Comparison of murine cirrhosis models induced by hepatotoxin administration and common bile duct ligation. World J. Gastroenterol. 2005;11:4167–4172. doi: 10.3748/wjg.v11.i27.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colle I, De Vriese A, Van Vlierberghe H, Lameire N, De Vos M. Systemic and splanchnic haemodynamic effects of sildenafil in an in vivo animal model of cirrhosis, support for a risk in cirrhotic patients. Liver Int. 2004a;24:63–68. doi: 10.1111/j.1478-3231.2004.00892.x. [DOI] [PubMed] [Google Scholar]
- Colle I, De Vriese A, Van Vlierberghe H, Lameire N, De Vos M. Vascular hyporesponsiveness in the mesenteric artery of rats with cirrhosis and portal hypertension: an in vivo study. Eur. J. Gastroenterol. Hepatol. 2004b;16:139–145. doi: 10.1097/00042737-200402000-00004. [DOI] [PubMed] [Google Scholar]
- Colombato LA, Albillos A, Groszmann RJ. Temporal relationship of peripheral plasma volume expansion and the hyperdynamic circulatory state in portal hypertensive rats. Hepatology. 1992;15:323–328. doi: 10.1002/hep.1840150224. [DOI] [PubMed] [Google Scholar]
- Fernandez M, Vizzutti F, Garcia-Pagan JC, Rodes J, Bosch J. Anti-VEGF receptor-2 monoclonal antibody prevents portal-systemic collateral vessel formation in portal hypertensive mice. Gastroenterology. 2004;126:886–894. doi: 10.1053/j.gastro.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Franco D, Gigou M, Szekely AM, Bismuth H. Portal hypertension after bile duct obstruction: effect of bile diversion on portal pressure in the rat. Arch. Surg. 1979;114:1064–1067. doi: 10.1001/archsurg.1979.01370330086016. [DOI] [PubMed] [Google Scholar]
- Garro AJ, Lieber CS. Alcohol and cancer. Annu. Rev. Pharmacol. Toxicol. 1990;30:219–249. doi: 10.1146/annurev.pa.30.040190.001251. [DOI] [PubMed] [Google Scholar]
- Georgiev P, Navarini AA, Eloranta JJ, et al. Cholestasis protects the liver from ischaemic injury and post-ischaemic inflammation in the mouse. Gut. 2007;56:121–128. doi: 10.1136/gut.2006.097170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groszmann RJ, Vorobioff J, Riley E. Splanchnic hemodynamics in portal hypertensive rats: measurements with gamma-labeled microspheres. Am. J. Physiol. 1982;242:G156–G160. doi: 10.1152/ajpgi.1982.242.2.G156. [DOI] [PubMed] [Google Scholar]
- Iwakiri Y, Cadelina G, Sessa WC, Groszmann RJ. Mice with targeted deletion of eNOS develop hyperdynamic circulation associated with portal hypertension. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G1074–G1081. doi: 10.1152/ajpgi.00145.2002. [DOI] [PubMed] [Google Scholar]
- Janakat S, Al-Merie H. Optimization of the dose and route of injection, and characterisation of the time course of carbon tetrachloride-induced hepatotoxicity in the rat. J. Pharmacol. Toxicol. Methods. 2002;48:41–44. doi: 10.1016/S1056-8719(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Jimenez W, Claria J, Arroyo V, Rodes J. Carbon tetrachloride induced cirrhosis in rats: a useful tool for investigating the pathogenesis of ascites in chronic liver disease. J. Gastroenterol. Hepatol. 1992;7:90–97. doi: 10.1111/j.1440-1746.1992.tb00940.x. [DOI] [PubMed] [Google Scholar]
