Abstract
(Pre)neoplastic lesions in livers of rats induced by diethylnitrosamine are characterized by elevated activity of the first irreversible enzyme of the oxidative branch of the pentose phosphate pathway (PPP), glucose-6-phosphate dehydrogenase (G6PD), for production of NADPH. In the present study, the activity of G6PD, and the other NADPH-producing enzymes, phosphogluconate dehydrogenase (PGD), isocitrate dehydrogenase (ICD) and malate dehydrogenase (MD) was investigated in (pre)neoplastic lesions by metabolic mapping. Transketolase (TKT), the reversible rate-limiting enzyme of the non-oxidative branch of the PPP, mainly responsible for ribose production, was studied as well. Activity of G6PD in (pre)neoplastic lesions was highest, whereas activity of PGD and ICD was only 10% and of MD 5% of G6PD activity, respectively. Glucose-6-phosphate dehydrogenase activity in (pre)neoplastic lesions was increased 25 times compared with extralesional parenchyma, which was also the highest activity increase of the four NADPH-producing dehydrogenases. Transketolase activity was 0.1% of G6PD activity in lesions and was increased 2.5-fold as compared with normal parenchyma. Transketolase activity was localized by electron microscopy exclusively at membranes of granular endoplasmic reticulum in rat hepatoma cells where G6PD activity is localized as well. It is concluded that NADPH in (pre)neoplastic lesions is mainly produced by G6PD, whereas elevated TKT activity in (pre)neoplastic lesions is responsible for ribose formation with concomitant energy supply by glycolysis. The similar localization of G6PD and TKT activity suggests the channelling of substrates at this site to optimize the efficiency of NADPH and ribose synthesis.
Keywords: glucose-6-phosphate dehydrogenase, hepatoma, isocitrate dehydrogenase, malate dehydrogenase, phosphogluconate dehydrogenase, transketolase
The pentose phosphate pathway (PPP) produces ribose, a building block of nucleotides and nucleic acids, and NADPH, the major reducing compound in the cytoplasm (Luzzatto & Battistuzzi 1985; Kletzien et al. 1994). The PPP consists of an oxidative branch with the first enzyme glucose-6-phosphate dehydrogenase (G6PD; EC 1.1.1.49) and a non-oxidative branch with the regulatory enzyme transketolase (TKT; EC 2.2.1.1) that catalyzes a number of steps in this branch of the PPP. Both branches produce ribose, but only the oxidative branch produces NADPH. Glucose-6-phosphate dehydrogenase activity is upregulated in cancer cells (Weber 1977; Bannasch et al. 1981; Van Driel et al. 1997; Van Noorden et al. 1997). There is increasing evidence that G6PD activity is of major importance for NADPH production for biosynthesis and the defence against oxidative stress rather than for ribose production during proliferation (Pandolfi et al. 1995; Spolarics 1998; Biagiotti et al. 2000; Koehler & Van Noorden 2003). We have demonstrated that G6PD activity is strongly elevated in chemically induced hepatocellular carcinoma in rat liver and is indeed accompanied by high levels of reduced glutathione (De Jong et al. 2001; Frederiks et al. 2007a,b). This suggests an important role of G6PD in maintaining the antioxidant capacity of cancer cells, but there are also indications that NADPH induces tumour growth (Kuo et al. 2000). Besides the activity of G6PD, activity of phosphogluconate dehydrogenase (PGD), the third enzyme of the oxidative branch of the PPP that also produces NADPH, was increased in (pre)neoplastic lesions in rat liver as well, but to a less extent (Frederiks et al. 2003).
TKT is the rate-limiting enzyme and catalyses several reactions in the non-oxidative branch of the PPP. Together with transaldolase, TKT serves as a reversible link between the oxidative part of the PPP and glycolysis, allowing the cell to adapt to a variety of metabolic needs under different environmental conditions (Horecker 2002). Boros et al. (1997) found that over 85% of ribose recovered from nucleic acids in pancreatic adenocarcinoma cells is generated directly or indirectly by the non-oxidative branch of the PPP. This implicates that TKT may play an important role in the proliferation of cancer cells. This was supported by studies of Raïs et al. (1999) showing a dramatic inhibition of cancer cell proliferation by specific TKT inhibitors. Moreover, Zhang et al. (2007) detected a direct relation between total TKT activity and proliferation of human hepatoma cells using inhibition of the transcript TKT-L1 by RNAi, which is the messenger of an isoenzyme of TKT. Three TKT genes have been identified in the human genome, TKT and transketolase-like 1 and 2 (TKT-L1 and TKT-L2). Expression of the gene and the protein of TKT-L1 is elevated in cancer cells (Coy et al. 2005). As far as we know, TKT activity has not been determined in tumours.
Ramos-Montoya et al. (2006) have recently reported that maintenance of the oxidative and non-oxidative activity balance of the PPP is critical for cancer cell survival and vulnerable to chemotherapeutic intervention.
