Abstract
The HMGN proteins are a group of non-histone nuclear proteins that associate with the core nucleosome and alter the structure of the chromatin fiber. We investigated the distribution of the three best characterized HMGN family members, HMGN1, HMGN2 and HMGN3 during mouse eye development. HMGN1 protein is evenly distributed in all ocular structures of 10.5 days post coitum (dpc) mouse embryos however, by 13.5 dpc, relatively less HMGN1 is detected in the newly formed lens fiber cells compared to other cell types. In the adult, HMGN1 is detected throughout the retina and lens, although in the cornea, HMGN1 protein is predominately located in the epithelium. HMGN2 is also abundant in all ocular structures of mouse embryos, however, unlike HMGN1, intense immunolabeling is maintained in the lens fiber cells at 13.5 dpc. In the adult eye, HMGN2 protein is still found in all lens nuclei while in the cornea, HMGN2 protein is mostly restricted to the epithelium. In contrast, the first detection of HMGN3 in the eye is in the presumptive corneal epithelium and lens fiber cells at 13.5 dpc. In the lens, HMGN3 remained lens fiber cell preferred into adulthood. In the cornea, HMGN3 is transiently upregulated in the stroma and endothelium at birth while its expression is restricted to the corneal epithelium in adulthood. In the retina, HMGN3 upregulates around two weeks of age and is found at relatively high levels in the inner nuclear and ganglion cell layers of the adult retina. RT-PCR analysis determined that the predominant HMGN3 splice form found in ocular tissues is HMGN3b which lacks the chromatin unfolding domain although HMGN3a mRNA is also detected. These results demonstrate that the HMGN class of chromatin proteins has a dynamic expression pattern in the developing eye.
1. Results and discussion
The development of multicellular organisms requires the tight control of differential gene expression during embryogenesis. In recent years, it has been demonstrated that precise control of the chromatin structure of specific genes is crucial for the maintenance of the pluripotency of the inner cell mass (Boyer et al., 2006), while remodeling of chromatin is crucial for the commitment of these cells to specific lineages during development (Kondo, 2006), and their final terminal differentiation (Ehrlich, 2003; Hsieh and Gage, 2004; Palacios and Puri, 2006; Wilson et al., 2005; Zhou et al., 2005). The molecular mechanisms controlling the chromatin structure of genes has been under intense investigation and has been found to result from numerous epigenetic modifications including the differential regulation of histone acetylation, methylation and phosphorylation, DNA methylation and the use of histone variants in the nucleosomal structure (Wegel and Shaw, 2005).
Non-histone components of the chromatin are also important modulators of local chromatin structure and include members of the High Mobility Group N (HMGN) protein family (Bustin, 2001). HMGNs are relatively small, ~10 kDa, basic proteins that bind the 147-bp generic core nucleosome without preference for the underlying DNA sequence via a highly conserved, 30 amino acid long stretch of arginine, lysine and proline residues denoted the nucleosomal-binding domain (NBD) (Bustin, 2001). The best characterized members of this family, HMGN1 (formerly HMG-14) and HMGN2 (formerly HMG-17), are located in a punctate pattern in cell nuclei and are co-localized with chromosomal segments enriched in nascent transcripts. In living cells, these proteins are only able to interact with chromatin in the interphase nucleus (Cherukuri et al., 2008) where they move rapidly and only transiently associate with chromatin (Catez et al., 2004; Phair and Misteli, 2000). Binding of HMGN1 or HMGN2 proteins to chromatin facilitates DNA transcription, replication, and DNA repair, likely due to the ability of HMGN proteins to decompact chromatin and increase DNA accessibility (Bustin, 2001). Notably, HMGN1 inhibits the phosphorylation of histone H3 by MSK1 while HMGN2 does not, showing that different family members probably have distinct biological functions (Ueda et al., 2006).
Currently, the HMGN protein family (as defined by the presence of the NBD) consists of five members, the extensively studied HMGN1 and 2 (Bustin, 2001) as well as HMGN3 (also reported as the thyroid hormone receptor binding protein Trip7) (Amano et al., 2002; West et al., 2001), HMGN4 (Birger et al., 2001) and NBP45/NSBP1 (Shirakawa et al., 2000). HMGN1 is highly expressed in early mouse embryos (Mohamed et al., 2001), with levels generally downregulating, except in self renewing cell populations, as development proceeds (Furusawa et al., 2006). Anti-sense manipulation of HMGN1 levels in early mouse and Xenopus development leads to developmental delays (Mohamed et al., 2001) and defective patterning (Korner et al., 2003). HMGN2 is also found in early mouse embryos (Mohamed et al., 2001) and like HMGN1, anti-sense manipulation leads to early embryonic abnormalities (Korner et al., 2003; Mohamed et al., 2001). Recently, HMGN2 was shown to play a role in modulating the function of the homeodomain transcription factor, Pitx2, during development in response to Wnt/βcatenin signaling (Amen et al., 2008). HMGN3 expression appears to be more tissue restricted (West et al., 2001) and in frogs, its levels upregulate during intestinal differentiation, apparently under the control of the thyroid hormone receptor (Amano et al., 2002).
