Abstract
Background
As many HIV seropositive individuals are now living longer after infection due to highly active antiretroviral therapy, aging related manifestations of cerebral small vessel ischemic vascular disease, such as brain white matter hyperintensities, are becoming increasingly important in this population.
Goals
This study is designed to determine the relationship between white matter hyperintensities and cortical volumes in HIV seropositive individuals.
Materials and Methods
Voxel-based-morphometry was used to compare cortical volumes between 62 HIV seropositive individuals participating in the Hawaii Aging with HIV cohort study, 30 with moderate white matter hyperintensities and 32 with minimal or no white matter hyperintensities.
Results
Presence of moderate white matter hyperintensities was associated with decreased cortical volumes in the frontal lobes bilaterally.
Conclusion
These findings suggest that age related white matter hyperintensities are associated with reduced frontal gray matter volumes in HIV seropositive individuals, supporting the hypothesis that the frontal lobes may have greater susceptibility to the effects of small vessel ischemic vascular disease.
Keywords: Leukoaraiosis, Ischemic vascular disease, Aging, HIV infection
Introduction
Human immunodeficiency virus (HIV) infection in older patients (age > 50 years) is becoming increasingly common in developed countries as HIV seropositive individuals live longer because of potent antiretroviral treatment (1). Simultaneously, the development and expression of aging related brain changes among HIV-infected patients is evolving. Typical aging related brain changes are now commonly observed in older patients with HIV infection, including those due to cerebral manifestations of small vessel ischemic vascular disease such as white matter hyperintensities (WMHs) (2-4).
White matter hyperintensities are considered to result from disruption of small penetrating arteries in the brain and are commonly observed in otherwise normal elderly individuals (5). The effects of small vessel ischemic vascular disease are not uniformly distributed throughout the brain and the frontal lobes may be particularly vulnerable. For example, previous studies of normal elderly populations demonstrate that presence of WMHs is associated both structurally with frontal lobe atrophy (5,-8) and functionally with worse performance on neuropsychological tests of frontal lobe functions (6,9-12). In individuals meeting clinical criteria for Alzheimer’s disease, other studies have reported a frontal lobe predominance for location of WMHs as well as correlations between WMHs and loss of frontal lobe cortical volumes (5,13). These results further support the increased vulnerability of the frontal lobes to small vessel ischemic vascular disease.
The objective of this study is to determine the relationship between brain gray matter volumes and white matter hyperintensities in patients with HIV infection. We hypothesized that presence of moderately severe white matter hyperintensities in HIV seropositive individuals would be associated with reduced gray matter volumes, particularly in the frontal lobes. The results of this study support the hypothesis that the frontal lobes are particularly vulnerable to the effects of small vessel ischemic vascular disease.
Materials and Methods
Study Population
The Hawaii Aging with HIV Cohort (HAHC) is a community-based study of aging in HIV seropositive individuals. Details of enrollment and clinical characterization are published elsewhere (14). Briefly, participants were enrolled if HIV seropositive and either age greater than 50 years (older group) or between 20-39 years (younger group), and major exclusion criteria are not present. Primary exclusion criteria include head injury, learning disability, major neurological or psychiatric disease, or brain opportunistic disease. Baseline and annual evaluations include a neurological examination, medical intake with demographic data, risk behavior inventory, HIV-1 laboratory parameters (viral load, CD4 count, and lowest ever CD4 count), medication histories, and co-morbid illnesses. Cohort participants were followed over a 5 year period from 2001 to 2006. This study included a total of 62 HAHCS participants who underwent magnetic resonance imaging (MRI) of the brain as part of their participation in the HAHCS. Institutional review board approval to conduct the study was obtained at the University of Hawaii.