In the present study, we investigated this balance by quantitative histochemical analysis of the activity of G6PD, PGD and the other NADPH-producing dehydrogenases, isocitrate dehydrogenase (ICD) and malate dehydrogenase (MD; also called malic enzyme) and that of TKT in chemically induced hepatomas in rat liver in comparison with adjacent normal liver parenchyma. We performed quantitative histochemical analysis of the activity of the enzymes and not immunohistochemistry and/or in situ hybridization to elucidate metabolic changes in cancer cells because the ultimate activity of enzymes depends on many regulatory steps from transcription of the genes to posttranslational regulation of the activity (Boonacker et al. 2004).
Materials and methods
Induction of neoplasms in liver
(Pre)neoplastic lesions were induced in livers of five male Wistar rats of 250–300 g by administration of 0.01% diethylnitrosamine (DENA; Sigma, St Louis, MO, USA) via drinking water for 9 weeks (Wu et al. 1996; De Jong et al. 2001). Principles of laboratory animal care were followed and, according to the Dutch law, the animal welfare committee of the Academic Medical Center approved the study. Animals were sacrificed with an overdose of barbiturates. Livers were immediately removed and dissected in pieces of 0.5 cm3. Liver fragments were frozen in liquid nitrogen and stored at −80 °C until used. Cryostat sections, 8 μm thick, were cut at −25 °C on a motor-driven Bright cryostat (Huntington, UK) fitted with a rotary retracting microtome, picked up on clean glass slides, and stored at −25 °C until used (Van Noorden & Frederiks 1992).
Cell line
FTO-2B cells were derived from rat hepatoma and were kindly supplied by Prof Dr W.H. Lamers (Amsterdam Liver Center, Academic Medical Center, Amsterdam, The Netherlands). Cells were kept in a 1:1 mixture of F12 medium and DMEM with 4.5 g/l glucose (Gibco, BRL; Paisley, Scotland) containing 10% foetal calf serum, 100 IU penicillin/ml and 100 mg streptomycin/ml (Life Technologies; Breda, The Netherlands). Cells were cultured at 37 °C in a humidified atmosphere of 5% CO2, washed in phosphate-buffered saline (PBS), and detached using 0.05% trypsin. Finally, cells were suspended in 100 mM phosphate buffer (PB), pH 7.4, and incubated for the ultrastructural localization of TKT activity.
Demonstration of G6PD, PGD, ICD and MD activity
Cryostat sections of rat liver were allowed to dry at room temperature for 5 min and were then incubated for the demonstration of G6PD, PGD, ICD and MD activity, according to Van Noorden and Frederiks (1992) and as recently demonstrated in adrenal gland (Frederiks et al. 2007a). Incubation medium contained 18% polyvinyl alcohol (PVA, weight average Mr 70,000–100,000; Sigma) in 0.1 M PBS, pH 7.4, 0.32 mM 1-methoxyphenazine methosulphate (Serva; Heidelberg, Germany), 0.5 mM NADP (Boehringer; Mannheim, Germany), 5 mM sodium azide, 5 mM MgCl2, 5 mM nitro blue tetrazolium salt (nitro BT; Sigma) and the respective substrate. For G6PD, PGD, ICD and MD, the following substrate concentrations were used, 10 mM glucose-6-phosphate (G6P; Boehringer), 10 mM 6-phosphogluconate (PG; Boehringer), 20 mM d,l-isocitrate (Sigma) and 100 mM L-malate (Serva), respectively. The media were freshly prepared just before incubation and nitro BT was added after being dissolved in a heated mixture of dimethylformamide and ethanol (final dilution of each solvent in the medium was 2% v/v). For the demonstration of G6PD, PGD, ICD and MD activity, sections were incubated for 5 min and 30 min at room temperature. Then, sections were rinsed with warm PBS (0.1 M, pH 5.3, 65 °C) to remove the viscous incubation medium and to stop the reaction immediately. Afterwards, sections were embedded in glycerin–gelatin. Control reactions were performed in the absence of substrate and NADP+ for G6PD, ICD and MD and in the absence of PG for PGD (Butcher & Van Noorden 1985). The control reaction was subtracted from the test reaction and test minus control was taken as a measure for the actual enzyme activity.
Demonstration of TKT activity
TKT activity was localized for light microscopical purposes by a modification of the dehydrogenase method with a tetrazolium salt and PVA as described by Boren et al. (2006). Total activity of the three TKT proteins, TKT, TKT-L1 and TKT-L2, was determined because activity assays cannot discriminate between the activity of the three proteins (Hu et al. 2007; Zhang et al. 2007). The substrates for the histochemical reaction were the same as for the biochemical method to determine TKT activity in homogenates (Smeets et al. 1971). The reaction was based on the transfer of the ketol moiety from xylulose-5-phosphate to ribose-5-phosphate, obtaining sedoheptulose-7-phosphate and glyceraldehyde-3-phosphate. Monitoring TKT activity can be achieved by coupling the reaction to glyceraldehyde-3-phosphate dehydrogenase (GAPD) with the formation of NADH, which is the first product in the chain of electron transfers that leads to formazan formation precipitated at the exact place where the reaction has taken place. Therefore, GAPD is an auxiliary enzyme in the present assay to detect TKT activity as described previously for other enzymes that need an auxiliary enzyme like hexokinase, creatine kinase and phosphofructokinase (Frederiks et al. 1987). Incubation media were prepared using 50 mM Tris-HCl buffer, pH 7.6, containing 18% (w/v) PVA, 5 mM sodium azide, 7.5 mM NAD, 3.7 mM KH2PO4, 5 mM MgCl2, 0.32 mM 1-methoxyphenazine methosulphate, 100 μl substrate mixture (see below) per ml incubation medium and 5 mM nitro BT (test reaction). The substrate mixture was prepared by dissolving 50 mM ribose-5-phosphate in 50 mM Tris-HCl, pH 7.6, and adding 0.05 IU ribulose-5-phosphate epimerase and 0.25 IU phosphoriboisomerase. The substrate mixture was continuously stirred and heated at 37 °C for 1 h and then kept at −20 °C until use. All compounds were obtained from Sigma.