The importance of HMGN proteins during the development of the eye has been suggested by prior studies showing that the expression of HMGN1 is upregulated four fold in the lens of mimecan null mice (Tasheva et al., 2004) while the cornea of HMGN1 null mice is poorly stratified and expresses reduced levels of corneal differentiation markers (Birger et al., 2006). SAGE analysis has indicated that HMGN2 mRNA levels decrease during postnatal corneal development (Norman et al., 2004). HMGN3 mRNA levels are higher in the adult mouse eye than any other tissue investigated (West et al., 2001) and its expression is downregulated 4.6 fold in lenses from heat shock factor 4 knockout mice which exhibit juvenile onset cataract (Min et al., 2004). HMGN3 is also present at high levels in the inner nuclear layer of the adult mouse retina where it may regulate the expression of the glycine transporter-1 gene (West et al., 2004). Here we investigate the expression pattern of HMGN1, HMGN2 and HMGN3 during murine eye development.
1.1 Expression of HMGN1 in the embryonic and adult mouse eye
HMGN1 expression is ubiquitous in the optic vesicle and surface ectoderm at 9.5 days post coitum (dpc) (data not shown). By 10.5 dpc, the mouse eye is composed of the three main structures, the presumptive corneal epithelium, lens vesicle, and optic cup, and HMGN1 protein is still evenly distributed in the cell nuclei of all of these structures (Figure 1A, B). As the eye continues to develop from 10.5dpc to 13.5dpc, HMGN1 protein remains prominent in most ocular structures although its relative levels decrease in lens fiber cells by 13.5 dpc (Figure 1C, D). At birth, HMGN1 levels are relatively lower in the retinal ganglion cells which are the first neural cells formed in the retina (de Melo et al., 2003) as compared to the undifferentiated retina neuroepithelium (Figure 1 E, F). HMGN1 is evenly distributed between the corneal epithelium, stroma and endothelium at birth (not shown), however, HMGN1 staining is relatively weak in the corneal stroma and endothelium of the adult although prominent staining remains in the corneal epithelium (Figure 1 G, H). This distribution is consistent with the prior report that HMGN1 null mice have defects in the stratification of the corneal epithelium