Assessment of White Matter Hyperintensities
MRI examinations were performed in a GE Sigma 1.5-Tesla scanner. In all participants, axial T1, T2, and FLAIR weighted images were acquired, with a slice thickness of 5mm. Two neurologists (A.M. and B.N.) classified the severity and distribution of white matter hyperintensities according to the Rotterdam Scan Study (RSS) scale (15, 16). At the time of reviewing the MRI images, the raters were blinded to the patient’s identity, demographic factors including ethnicity, sex, hypertension, diabetes, and had not participated in the patients’ clinical care. Participants were considered to have a moderate degree of WMHs if either subcortical white matter lesions were present or the severity of the periventricular white matter lesions was graded as 2 or higher in any of the three periventricular regions according to the Rotterdam Scan Study (RSS) scale (16). Subjects were considered to have a minimal degree of WMHs if no subcortical white matter lesions were present and ratings for severity of periventricular white matter lesions were 1 or less in each of the three periventricular regions according to the Rotterdam Scan Study (RSS) scale (16).
Voxel-Based-Morphometry (VBM)
All MRI images were processed using Statistical Parametric Mapping (SPM2) (17). MRI images were processed based on an optimized VBM protocol (18). First the images were coregistered and spatially normalized using a twelve-parameter affine transformation to the standard coordinate system of Talairach and Tournoux. Global normalization by proportional scaling was used. The normalized images were then segmented into gray matter, white matter and cerebrospinal fluid volumes. All gray matter volumes were then smoothed with a 12 mm full width at half-maximum three dimensional Gaussian smoothing filter.
Statistical Analysis
Demographic factors, vascular risk factors, and HIV infection-related factors were compared between HIV seropositive individuals with moderate and minimal degrees of WMHs using two-tailed t-tests for continuous variables and chi-square analysis for categorical variables. A generalized linear model was then used to compare the volume of gray matter on a voxel-by-voxel basis using Voxel-Based-Morphometry between the groups of individuals with moderate and minimal WMHs. A non-adjusted p-value for statistical significance of 0.05 was used.
Results
A total of 62 HIV seropositive individuals were included in the study, 30 with moderate WMHs and 32 with minimal WMHs. Presence of moderate WMHs was associated with greater mean age in years (54.59, S.D. = 6.38; 42.70, S.D. = 8.56; p < 0.001) and greater mean years of education (14.77, S.D. = 2.34; 13.13, S.D. = 1.76; p = 0.003) compared to those with minimal WMHs (Table 1). A trend towards greater mean systolic blood pressure in mm Hg in participants with moderate WMHs compared to those with minimal WMHs was also identified (133.07, S.D. = 16.17; 126.41, S.D. = 14.99; p = 0.098). No differences were detected for other demographic variables, vascular risk factors, or HIV infection-related factors.
Table 1.
Comparison of Demographic Characteristics, Vascular Risk Factors, and HIV Infection Related Factors
| Moderate WMH | Minimal WMH | Significance | |
|---|---|---|---|
| Volume of subcortical WMH (cm3) | 5.88 (6.81)* | 0.00 (0.00) | p < 0.001 |
| Periventricular WMH severity | 3.03 (1.03) | 1.41 (1.01) | p < 0.001 |
| Age (years) | 54.59 (6.38) | 42.70 (8.56) | p < 0.001 |
| Years of formal education | 14.77 (2.34) | 13.13 (1.76) | p = 0.003 |
| Viral Load (1000 copies/mL) | 122.85 (291.91) | 185.98 (441.86) | N.S. |
| CD4 cell count (cells per mL) | 314.77 (183.67) | 333.81 (304.65) | N.S. |
| CD4 nadir (cells per mL) | 125.71 (121.07) | 134.93 (148.58) | N.S. |
| Systolic Blood Pressure | 133.07 (16.17) | 126.41 (14.99) | p = 0.098 |
| Smoking (pack-years) | 12.50 (15.35) | 13.81 (12.17) | N.S. |
| Gender (Men/Women) | 26/4** | 29/3 | N.S. |
| On HAART (yes/no) | 24/6 | 25/7 | N.S. |
WMH = White matter hyperintensity.
N.S. = non-significant (p > 0.05).
HAART = highly active anti-retroviral therapy.
Continuous variables are reported as: mean (standard deviation).
Categorical variables are reported as the number present in each category, with categories separated by a forward slash.
The relationship between white matter hyperintensities and brain cortical volumes was evaluated using VBM. Figures 1 illustrates specific pixels representing areas of decreased cortical volume in HIV seropositive patients with moderate compared to minimal WMHs (p < 0.01). These pixels correspond to regions within the frontal lobes bilaterally.