Control reactions were performed by using incubation media that lacked the substrate mixture, but in the presence of 10 mM ADP. Incubations were performed for 30 min at room temperature. To stop the reaction, sections were rinsed with PBS, pH 5.0, at 65 °C and then mounted in glycerin–gelatin. The specific reaction was defined as the test minus control reaction.
Electron microscopical procedure for the localization of TKT activity
The copper iron method was used to demonstrate TKT activity as described previously (Boren et al. 2006). FTO-2B cells were permeabilized with glutaraldehyde as described previously. The incubation medium to detect TKT activity was prepared in 50 mM Tris-HCl buffer, pH 7.6, containing 6% PVA. Test incubation media contained (concentration in mM) 5 sodium azide, 5 MgCl2, 3.7 KH2PO4, 10 potassium ferricyanide, 30 sodium citrate, 30 cupric sulphate, 0.32 methoxyphenazine methosulphate, 7.5 NAD, and 100 μl substrate mixture per ml incubation medium (prepared as described above). Control reactions were performed using media lacking the substrate mixture and in the presence of 10 mM ADP. Incubations were performed for 1 h at 37 °C under continuous agitation. The incubation was stopped by addition of 5 ml cold PBS. Several washes with PBS were performed to remove incubation media.
After incubation, cells were fixed immediately using 4% paraformaldehyde and 1% (v/v) glutaraldehyde in 100 mM cacodylate buffer, pH 7.4, at 4 °C for 2–48 h. After fixation, cells were rinsed with 100 mM cacodylate buffer, pH 7.4, for 40 min, postfixed with 1% OsO4 in 100 mM cacodylate buffer, pH 7.4, for 60 min at 4 °C or with 1% OsO4 and 1.5% potassium ferrocyanide in 100 mM PBS, pH 7.4, for 2 h at 4 °C and thoroughly rinsed with bidistilled water at 4 °C. Afterwards, samples were dehydrated and embedded in epoxy resin LX-112 according to standard procedures. Semithin sections (1–2 μm thick) were cut on a LKB Pyramitone and stained with methylene blue to check the quality of the cell preparations. Ultrathin sections (30–70 nm thick) were cut on an ULTRACUT E ultramicrotome (Leica Microsystems; Wezlar, Germany) and studied with an EM-10C transmission electron microscope (Zeiss; Oberkochen, Germany).
Image analysis
Formazan in (pre)neoplastic lesions and extralesional parenchyma in rat liver was measured by image analysis according to Chieco et al. (1994) using a Vanox-T photomicroscope (Olympus; Tokyo, Japan) with a x2 objective (numerical aperture, 0.08). Experiments were performed in triplicate, two preparations were incubated per experiment, and 10 areas per section were investigated. Sections were illuminated with white light from a stabilized power supply after filtering by infrared blocking filters (Jonker et al. 1997) and a monochromatic filter of the isobestic wavelength of nitro BT formazan (585 nm; Van Noorden & Frederiks 1992). Images of the sections were captured using a CCD camera that was attached to a frame grabber (scion image 1.59 for Mac; scion, Frederick, MD, USA) and a computer (8100; Apple Macintosh, Cupertino, CA, USA). Grey values were converted to absorbance values by using a set of neutral density filters (Jonker et al. 1997). Absorbance values of control reactions were subtracted from test values to obtain specific activity (Butcher & Van Noorden 1985).
Statistical analysis
Statistical processing of data was performed using excel 97 (Microsoft; San Jose, CA, USA) and spss 8.0 for Windows (spss; Chicago, IL, USA). Data were expressed as mean values ± SD of three individual experiments. SD of each individual experiment was 5–10% of the mean value. A paired Student's t-test was used to determine statistical differences. Differences were considered statistically significant when P < 0.05.
Results
Quantitative histochemical analysis of G6PD, PGD, ICD and MD activity
In all livers investigated, foci were found that were classified as either preneoplastic or neoplastic based on the presence or absence of glycogen as detected by PAS staining (Bannasch et al. 1981; Hacker et al. 1982) in serial sections. Activity of all enzymes investigated was not different in preneoplastic and neoplastic lesions.