and its differentiation (Birger et al., 2006). HMGN1 levels decrease but are still detectable in all adult lens cell nuclei (Figure 1 I, J). In the adult retina, HMGN1 was detected at relatively low levels in all cell nuclei. (Figure 1 K, L). In contrast, HMGN1 mRNA was generally found at lower levels in the embryonic and newborn eye as compared to the adult (Figure 4).
Figure 1. HMGN 1 expression in the developing and adult mouse eye.
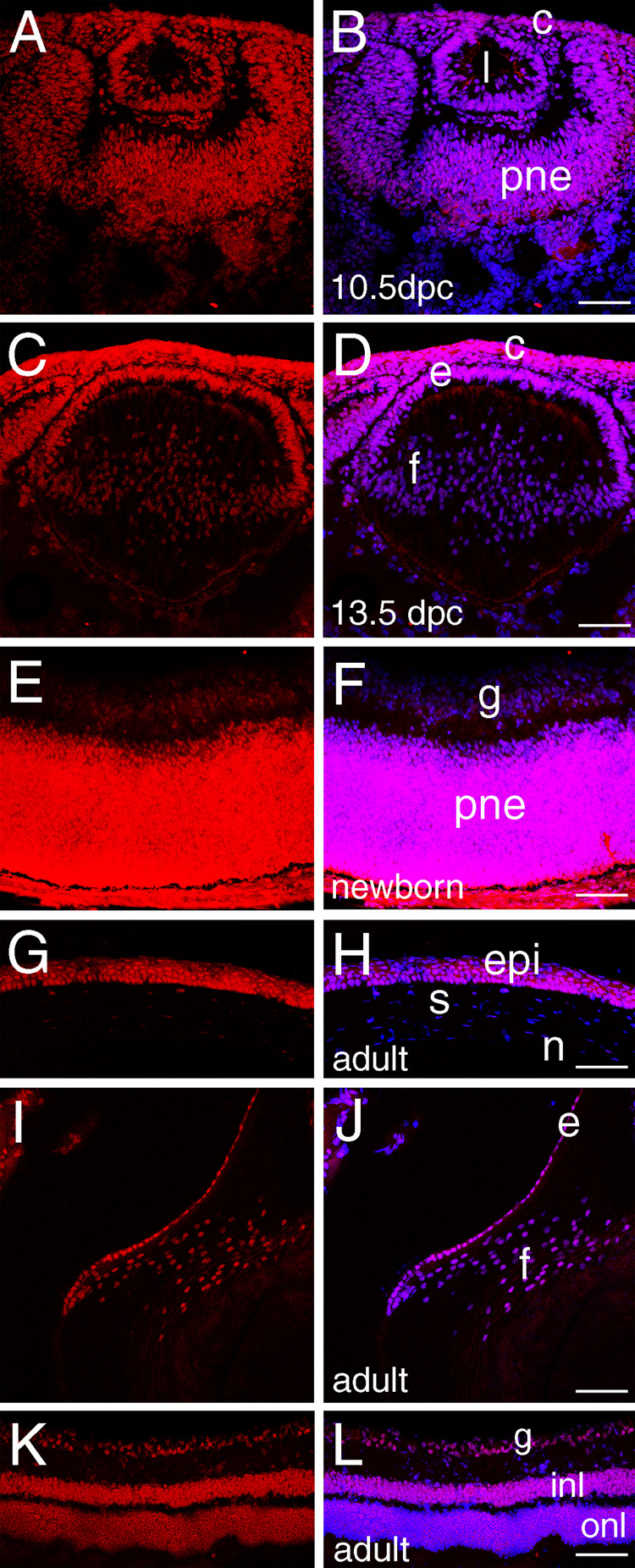
A, C, E, G, I, K-HMGN1 staining (red); B, D, F, H, J, L- merge of HMGN1 (red) and DNA (blue), overlap is pink. A, B) At 10.5 dpc, HMGN1 protein expression is seen in all tissues of the developing mouse eye. C, D) At 13.5 dpc, expression in the lens fiber cells begins to decrease relative to other cells of the eye. E, F) In the newborn retina, HMGN1 is restricted to the proliferating neuroepithelium with much lower levels observable in the ganglion cells. G, H) In the adult cornea, HMGN1 appears restricted to the epithelium. I, J) In the adult lens, HMGN1 is prominent in the lens epithelium, however fiber cell nuclei have some detectable protein. K, L) In the adult retina, HMGN1 levels decrease overall compared to the newborn proliferating neuroepithelium although protein is still detectable in all retinal layers. Abbreviations- c, cornea; l, lens vesicle; pne- proliferative retinal neuroepithelium; e, lens epithelium; f, lens fiber cells; epi, cornea epithelium; s, cornea stroma; n, cornea endothelium; g, ganglion cells; onl, outer nuclear layer; inl, inner nuclear layer; Scale bars, all 77 µm except for panel F where the bar=72 µm.
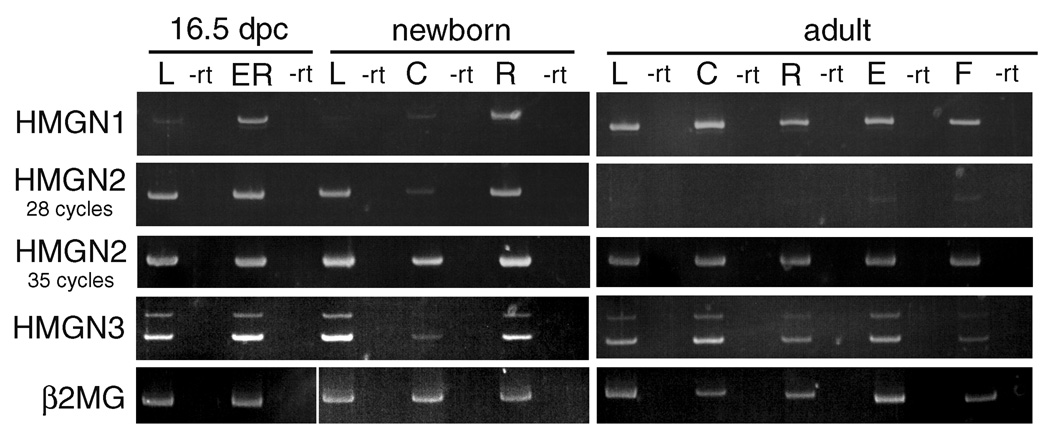
Figure 4.
RT-PCR analysis of HMGN gene expression in the developing eye. All PCR reactions were performed at cycle numbers in the linear amplification range of the sample with the highest expression level. For HMGN2, rt-PCR reactions were also performed for 35 cycles to detect the lower level of message present in adult ocular tissues. L- lens; ER- eye remaining after removal of lens; C- cornea; R- retina; E- lens epithelium; F- lens fibers. –rt, identical reactions performed without reverse transcriptase of the sample found at the lane on the left; β2MG- β2-microglobulin used as a control for RNA quality.