Figure 1.
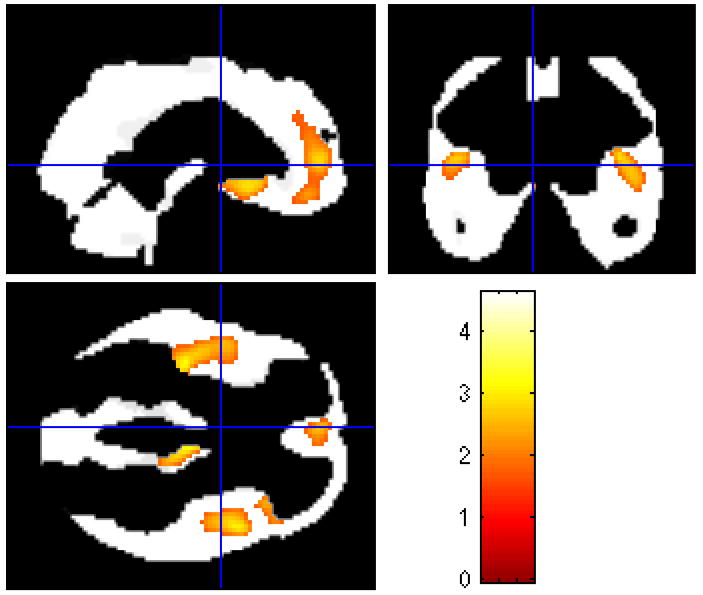
Statistical map (with t-scores linked to shading scale) derived from normalized brain gray matter volumes.
Orthogonal views of statistical maps of normalized brain gray matter volume data indicating bilateral regions of decreased gray matter volume in the frontal lobes of HIV seropositive patients with moderate white matter hyperintensities compared to those with minimal white matter hyperintensities.
Discussion
Cerebral manifestations of small vessel ischemic vascular disease such as WMHs are of increasing importance for HIV seropositive individuals as the prevalence of older and elderly patients continues to increase due to increased longevity with highly active anti-retroviral therapy (HAART). This investigation into the relationship between cortical atrophy and WMHs in HIV seropositive individuals found areas of significantly reduced cortical volume in the frontal lobes of HIV seropositive individuals with moderate WMHs compared to those with minimal WMHs. Given the relationship between WMHs and frontal lobe cortical atrophy in the general population (5-8), these findings extend knowledge regarding the increased vulnerability of the frontal lobes to the effects of small vessel ischemic vascular disease to HIV seropositive individuals.
In the general population WMHs are regarded as one of several manifestations of small vessel ischemic vascular disease of the brain (2-4,25). Several population based studies have demonstrated that these lesions occur frequently among older individuals and are associated with vascular risk factors (2-4,25,26). Consequently, in normal elderly populations presence of these lesions is often considered a reliable marker for small vessel ischemic vascular disease. Additionally, presence of these lesions is now considered an important component of commonly used clinical criteria for vascular dementia (26,30-33), further indicating the clinical relevance of these lesions.
Neuroimaging studies using magnetic resonance imaging and positron emission tomography have demonstrated significant associations between presence of WMHs and measures of brain atrophy in the general population (6,19,20). WMHs have been associated with several markers of brain atrophy including: larger ventricular volumes (6), reduced whole brain volumes (6) and reduced cortical blood volumes (21). Additionally, the affect of presence of WMHs on cortical gray matter is not uniformly distributed and a predilection for reduction in frontal lobe gray matter volumes compared to other brain regions is now well established in normal elderly populations (5,13,21).