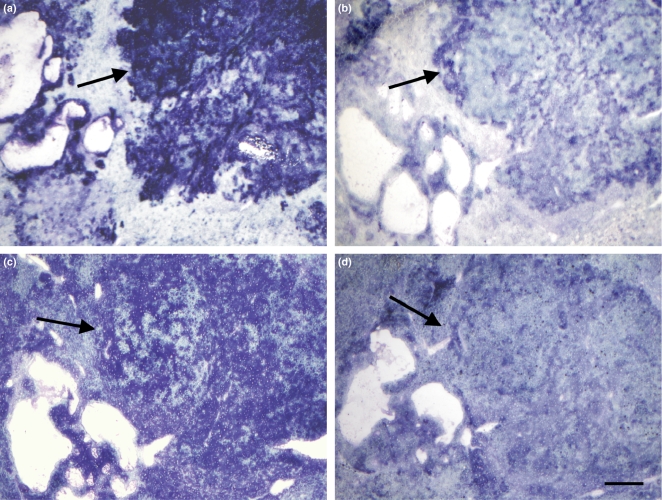
The activity of all four NADPH-producing dehydrogenases was similarly localized in hepatoma-containing rat livers (Figure 1). Activity of all dehydrogenases was highest in (pre)neoplastic lesions in rat liver. However, activity levels were distinctly different of the four dehydrogenases especially in (pre)neoplastic lesions. Only small amounts of final reaction product were formed in these livers after incubation in the absence of substrate and coenzyme.
Figure 1.
Light micrographs of cryostat sections of liver of rat treated with diethylnitrosamine incubated for the demonstration of the activity of glucose-6-phosphate dehydrogenase (a), phosphogluconate dehydrogenase (b), isocitrate dehydrogenase (c) and malate dehydrogenase (d). Increased activities are found in (pre)neoplastic lesions (arrows). Preneoplastic and neoplastic lesions as detected by glycogen staining in serial sections were not different with respect to the activity of the dehydrogenases. Bar is 100 μm.
Table 1 shows that G6PD provides at least two-thirds of NADPH in (pre)neoplastic lesions in rat liver. Phosphogluconate dehydrogenase, ICD and MD together contribute one-third to NADPH synthesis. The relative activity of G6PD, PGD, ICD and MD in (pre)neoplastic lesions compared to extralesional parenchyma was 24.9, 5.7, 2.8 and 5.3-fold higher, respectively.
Table 1.
Relative activity of the NADPH-producing enzymes glucose-6-phosphate dehydrogenase (G6PD), phosphogluconate dehydrogenase (PGD), isocitrate dehydrogenase (ICD) and malate dehydrogenase (MD) as demonstrated in (pre)neoplastic lesions and related to extralesional parenchyma
| Enzyme | Activity (%) | Fold increase in lesions vs. extralesional parenchyma |
|---|---|---|
| G6PD | 100 ± 21.3 | 24.9 |
| PGD | 11.6 ± 2.8 | 5.7 |
| ICD | 12.6 ± 3.0 | 2.8 |
| MD | 4.8 ± 1.5 | 5.3 |
| TKT | 0.1 | 2.5 |
Activity of G6PD is set at 100%. All values are statistically significantly different from G6PD activity. TKT, transketolase.
Localization and quantification of TKT activity
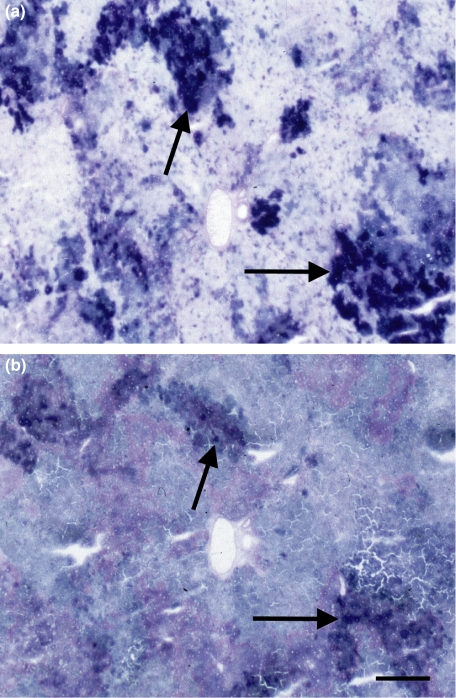
Figure 2 shows that TKT activity has a similar distribution pattern with highest activity in (pre)neoplastic lesions as G6PD and therefore of the four dehydrogenases. Transketolase activity was only 0.1% of G6PD activity in these areas. The relative increase in TKT activity in (pre)neoplastic lesions compared with that in extralesional parenchyma was 2.5 (Table 1).
Figure 2.
Light micrographs of cryostat sections of liver of rat treated with diethylnitrosamine, incubated for the demonstration of glucose-6-phosphate dehydrogenase activity (a) and transketolase activity (b). Increased activities are found in (pre)neoplastic lesions (arrows). Bar is 200 μm.