1.2 Expression of HMGN2 in embryonic and adult mouse eye
The expression pattern of HMGN2 is similar to that observed for HMGN1 in the 10.5 dpc mouse eye (Figure 2A, B) with high levels of apparently ubiquitous expression in the lens vesicle, presumptive cornea and optic cup. This similarity is still seen at 13.5 dpc, however, unlike HMGN1, HMGN2 expression remains prominent in 13.5 dpc lens fiber cells (Figure 2 C, D). In newborns, HMGN2 is found in all cells of the cornea (not shown), lens (not shown) and retina (Figure 2E, F). However, in adults, HMGN2 is less abundant in the corneal stroma and endothelium as compared to the epithelium (Figure 2 G, H). In the lens, HMGN2 protein is found in both the epithelium and fibers, although the nuclear staining in fiber cells is less intense (Figure 2, I, J). In the retina, high levels of HMGN2 staining are detected in the inner and outer nuclear layers, although much of the signal does not appear to be found in the cell nuclei. In contrast, HMGN2 is localized in the nuclei of retinal ganglion cells (Figure 2 K, L). RT-PCR analysis of HMGN2 expression in the eye detected higher levels of HMGN2 mRNA in embryonic as compared to adult eye tissues (Figure 4).
Figure 2. HMGN 2 expression in the embryonic and adult mouse eye.
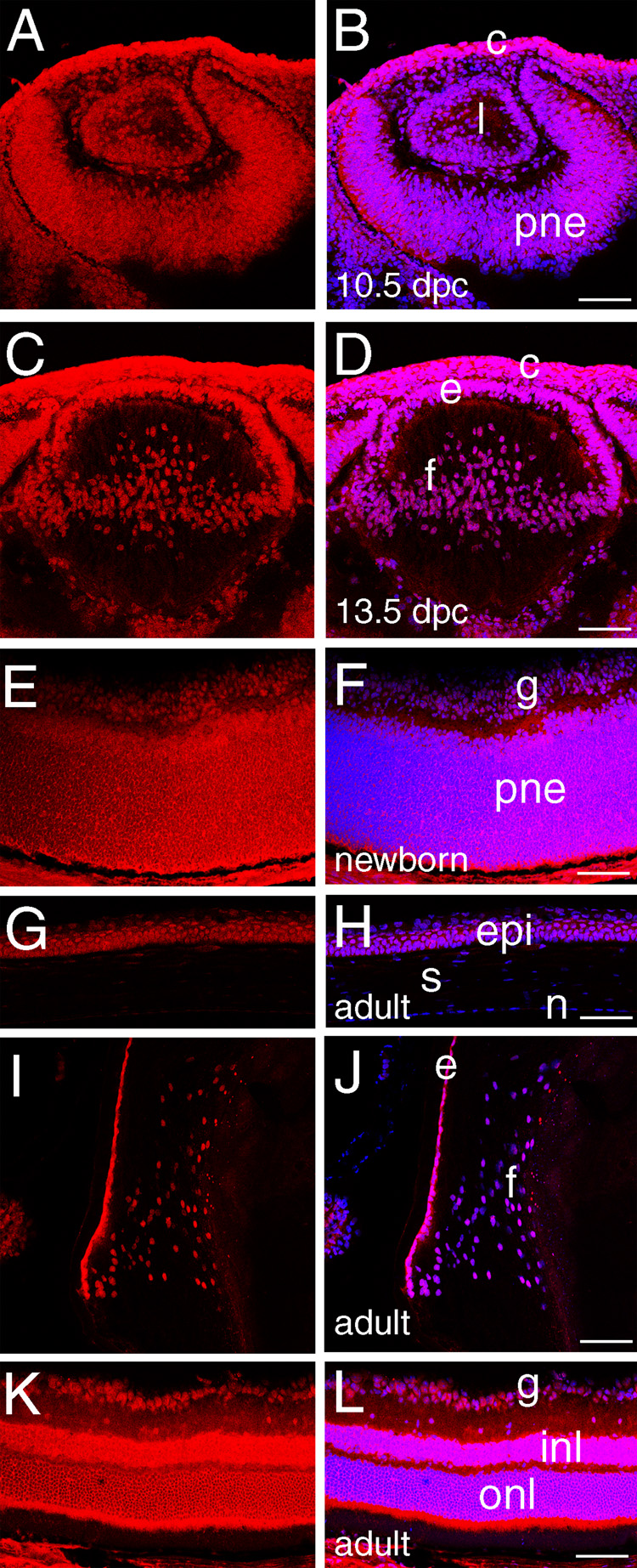
A, C, E, G, I, K-HMGN2 staining (red); B, D, F, H, J, L- merge of HMGN2 (red) and DNA (blue), overlap is pink. A,B) At 10.5 dpc, HMGN2 is found in all eye structures although the highest levels are seen in the presumptive corneal epithelium. C, D) At 13.5 dpc, HMGN2 levels are maintained throughout the eye. E,F) In the newborn retina, HMGN2 is found in all cell nuclei. G, H) In the adult cornea, HMGN2 protein appears restricted to the corneal epithelium. I, J) In the adult lens, HMGN2 levels are relatively higher in the epithelium than the fiber cells. K, L HMGN2 levels remain relatively similar in all retinal nuclei. epi, cornea epithelium Abbreviations: c, cornea; l, lens vesicle; pne, proliferative retinal neuroepithelium; e, lens epithelium; f, lens fiber cells; s, cornea stroma; n, cornea endothelium; e, lens epithelium; f, lens fiber cells; p, proliferating retina; g, ganglion cells; opl, outer plexiform layer; onl, outer nuclear layer; ipl, inner plexiform layer; inl, inner nuclear layer. Scale bars equal to 77 µm.