Despite all that is known, the underlying etiology of gray matter atrophy associated with presence of WMH’s has not yet been established and several theories exist. First, cortical gray matter loss has been suggested to occur as a result of Wallerian degeneration after damage to axons through small vessel ischemic disease (5). Another possibility is axonal denervation causing neuronal loss in the gray matter as a result of axonal damage in the white matter (5). Finally, it has been suggested that WMH’s do not directly cause cortical gray matter loss, but are a marker for the effects of reduced cerebral blood flow to the cortex due to small vessel vascular disease with resulting cortical hypoperfusion as the direct cause of neuronal loss and cortical atrophy (5). Additionally, several types of WMHs can be distinguished that may have different underlying associated pathophysiologies: periventricular changes may be associated mainly with disruption of the subependymal lining and gliosis (5), punctate subcortical lesions with tissue hypoperfusion due to thickened arteriolar walls (5), and large patchy lesions with more extensive tissue ischemia (23). An additional possibility is that primary cortical apoptosis, which is known to occur in HIV-associated dementia, may contribute to brain atrophy as well (24).
Clinically, several studies have demonstrated associations between presence of these lesions and worse performance on cognitive tasks as well as an increased risk for cognitive decline and development of dementia (22, 27-29). While the association between presence of WMHs and cognitive decline in the general population is well recognized, the relationship between WMHs and cognitive performance in individuals living with HIV infection is still somewhat controversial. WMHs have been reported to be associated with some cognitive measures, including worse performance on tests of psychomotor speed and verbal memory (34). Additionally, decreased white matter volume in HIV seropositive individuals has been associated with dementia (35). However, in other studies, no relationship was identified between presence of WMHs and cognitive performance (36-38). Additionally, there is evidence that development of dementia in HIV infection may be associated with atrophy of other brain regions such as the basal ganglia and hippocampus (39). However, further support for the role of white matter damage in development of cognitive impairment and dementia in HIV infection has been recently demonstrated using magnetic resonance spectroscopy (40-44). The findings of this study add support to the role for WMHs in development of cognitive impairment and dementia in HIV infection through the identified associations with frontal lobe atrophy.
This study is limited by several factors including: the use of a visual rating of WMHs, the lack of pathological examination of the brains, the lack of MRI scans on HIV-seronegative controls for comparison, and confounding differences in mean ages between the study groups. Because HIV infection may also cause the appearance of white matter hyperintensities, the lack of information from pathological examination of the participants’ brains prevents definitive determination of the etiology of the white matter lesions identified in this study. However, the finding of associated frontal lobe atrophy in this study strengthens the hypothesis that small vessel ischemic vascular disease contributes to development of white matter hyperintensities in aging HIV seropositive individuals. Additionally, previous studies have demonstrated that treatment of HIV infection with HAART and the resulting clinical improvement, as measured by increased CD4 cell count and decreased viral load, are associated with regression of HIV induced white matter abnormalities on MRI (45). This would be consistent with a decreased contribution of HIV induced white matter abnormalities to the white matter hyperintensities identified in this study, since all participants included in this study were receiving HAART and no differences in mean CD4 cell count or mean viral load existed between participants with and without white matter hyperintensities. In our study, the group with moderate WMHs was significantly older than the group with minimal WMHs, and further study with age-matched controls would be useful to confirm that these findings are not related to an affect of the aging process. Additionally, our results may be strengthened by the use of a computer based quantitative method of measuring brain WMHs such as that used by Bartzokis et al (46), as well as a more sophisticated quantification of gray matter volumes such as cortical thickness mapping. The main strength of the study is that we have extended associations between WMHs and gray matter atrophy to individuals living with HIV infection (5,13,21), and confirmed that as reported in the general population this association appears to preferentially affect frontal lobe regions.
As HIV seropositive individuals are more commonly reaching older and elderly ages, understanding cerebral small vessel ischemic vascular disease and its effects on brain structure and function is becoming increasingly more important in this population. The results of this study are consistent with those reported in normal elderly populations and provide further support for increased susceptibility of the frontal lobes to the effects of small vessel ischemic vascular disease. Further investigation using techniques such as cortical thickness mapping and computerized quantification of white matter hyperintensities are indicated, as well as neuropsychological testing to determine if the identified structural changes effect cognitive performance. These findings may also be useful to clinicians caring for aging HIV seropositive individuals.