- Katsuta Y, Zhang XJ, Ohsuga M, et al. Hemodynamic feature of advanced cirrhosis due to chronic bile duct ligation. J. Nippon Med. Sch. 2005;72:217–225. doi: 10.1272/jnms.72.217. [DOI] [PubMed] [Google Scholar]
- Koop DR, Coon MJ. Ethanol oxidation and toxicity: role of alcohol P-450 oxygenase. Alcohol. Clin. Exp. Res. 1986;10:44S–49S. doi: 10.1111/j.1530-0277.1986.tb05179.x. [DOI] [PubMed] [Google Scholar]
- Kountouras J, Billing BH, Scheuer PJ. Prolonged bile duct obstruction: a new experimental model for cirrhosis in rat. Br. J. Exp. Pathol. 1984;65:305–311. [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Girod C, Braillon A, et al. Hemodynamic characterization of chronic bile-duct ligated rats: effect of phenobarbital sodium. Am. J. Physiol. 1986;251:G176–G180. doi: 10.1152/ajpgi.1986.251.2.G176. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Alcohol and the liver: 1984 update. Hepatology. 1984;4:1243–1260. doi: 10.1002/hep.1840040625. [DOI] [PubMed] [Google Scholar]
- Mennon A, Soroka CJ, Cai SY, et al. Mrp 4−/− mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology. 2006;43:1013–1021. doi: 10.1002/hep.21158. [DOI] [PubMed] [Google Scholar]
- Natsume M, Tsuji H, Harada A, et al. Attenuated liver fibrosis and depressed serum albumin levels in carbon-tetrachloride IL-6-deficient mice. J. Leukoc. Biol. 1999;66:601–608. [PubMed] [Google Scholar]
- Niggemann P, Murata S, Naito Z, Kumazaki T. A comparative study of the microcirculatory changes in the developing liver cirrhosis between the central and peripheral parts of the main lobe in mice. Hepatol Res. 2004;28:41–48. doi: 10.1016/j.hepres.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Perez Tamayo R. Is cirrhosis of the liver experimentally produced by CCl4 and adequate model of human cirrhosis? Hepatology. 1983;3:112–120. doi: 10.1002/hep.1840030118. [DOI] [PubMed] [Google Scholar]
- Proctor E, Chatamra K. High yield micronodular cirrhosis in the rat. Gastroenterology. 1982;83:1181–1190. [PubMed] [Google Scholar]
- Sikuler E, Buchs AE, Yaari A, Keynan A. Hemodynamic characterization of conscious and ketamine-anesthetized bile duct-ligated rats. Am. J. Physiol. 1991;260:G161–G166. doi: 10.1152/ajpgi.1991.260.1.G161. [DOI] [PubMed] [Google Scholar]
- Stewart SF, Day CP. The management of alcoholic liver disease. J. Hepatol. 2003;38:2–13. doi: 10.1016/s0168-8278(02)00427-0. [DOI] [PubMed] [Google Scholar]
- Van de Casteele M, Sägesser H, Zimmermann H, Reichen J. Characterisation of portal hypertension models by microspheres in aneasthtised rats: a comparison of flow. Pharmacol. Ther. 2001;35:35–43. doi: 10.1016/s0163-7258(01)00123-1. [DOI] [PubMed] [Google Scholar]
- Vorobioff J, Bredfeldt JE, Groszmann RJ. Hyperdynamic circulation in portal-hypertensive rat model: a primary factor for maintenance of chronic portal hypertension. Am. J. Physiol. 1983;244:G52–G57. doi: 10.1152/ajpgi.1983.244.1.G52. [DOI] [PubMed] [Google Scholar]
- Vorobioff J, Bredfeldt JE, Groszmann RJ. Increased blood flow through the portal system in cirrhotic rats. Gastroenterology. 1994;87:1120–1126. [PubMed] [Google Scholar]
- Zimmerman HJ. Effects of alcohol on other hepatotoxins. Alcohol. Clin. Exp. Res. 1986;10:3–15. doi: 10.1111/j.1530-0277.1986.tb05605.x. [DOI] [PubMed] [Google Scholar]