1.3 Expression of HMGN3 in embryonic and adult mouse eye
In contrast to HMGN1 and HMGN2, HMGN3 is not detected in the eye prior to 12.5 dpc (data not shown). At 12.5 dpc, a low level of HMGN3 expression was detected in the presumptive corneal epithelium but it is absent from the retina and lens at this stage (data not shown). At 13.5 dpc, HMGN3 protein is maintained in the presumptive corneal epithelium, while expression is first detected at very low levels in other ocular structures (Figure 3 A, B). At 14.5 dpc, the levels of HMGN3 increase in the corneal epithelium and first initiate in the developing corneal stroma, in addition to upregulating in all lens cells, most notably the lens fibers (Figure 3C, D). By birth, HMGN3 staining is prominent in all regions of the cornea (Figure 3 E, F), and retina (Figure 3 G, H), while staining is fiber cell preferred in the lens (data not shown). In the adult cornea, HMGN3 protein is only detectable in the epithelium (Figure 3 I, J), while HMGN3 is highly fiber cell preferred in the lens (Figure 3 K, L). In the retina, HMGN3 expression becomes transiently prominent in all retinal layers at two weeks after birth (data not shown), before its levels decrease in the outer nuclear layer as compared to the inner nuclear layer and ganglion cell layer (Figure 3 M, N) consistent with our prior report (West et al., 2004). RT-PCR analysis of HMGN3 using a primer set able to distinguish the HMGN3a (213 bp product) from the HMGN3b (164 bp product) splice variant lacking the chromatin unfolding domain (CHUD) which modulates histone acetylation in other HMGN family members (Ueda et al., 2006), found that the HMGN3b form predominates in all tissues examined (Figure 4), consistent with prior observations in other tissues (West et al., 2001).
Figure 3. HMGN 3 expression in the embryonic and adult mouse eye.
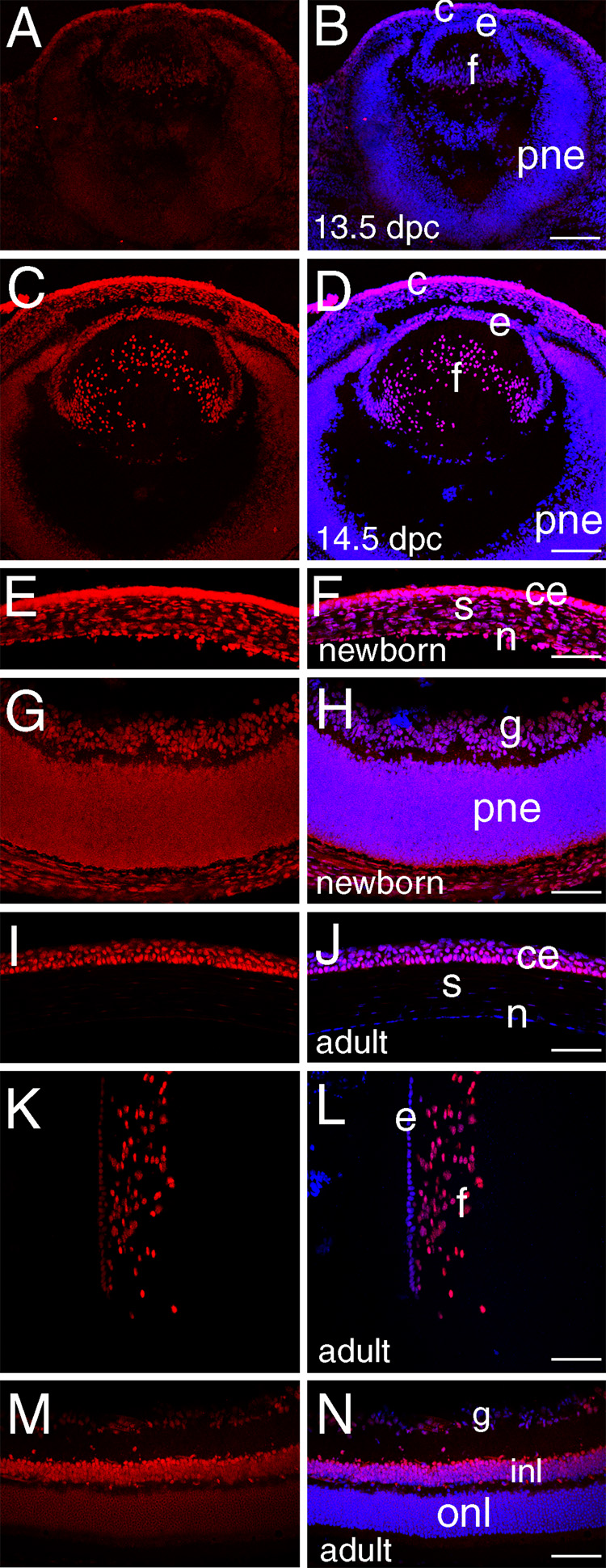
A, C, E, G, I, K, M- HMGN3 staining (red); B, D, F, H, J, L, N- merge of HMGN3 (red) and DNA (blue), overlap is pink. A, B) At 13.5 dpc, HMGN 3 protein expression is at low to undetectable levels in all ocular tissues except the presumptive corneal epithelium. C, D) At 14.5 dpc, noticeable levels of HMGN3 can be seen in the lens fiber cells as well as remaining high in the presumptive corneal epithelium. E,F) At birth, HMGN3 is found prominently in all layers of the cornea G, H) At birth, HMGN3 is present at moderate levels in both the ganglion cells and proliferating retinal neuroepithelium I, J) In the adult cornea, HMGN3 protein is only detected in the cornea epithelium with higher levels in basal cells as compared to stratified cells. K, L) In