Ultrastructural localization of TKT activity in FTO-2B cells
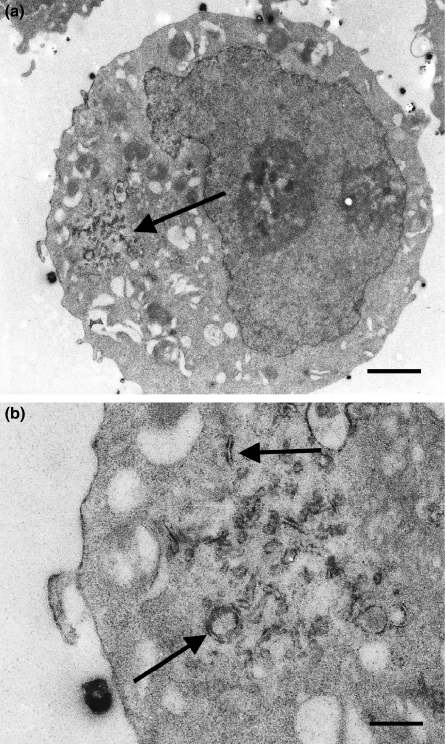
Electron-dense final reaction product generated by TKT activity was present only at membranes of granular endoplasmic reticulum (GER) in hepatoma cells (Figure 3).
Figure 3.
Electron micrographs of rat hepatoma cells incubated for the ultrastructural localization of transketolase activity. Electron dense final reaction product is observed at membranes of granular endoplasmic reticulum (arrows). (a) Bar is 2.0 μm; (b) Bar is 0.5 μm.
Discussion
The present study shows that the activity of all four NADPH-producing dehydrogenases G6PD, PGD, ICD and MD was elevated in (pre)neoplastic lesions in livers of rats treated with DENA compared to extralesional parenchyma. However, G6PD, the rate-limiting enzyme of the oxidative branch of the PPP, is by far the largest provider with at least two-thirds of the total NADPH supply (Table 1). The large increase of G6PD activity in foci of (pre)neoplastic cells has been demonstrated in the past (Hacker et al. 1982; Baba et al. 1989; Frederiks et al. 2003). Moreover, kinetic parameters of G6PD and PGD are affected in (pre)neoplastic lesions (Frederiks et al. 2003; Koehler & Van Noorden 2003). Fluxes calculated on the basis of these kinetic parameters of G6PD and PGD at physiological substrate concentrations are similar in liver parenchyma but elevated more than 10-fold for G6PD and 3-fold for PGD in the lesions. It is unlikely that there is accumulation of G6PD product or PGD substrate because an elevation in PGD substrate concentration probably leads to an increased flux through this enzyme.
Upregulation of especially G6PD activity in chemically induced (pre)neoplastic lesions in livers provides reducing power for regeneration of reduced glutathione and other detoxification processes (Winzer et al. 2002; Frederiks et al. 2003; Koehler & Van Noorden 2003). On the other hand, proliferation of cancer cells was described to be slowed down by the G6PD inhibitor dehydroepiandrosterone (Pascale et al. 1995; Melvin et al. 1997; Raïs et al. 1999; Ramos-Montoya et al. 2006). Thus, NADPH produced by G6PD seems to play a role in proliferation as well, which is in agreement with studies of Kuo et al. (2000) suggesting that NADPH induces tumour growth by the effect of the redox state on transcription factors.
The second finding of the present study is that TKT activity was also increased in (pre)neoplastic lesions. Boros et al. (1997) demonstrated that the majority of ribose recovered from nucleic acids in cancer cells was synthesized directly or indirectly via the non-oxidative PPP controlled by TKT activity. Inhibition of TKT activity has been shown to reduce the proliferation rate of pancreatic adenocarcinoma cells (Boros et al. 1997, 1998), Ehrlich ascites tumour cells in vitro (Raïs et al. 1999) and in vivo (Comin-Andiux et al. 2001), human hepatoma cells (Zhang et al. 2007) and human colon cancer cells (Hu et al. 2007). The importance of TKT for cancer cell proliferation was further supported by findings of Comin-Andiux et al. (2001) showing an elevated proliferation rate of Ehrlich ascites cancer cells induced by thiamine, a cofactor of TKT.
Studies of Coy et al. (2005) suggest that a mutation in the human TKT-L1 gene is responsible for elevated TKT activity in cancer cells and decreased TKT activity in patients with neurodegenerative diseases and diabetes. Elevated expression of TKT-L1 is a predictor of clinical outcome in patients with colon and urothelial cancers (Langbein et al. 2006), gastric cancer (Staiger et al. 2006), ovarian cancer (Krockenberger et al. 2007) and breast cancer (Foldi et al. 2007). A direct correlation between TKT-L1 mRNA and TKT activity in cancer cells was recently demonstrated in human hepatoma (Zhang et al. 2007) and colon cancer cell lines (Hu et al. 2007). Moreover, TKT activity in cancer cell proliferation may not only be related with the synthesis of ribose phosphate but also with the supply of energy by anaerobic degradation of glucose (Warburg et al. 1924; Coy et al. 2005; Langbein et al. 2006). The present study is the first to show increased TKT activity in situ in (pre)neoplastic lesions in livers of rats. Increased TKT activity may be a consequence of upregulation of TKT-L1 in combination with up- or downregulation of TKT in (pre)neoplastic lesions. As we localized total TKT activity, we are not able to draw any conclusions on the relative contributions of TKT and TKT-L1 in liver parenchyma, preneoplastic lesions or neoplastic lesions. Nevertheless, we provide information on the functional status of the non-oxidative PPP under the different conditions.