Acknowledgments
This work supported by the Ph.D. in Clinical Research Program at the University of Hawaii, 1K07GM072A84. Additional support from NINDS grant 1U54NS43049, NCRR grant P20 RR11091, and RCMI grant G12 RR/AI 03061. Special appreciation extended to our valued participants, staff, and community physicians.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goodkin K, Wilkie FL, Concha M, et al. Aging and neuro-AIDS conditions and the changing spectrum of HIV-1 associated morbidity and mortality. J Clin Epidemiol. 2001;54(Suppl 1):S35–43. doi: 10.1016/s0895-4356(01)00445-0. [DOI] [PubMed] [Google Scholar]
- 2.Inzitari D. Leukoaraiosis. An independent risk factor for stroke? Stroke. 2003;34:2067–2071. doi: 10.1161/01.STR.0000080934.68280.82. [DOI] [PubMed] [Google Scholar]
- 3.Basile AM, Pantoni L, Pracucci G, et al. Age, Hypertension, and Lacunar Stroke Are the Major Determinants of the Severity of Age-Related White Matter Changes. The LADIS (Leukoaraiosis and Disability in the Elderly) Study. Cerebrovasc Dis. 2006;21:315–322. doi: 10.1159/000091536. [DOI] [PubMed] [Google Scholar]
- 4.Ovbiagele B, Saver JL. Cerebral White Matter Hyperintensities on MRI: Current Concepts and Therapeutic Implications. Cerebrovasc Dis. 2006;22:83–90. doi: 10.1159/000093235. [DOI] [PubMed] [Google Scholar]
- 5.Rossi R, Boccardi M, Sabattoli F, et al. Topographic correspondence between white matter hyperintensities and brain atrophy. J Neurol. 2006;253:919–927. doi: 10.1007/s00415-006-0133-z. [DOI] [PubMed] [Google Scholar]
- 6.DeCarli C, Murphy DG, Tranh M, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- 7.Mirsen TR, Lee DH, Wong CJ, et al. Clinical correlates of white matter changes on magnetic resonance imaging scans of the brain. Arch Neurol. 1991;48:1015–1021. doi: 10.1001/archneur.1991.00530220031015. [DOI] [PubMed] [Google Scholar]
- 8.Ylikoski A, Erkinjuntti T, Raininko R, et al. White matter hyperintensities on MRI in the neurologically nondiseased elderly. Stroke. 1995;26:1171–1177. doi: 10.1161/01.str.26.7.1171. [DOI] [PubMed] [Google Scholar]
- 9.Boone KB, Miller BL, Lesser Im, et al. Neuropsychological correlates of white-matter lesions in healthy elderly subjects. A threshold effect. Arch Neurol. 1992;49:549–554. doi: 10.1001/archneur.1992.00530290141024. [DOI] [PubMed] [Google Scholar]
- 10.Mendez MF, Cummings JL. Dementia: A Clinical Approach. third. Philadelphia, PA: Elsevier Science Publishing Company; 2003. [Google Scholar]
- 11.Longstreth WT, Jr, Bernick C, Manolio TA, et al. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol. 1998;55:1217–1225. doi: 10.1001/archneur.55.9.1217. [DOI] [PubMed] [Google Scholar]
- 12.Vermeer SE, Den Heijer T, Koudstaal PJ, et al. Rotterdam Scan Study. Incidence and risk factors of silent brain infarcts in the population-based Rótterdam Scan Study. Stroke. 2003;34:392–396. doi: 10.1161/01.str.0000052631.98405.15. [DOI] [PubMed] [Google Scholar]
- 13.Capizzano AA, Acion L, Bekinschtein T, et al. White matter hyperintensities are significantly associated with cortical atrophy in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75:822–827. doi: 10.1136/jnnp.2003.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valcour V, Shikuma C, Shiramizu B, et al. Higher frequency of dementia in older HIV+ individuals. The Hawaii Aging with HIV Cohort. Neurology. 2004;63:822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMurtray AM, Nakamoto B, Shikuma C, et al. Small Vessel Vascular Disease in HIV Infection: The Hawaii Aging with HIV Cohort Study. Cerebrovasc Dis. 2007;24:236–241. doi: 10.1159/000104484. [DOI] [PubMed] [Google Scholar]
- 16.de Leeuw F-E, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonante imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.www.fil.ion.ucl.ac.uk/spm