the adult lens, HMGN3 is found at higher levels in the fibers as compared to the epithelium. M, N) In the adult retina, HMGN3 is found predominantly in the inner nuclear layer. e, lens epithelium; f, lens fiber cells; pne, proliferating retinal neuroepithelium; c- cornea; ce- corneal epithelium; s- corneal stroma; n- corneal epithelium, g- retinal ganglion cells; inl- inner nuclear layer of retina; onl-outer nuclear layer of retina. Scale bars equal to 77 except for panel D where scale bar equals 154 µm.
1.4 Conclusion
Here we demonstrate that HMGN 1, 2 and 3 have different distributions in the eye during development. This is consistent with prior evidence suggesting that they play distinct roles in chromatin structure (Bustin, 2001; Ueda et al., 2006; West et al., 2004). Since HMGN1 has previously been shown to be important for corneal development (Birger et al., 2006) while HMGN3 likely regulates glycine transporter one expression in the retina (West et al., 2004), these proteins probably have distinct functions in the development of the eye.
2. Experimental Procedures
2.1 Mice
Mice (strain C57Bl/6Har) were obtained from Harlan Laboratories (Indianapolis, Indiana) and maintained in-house in a specific pathogen free animal facility. Embryos were obtained from timed matings with noon on the day that the vaginal plug was discovered defined as 0.5 days post coitum (dpc). All animal experiments were performed with the approval of the University of Delaware Institutional Animal Care and Use Committee and conform to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2 Immunofluorescent localization of HMGNs
Heads (embryonic mice) or eyes (birth until adulthood) were embedded in tissue freezing medium and 16-µm sections were generated as previously described (Reed et al., 2001). The tissue was blocked with 1% bovine serum albumen in PBS for one hour at room temperature then treated with antibodies previously shown to be specific for either HMGN1 (1:3000 dilution, (Bustin, 1989)), HMGN2 (1:3000 dilution, (Bustin, 1989)) or HMGN3 (raised against peptide 2752 which is unique to HMGN3 and will detect both HMGN3 splice forms, 1:5000 dilution (West et al., 2001)) for one hour at room temperature. Following two room temperature washes, antibody binding to the sections was visualized by treating sections with AlexaFluor 568 conjugated anti-rabbit IgG, (1:200 dilution, Invitrogen/Molecular Probes) with Draq V (1:5000 dilution, BioStatus LTD) added to visualize cell nuclei. Confocal microscopy was performed with a Zeiss 510 LSM confocal microscope configured with argon/krypton and helium/neon lasers in fast line scanning mode.