Metabolic control analysis performed on the regulatory enzymes of the PPP, G6PD and TKT revealed that the non-oxidative branch of the PPP is more important for tumour growth than the oxidative branch (Boren et al. 2002). On the other hand, the obtained results in the present study also show that although both G6PD and TKT activity increase in (pre)neoplastic cells, the relative increase of the oxidative enzyme (G6PD) is 10 times higher than that of the non-oxidative enzyme (TKT) and G6PD activity is 1000 times higher than TKT activity. This is in accordance with findings of Ramos-Montoya et al. (2006) demonstrating the importance of a forced balance of oxidative/non-oxidative branch activity in the direction of oxidative in order to sustain a high proliferation of cancer cells. These authors concluded that the activity of enzymes of the irreversible branch of the PPP (G6PD and PGD) is triggered by the need of NADPH, whereas reversibility of the non-oxidative branch of the PPP permits TKT to regulate the ribose pool. Thus, the non-oxidative branch of the PPP acts as a buffer: when NADPH synthesis is required, the excess of pentoses generated by G6P is converted to glycolysis intermediates by TKT and transaldolase reactions, whereas pentose phosphates are synthesized from glycolysis intermediates when high nucleotide synthesis is required.
In conclusion, we have described in the present study elevated activity of the oxidative and non-oxidative branches of the PPP. Moreover, we have shown the ultrastructural localization of TKT activity at membranes of GER in rat hepatoma cells. The similar intracellular localization of TKT activity, G6PD activity (Frederiks et al. 2007b) and likely PGD activity (Frederiks et al. 2007a) suggests that enzymes involved in the oxidative and non-oxidative branch of the PPP and possibly the entire PPP are present in metabolic complexes at this site. This may allow channelling of substrates in order to optimize the efficiency of the PPP.
Acknowledgments
The authors are grateful to Prof Dr C.J.F. Van Noorden for critical reviewing the manuscript, Dr C.-G. Wu for providing the rat specimens, the students Henrique Jorge and Rawichot Banditsaowapak for performing experiments, Mr J. Peeterse for photographic work, and Ms T.M.S. Pierik for the careful preparation of the manuscript.
This work was supported by SAF 2005-01626 from the Ministerio de Ciencia y Tecnologia, Spain and the European Union FEDER funds.
References
- Baba H, Yamamoto R, Jishi H, Tatsuta M, Wada A. Role of glucose-6-phosphate dehydrogenase in enhanced proliferation of pre-neoplastic and neoplastic cells in rat liver induced by N-nitrosomorpholine. Int. J. Cancer. 1989;43:892–895. doi: 10.1002/ijc.2910430526. [DOI] [PubMed] [Google Scholar]
- Bannasch P, Benner U, Hacker H-J, et al. Cytochemical and biochemical microanalysis of carcinogenesis. Histochem. J. 1981;13:79–820. doi: 10.1007/BF01003291. [DOI] [PubMed] [Google Scholar]
- Biagiotti E, Bosch KS, Ninfali P, Frederiks WM, Van Noorden CJF. Posttranslational regulation of glucose-6-phosphate dehydrogenase activity in tongue epithelium. J. Histochem. Cytochem. 2000;48:971–977. doi: 10.1177/002215540004800710. [DOI] [PubMed] [Google Scholar]
- Boonacker E, Stap J, Koehler A, Van Noorden CJF. The need for metabolic mapping in living cells and tissues. Acta Histochem. 2004;106:89–96. doi: 10.1016/j.acthis.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Boren J, Montoya AR, de Atauri P, et al. Metabolic control analysis aimed at the ribose synthesis pathways of tumor cells: a new strategy for antitumor drug development. Mol. Biol. Rep. 2002;29:7–12. doi: 10.1023/a:1020333730485. [DOI] [PubMed] [Google Scholar]
- Boren J, Ramos-Montoya A, Bosch KS, et al. In situ localization of transketolase activity in epithelial cells of different rat tissues and subcellularly in liver parenchymal cells. J. Histochem. Cytochem. 2006;54:191–199. doi: 10.1369/jhc.5A6745.2005. [DOI] [PubMed] [Google Scholar]
- Boros LG, Puigjaner J, Cascante M, et al. Oxythiamine and dehydroepiandrosterone inhibit the nonoxidative synthesis of ribose and tumor cell proliferation. Cancer Res. 1997;57:4242–4248. [PubMed] [Google Scholar]
- Boros LG, Brandes JL, Yusuf FI, Cascante M, Williams RD, Schirmer WJ. Inhibition of the oxidative and nonoxidative pentose phosphate pathways by somatostatin: a possible mechanism of antitumor action. Med. Hypotheses. 1998;50:501–506. doi: 10.1016/s0306-9877(98)90271-7. [DOI] [PubMed] [Google Scholar]
- Butcher RG, Van Noorden CJF. Reaction rate studies of glucose-6-phosphate dehydrogenase activity in sections of rat liver using four tetrazolium salts. Histochem. J. 1985;17:993–1008. doi: 10.1007/BF01417948. [DOI] [PubMed] [Google Scholar]
- Chieco P, Jonker A, Melchiorri C, Vanni G, Van Noorden CJF. A user's guide for avoiding errors in absorbance image cytometry: a review with original experimental observations. Histochem. J. 1994;26:1–19. [PubMed] [Google Scholar]
- Comin-Andiux B, Boren J, Martinez S, et al. The effect of thiamine supplementation on tumour proliferation. A metabolic control analysis study. Eur. J. Biochem. 2001;268:4177–4182. doi: 10.1046/j.1432-1327.2001.02329.x. [DOI] [PubMed] [Google Scholar]
- Coy JF, Dressler D, Wilde J, Schubert P. Mutations in the transketolase-like gene TKTL1: clinical implications for neurodegenerative diseases, diabetes and cancer. Clin. Lab. 2005;51:257–273. [PubMed] [Google Scholar]
- De Jong JSSG, Frederiks WM, Van Noorden CJF. Oxygen insensitivity of the histochemical assay of glucose-6-phosphate dehydrogenase activity for the detection of (pre)neoplasm in rat liver. J. Histochem. Cytochem. 2001;49:565–571. doi: 10.1177/002215540104900503. [DOI] [PubMed] [Google Scholar]
- Foldi M, Stickeler E, Bau L, et al. Transketolase protein TKTL1 overexpression: a potential biomarker and therapeutic target in breast cancer. Oncol. Rep. 2007;17:841–845. [PubMed] [Google Scholar]
- Frederiks WM, Vreeling-Sindelárová H. Localization of glucose-6-phosphate dehydrogenase activity on ribosomes of granular endoplasmic reticulum, in peroxisomes and peripheral cytoplasm of rat liver parenchymal cells. Histochem. J. 2001;33:345–353. doi: 10.1023/a:1012427224822. [DOI] [PubMed] [Google Scholar]
- Frederiks WM, Marx F, Strootman F. Demonstration of creatine kinase in myocardial and skeletal muscle using the semipermeable membrane technique. J. Mol. Cell. Cardiol. 1987;19:311–317. doi: 10.1016/s0022-2828(87)80598-9. [DOI] [PubMed] [Google Scholar]
- Frederiks WM, Bosch KS, De Jong JS, Van Noorden CJF. Post-translational regulation of glucose-6-phosphate dehydrogenase activity in (pre)neoplastic lesions in rat liver. J. Histochem. Cytochem. 2003;51:105–112. doi: 10.1177/002215540305100112. [DOI] [PubMed] [Google Scholar]
- Frederiks WM, Kummerlin IPED, Bosch KS, Vreeling-Sindelárová H, Jonker A, Van Noorden CJF. NADPH production by the pentose phosphate pathway in the zona fasciculata of rat adrenal gland. J. Histochem. Cytochem. 2007a;55:975–980. doi: 10.1369/jhc.7A7222.2007. [DOI] [PubMed] [Google Scholar]
- Frederiks WM, Vreeling-Sindelárová H, Van Noorden CJF. Loss of peroxisomes causes oxygen insensitivity of the histochemical assay of glucose-6-phosphate dehydrogenase activity to detect cancer cells. J. Histochem. Cytochem. 2007b;55:175–181. doi: 10.1369/jhc.6A7068.2006. [DOI] [PubMed] [Google Scholar]
- Hacker HJ, Moore MA, Mayer D, Bannasch P. Correlative histochemistry of some enzymes of carbohydrate metabolism in preneoplastic and neoplastic lesions in the rat liver. Carcinogenesis. 1982;3:1265–1271. doi: 10.1093/carcin/3.11.1265. [DOI] [PubMed] [Google Scholar]
- Horecker BL. The pentose phosphate pathway. J. Biol. Chem. 2002;277:47965–47971. doi: 10.1074/jbc.X200007200. [DOI] [PubMed] [Google Scholar]
- Hu LH, Yang JH, Zhang DT, et al. The TKTL1 gene influences total transketolase activity and cell proliferation in human colon cancer LoVo cells. Anticancer Drugs. 2007;18:427–433. doi: 10.1097/CAD.0b013e328013d99e. [DOI] [PubMed] [Google Scholar]
- Jonker A, Geerts WJC, Chieco P, Moorman AFM, Lamers WH, Van Noorden CJF. Basic strategies for valid cytometry using image analysis. Histochem. J. 1997;29:347–364. doi: 10.1023/a:1026434816947. [DOI] [PubMed] [Google Scholar]
- Kletzien RF, Harris PK, Foellmi LA. Glucose-6-phosphate dehydrogenase: a “housekeeping” enzyme subject to tissue-specific regulation by hormones, nutrients, and oxidant stress. FASEB J. 1994;8:174–181. doi: 10.1096/fasebj.8.2.8119488. [DOI] [PubMed] [Google Scholar]
- Koehler A, Van Noorden CJF. Reduced nicotinamide adenine nucleotide phosphate and the higher incidence of pollution-induced liver cancer in female flounder. Environ. Toxicol. Chem. 2003;22:2703–2710. doi: 10.1897/02-364. [DOI] [PubMed] [Google Scholar]