2.3 RT-PCR analysis of HMGN expression in the eye
Whole eyes were removed from C57Bl/6Har mice and then further dissected into individual eye tissues. RNA was then isolated with the SV Total RNA Isolation kit (Promega). Reverse transcriptase PCR was performed on 100 ng of RNA from each tissue using the Super Script One-Step RT-PCR system (Invitrogen). The cycle numbers used were chosen in the range of linear amplification for each primer set. Reactions without reverse transcriptase were performed with For HMGN1, the primers used were 5'-GAT GTC TGT GGT CAT GGC AG -3' and 5'-ACT TGT TCC AGT GAC TCG GC -3' resulting in a 179 bp product. For HMGN 2, the primers used were 5' - AGG ACG AGC CAC AGA GAA GA -3' and 5' -CTG CAG GAT TAT TCG CAT CC -3' which yielded a 159 bp product. For both HMGN1 and HMGN2, the cycling conditions were 30 minutes at 50C, 15 minutes at 95 C, followed by 28 cycles of 94C, 30 seconds; 55C, 30 seconds; 72C, 1 minute and a 10 minute final extension step at 72C. PCR was also performed under the same cycling conditions but for 35 cycles for HMGN2 to detect the lower level of its mRNA in adult tissues. For HMGN3, the primers used were 5’ GAA ACC TGT TCC ACC AAA ACC 3’ and 5’ ACG ACA ATT CAC TCT CCC TCC 3’ and the cycling conditions were 30 minutes at 50C, 15 minutes at 95 C, followed by 34 cycles of 94C, 30 seconds; 55C, 30 seconds; 72C, 5 seconds and a 10 minute final extension step at 72C. This produces a product for HMGN3a of 213 base pairs, and HMGN3b of 164 base pairs.
Acknowledgments
Funding: National Eye Institute grant EY012221 to MKD and INBRE program grant P20 RR16472 supporting the University of Delaware Core Imaging facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amano T, Leu K, Yoshizato K, Shi YB. Thyroid hormone regulation of a transcriptional coactivator in Xenopus laevis: implication for a role in postembryonic tissue remodeling. Dev Dyn. 2002;223:526–535. doi: 10.1002/dvdy.10075. [DOI] [PubMed] [Google Scholar]
- Amen M, Espinoza HM, Cox C, Liang X, Wang J, Link TM, Brennan RG, Martin JF, Amendt BA. Chromatin-associated HMG-17 is a major regulator of homeodomain transcription factor activity modulated by Wnt/beta-catenin signaling. Nucleic Acids Res. 2008;36:462–476. doi: 10.1093/nar/gkm1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birger Y, Davis J, Furusawa T, Rand E, Piatigorsky J, Bustin M. A role for chromosomal protein HMGN1 in corneal maturation. Differentiation. 2006;74:19–29. doi: 10.1111/j.1432-0436.2006.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birger Y, Ito Y, West KL, Landsman D, Bustin M. HMGN4, a newly discovered nucleosome-binding protein encoded by an intronless gene. DNA Cell Biol. 2001;20:257–264. doi: 10.1089/104454901750232454. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Mathur D, Jaenisch R. Molecular control of pluripotency. Curr Opin Genet Dev. 2006;16:455–462. doi: 10.1016/j.gde.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Bustin M. Preparation and application of immunological probes for nucleosomes. Methods Enzymol. 1989;170:214–251. doi: 10.1016/0076-6879(89)70049-5. [DOI] [PubMed] [Google Scholar]
- Bustin M. Chromatin unfolding and activation by HMGN(*) chromosomal proteins. Trends Biochem Sci. 2001;26:431–437. doi: 10.1016/s0968-0004(01)01855-2. [DOI] [PubMed] [Google Scholar]
- Catez F, Yang H, Tracey KJ, Reeves R, Misteli T, Bustin M. Network of dynamic interactions between histone H1 and high-mobility-group proteins in chromatin. Mol Cell Biol. 2004;24:4321–4328. doi: 10.1128/MCB.24.10.4321-4328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherukuri S, Hock R, Ueda T, Catez F, Rochman M, Bustin M. Cell Cycle Dependent Binding of HMGN Proteins to Chromatin. Mol Biol Cell. 2008 doi: 10.1091/mbc.E07-10-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo J, Qiu X, Du G, Cristante L, Eisenstat DD. Dlx1, Dlx2, Pax6, Brn3b, and Chx10 homeobox gene expression defines the retinal ganglion and inner nuclear layers of the developing and adult mouse retina. J Comp Neurol. 2003;461:187–204. doi: 10.1002/cne.10674. [DOI] [PubMed] [Google Scholar]