- Krockenberger M, Honig A, Rieger L, et al. Transketolase-like 1 expression correlates with subtypes of ovarian cancer and the presence of distant metastases. Int. J. Gynecol. Cancer. 2007;17:101–106. doi: 10.1111/j.1525-1438.2007.00799.x. [DOI] [PubMed] [Google Scholar]
- Kuo WY, Lin JY, Tang TK. Human glucose-6-phosphate dehydrogenase (G6PD) gene transforms NIH 3T3 cells and induces tumors in nude mice. Int. J. Cancer. 2000;85:857–864. doi: 10.1002/(sici)1097-0215(20000315)85:6<857::aid-ijc20>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Langbein S, Zerilli M, Zur Hausen A, et al. Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br. J. Cancer. 2006;94:578–585. doi: 10.1038/sj.bjc.6602962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzatto L, Battistuzzi G. Glucose-6-phosphate dehydrogenase. Adv. Hum. Genet. 1985;14:217–329. doi: 10.1007/978-1-4615-9400-0_4. [DOI] [PubMed] [Google Scholar]
- Melvin WS, Boros LG, Muscarella P, et al. Dehydroepiandrosterone-sulfate inhibits pancreatic carcinoma cell proliferation in vitro and in vivo. Surgery. 1997;121:392–397. doi: 10.1016/s0039-6060(97)90308-1. [DOI] [PubMed] [Google Scholar]
- Pandolfi PP, Sonati F, Rivi R, Mason P, Grosveld F, Luzzatto L. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 1995;14:5209–5215. doi: 10.1002/j.1460-2075.1995.tb00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale SM, De Miglio MR, Nufris A, et al. Inhibition by dehydroepiandrosterone of growth and progression of persistent liver nodules in experimental rat liver. Int. J. Cancer. 1995;17:210–215. doi: 10.1002/ijc.2910620217. [DOI] [PubMed] [Google Scholar]
- Raïs B, Comin B, Puigjaner J, et al. Oxythiamine and dehydroepiandrosterone induce a G1 phase cycle arrest in Ehrlich's tumor cells through inhibition of the pentose cycle. FEBS Lett. 1999;456:113–118. doi: 10.1016/s0014-5793(99)00924-2. [DOI] [PubMed] [Google Scholar]
- Ramos-Montoya A, Lee WN, Bassilian S, et al. Pentose phosphate cycle oxidative and non-oxidative balance: a new vulnerable target for overcoming drug resistance in cancer. Int. J. Cancer. 2006;119:2733–2741. doi: 10.1002/ijc.22227. [DOI] [PubMed] [Google Scholar]
- Smeets EH, Muller H, De Wael J. A NADH-dependent transketolase assay in erythrocyte hemolysates. Clin. Chim. Acta. 1971;33:379–386. doi: 10.1016/0009-8981(71)90496-7. [DOI] [PubMed] [Google Scholar]
- Spolarics Z. Endotoxemia, pentose cycle, and the oxidant/antioxidant balance in the hepatic sinusoid. J. Leukocyte Biol. 1998;63:534–541. doi: 10.1002/jlb.63.5.534. [DOI] [PubMed] [Google Scholar]
- Staiger WI, Coy JF, Grobholz R, et al. Expression of the mutated transketolase TKTL1, a molecular marker in gastric cancer. Oncol. Rep. 2006;16:657–661. [PubMed] [Google Scholar]
- Van Driel BEM, De Goeij AFPM, Song J-Y, De Bruine AP, Van Noorden CJF. Development of oxygen insensitivity of the quantitative histochemical assay of G6PDH activity during colorectal carcinogenesis. J. Pathol. 1997;182:398. doi: 10.1002/(SICI)1096-9896(199708)182:4<398::AID-PATH869>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Van Noorden CJF, Frederiks WM. Enzyme Histochemistry. A Laboratory Manual of Current Methods. Oxford: BIOS Scientific Publishers; 1992. [Google Scholar]
- Van Noorden CJF, Bahns S, Koehler A. Adaptational changes in kinetic parameters of G6PDH but not of PGDH during contamination-induced carcinogenesis in livers of North Sea flatfish. Biochim. Biophys. Acta. 1997;1342:141–148. doi: 10.1016/s0167-4838(97)00061-7. [DOI] [PubMed] [Google Scholar]
- Warburg O, Posener K, Negelein E. Ueber den Stoffwechsel der Carcinomzelle. Biochem. Z. 1924;152:309–344. [Google Scholar]
- Weber G. Enzymology of cancer cells. N. Engl. J. Med. 1977;296:541–551. doi: 10.1056/NEJM197703102961005. [DOI] [PubMed] [Google Scholar]
- Winzer K, Van Noorden CJF, Koehler A. Sex-specific biotransformation and detoxification after xenobiotic exposure of primary cultured hepatocytes of European flounder (Platichtys flesus L.) Aquat. Toxicol. 2002;59:17–33. doi: 10.1016/s0166-445x(01)00213-2. [DOI] [PubMed] [Google Scholar]
- Wu CG, Hakvoort TB, Lamers WH, Chamuleau RAFM. Isolation of up- and down-regulated cDNAs associated with hepatocellular carcinoma by a subtraction-enhanced display technique. Biochim. Biophys. Acta. 1996;1315:169–175. doi: 10.1016/0925-4439(95)00117-4. [DOI] [PubMed] [Google Scholar]
- Zhang S, Yang JH, Guo CK, Cai PC. Gene silencing of TKTL1 by RNAi inhibits cell proliferation in human hepatoma cells. Cancer Lett. 2007;253:108–114. doi: 10.1016/j.canlet.2007.01.010. [DOI] [PubMed] [Google Scholar]