- Ehrlich M. Expression of various genes is controlled by DNA methylation during mammalian development. J Cell Biochem. 2003;88:899–910. doi: 10.1002/jcb.10464. [DOI] [PubMed] [Google Scholar]
- Furusawa T, Lim JH, Catez F, Birger Y, Mackem S, Bustin M. Down-regulation of nucleosomal binding protein HMGN1 expression during embryogenesis modulates Sox9 expression in chondrocytes. Mol Cell Biol. 2006;26:592–604. doi: 10.1128/MCB.26.2.592-604.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Gage FH. Epigenetic control of neural stem cell fate. Curr Opin Genet Dev. 2004;14:461–469. doi: 10.1016/j.gde.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kondo T. Epigenetic alchemy for cell fate conversion. Curr Opin Genet Dev. 2006;16:502–507. doi: 10.1016/j.gde.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Korner U, Bustin M, Scheer U, Hock R. Developmental role of HMGN proteins in Xenopus laevis. Mech Dev. 2003;120:1177–1192. doi: 10.1016/j.mod.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Min JN, Zhang Y, Moskophidis D, Mivechi NF. Unique contribution of heat shock transcription factor 4 in ocular lens development and fiber cell differentiation. Genesis. 2004;40:205–217. doi: 10.1002/gene.20087. [DOI] [PubMed] [Google Scholar]
- Mohamed OA, Bustin M, Clarke HJ. High-mobility group proteins 14 and 17 maintain the timing of early embryonic development in the mouse. Dev Biol. 2001;229:237–249. doi: 10.1006/dbio.2000.9942. [DOI] [PubMed] [Google Scholar]
- Norman B, Davis J, Piatigorsky J. Postnatal gene expression in the normal mouse cornea by SAGE. Invest Ophthalmol Vis Sci. 2004;45:429–440. doi: 10.1167/iovs.03-0449. [DOI] [PubMed] [Google Scholar]
- Palacios D, Puri PL. The epigenetic network regulating muscle development and regeneration. J Cell Physiol. 2006;207:1–11. doi: 10.1002/jcp.20489. [DOI] [PubMed] [Google Scholar]
- Phair RD, Misteli T. High mobility of proteins in the mammalian cell nucleus. Nature. 2000;404:604–609. doi: 10.1038/35007077. [DOI] [PubMed] [Google Scholar]
- Reed NA, Oh DJ, Czymmek KJ, Duncan MK. An immunohistochemical method for the detection of proteins in the vertebrate lens. J Immunol Methods. 2001;253:243–252. doi: 10.1016/s0022-1759(01)00374-x. [DOI] [PubMed] [Google Scholar]
- Shirakawa H, Landsman D, Postnikov YV, Bustin M. NBP-45, a novel nucleosomal binding protein with a tissue-specific and developmentally regulated expression. J Biol Chem. 2000;275:6368–6374. doi: 10.1074/jbc.275.9.6368. [DOI] [PubMed] [Google Scholar]
- Tasheva ES, Ke A, Deng Y, Jun C, Takemoto LJ, Koester A, Conrad GW. Differentially expressed genes in the lens of mimecan-null mice. Mol Vis. 2004;10:403–416. [PubMed] [Google Scholar]
- Ueda T, Postnikov YV, Bustin M. Distinct domains in high mobility group N variants modulate specific chromatin modifications. J Biol Chem. 2006;281:10182–10187. doi: 10.1074/jbc.M600821200. [DOI] [PubMed] [Google Scholar]
- Wegel E, Shaw P. Gene activation and deactivation related changes in the three-dimensional structure of chromatin. Chromosoma. 2005;114:331–337. doi: 10.1007/s00412-005-0015-7. [DOI] [PubMed] [Google Scholar]
- West KL, Castellini MA, Duncan MK, Bustin M. Chromosomal proteins HMGN3a and HMGN3b regulate the expression of glycine transporter 1. Mol Cell Biol. 2004;24:3747–3756. doi: 10.1128/MCB.24.9.3747-3756.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West KL, Ito Y, Birger Y, Postnikov Y, Shirakawa H, Bustin M. HMGN3a and HMGN3b, two protein isoforms with a tissue-specific expression pattern, expand the cellular repertoire of nucleosome-binding proteins. J Biol Chem. 2001;276:25959–25969. doi: 10.1074/jbc.M101692200. [DOI] [PubMed] [Google Scholar]
- Wilson CB, Makar KW, Shnyreva M, Fitzpatrick DR. DNA methylation and the expanding epigenetics of T cell lineage commitment. Semin Immunol. 2005;17:105–119. doi: 10.1016/j.smim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Zhou GL, Xin L, Liu DP, Liang CC. Remembering the cell fate during cellular differentiation. J Cell Biochem. 2005;96:962–970. doi: 10.1002/jcb.20572. [DOI] [PubMed] [Google Scholar]