- 18.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 19.Sultzer DL, Mahler ME, Cummings JL, et al. Cortical abnormalities associated with subcortical lesions in vascular dementia. Clinical and positron emission tomographic findings. Arch Neurol. 1995;52:773–780. doi: 10.1001/archneur.1995.00540320049012. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi W, Takagi S, Ide M, et al. Reduced cerebral glucose metabolism in subjects with incidental hyperintensities on magnetic resonance imaging. J Neurol Sci. 2000;176:21–27. doi: 10.1016/s0022-510x(00)00286-0. [DOI] [PubMed] [Google Scholar]
- 21.Wen W, Sachdev P, Shnier R, et al. Effect of white matter hyperintensities on cortical cerebral blood volume using perfusion MRI. NeuroImage. 2004;21:1350–1356. doi: 10.1016/j.neuroimage.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Tullberg M, Fletcher E, DeCarli C, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63:246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fazekas F, Schmidt R, Kleinert R, et al. The spectrum of age-associated brain abnormalities: their measurement and histopathological correlates. J Neural Transm Suppl. 1998;53:31–39. doi: 10.1007/978-3-7091-6467-9_4. [DOI] [PubMed] [Google Scholar]
- 24.Ozdener H. Molecular mechanisms of HIV-1 associated neurodegeneration. J Biosci. 2005;30:391–405. doi: 10.1007/BF02703676. [DOI] [PubMed] [Google Scholar]
- 25.Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Arch Neurol. 1987;44:21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- 26.Roman GC, Erkinjuntti T, Wallin A, et al. Subcortical ischemic vascular dementia. Lancet Neurol. 2002;1:426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 27.De Groot JC, De Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rótterdam Scan Study. Ann Neurol. 2000;47:145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- 28.Longstreth WT, Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 29.De Groot JC, De Leeuw FE, Oudkerk M, et al. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol. 2002;52:335–341. doi: 10.1002/ana.10294. [DOI] [PubMed] [Google Scholar]
- 30.Chui HC, Victoroff JI, Margolin D, et al. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 31.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 32.Hachinski VC, Lassen NA, Marshall J. Multi-infarct dementia. A cause of mental deterioration in the elderly. Lancet. 1974;2:207–210. doi: 10.1016/s0140-6736(74)91496-2. [DOI] [PubMed] [Google Scholar]
- 33.International statistical classification of diseases and related health problems, 1989 rev. Geneva: World Health Organization; 1992. pp. 311–388. [Google Scholar]
- 34.Harrison MJ, Newman SP, Hall-Craggs MA, et al. Evidence of CNS impairment in HIV infection: clinical, neuropsychological, EEG, and MRI/MRS study. J Neurol Neurosurg Psychiatr. 1998;65:301–307. doi: 10.1136/jnnp.65.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aylward EH, Brettschneider PD, McArthur JC, et al. Magnetic resonance imaging measurement of gray matter volume reductions in HIV dementia. Am J Psychiatr. 1995;152:987–994. doi: 10.1176/ajp.152.7.987. [DOI] [PubMed] [Google Scholar]
- 36.McArthur JC, Kumar AJ, Johnson DW, et al. Incidental white matter hyperintensities on magnetic resonance imaging in HIV-1 infection. J Acquir Immune Defic Syndr. 1990;3:252–9. [PubMed] [Google Scholar]
- 37.Levin HS, Williams DH, Borucki MJ, et al. Magnetic resonance imaging and neuropsychological findings in human immunodeficiency virus infection. J Acquir Immune Defic Syndr. 1990;3:757–762. [PubMed] [Google Scholar]
- 38.Dooneief G, Bello J, Todak G, et al. A prospective controlled study of magnetic resonance imaging of the brain in gay men and parenteral drug users with human immunodeficiency virus infection. Arch Neurol. 1992;49:38–43. doi: 10.1001/archneur.1992.00530250042014. [DOI] [PubMed] [Google Scholar]
- 39.Moore DJ, Masliah E, Rippeth JD, et al. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS. 2006;20:879–887. doi: 10.1097/01.aids.0000218552.69834.00. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y, Storey P, Cohen BA, et al. Diffusion alterations in corpus callosum of patients with HIV. AJNR. Am J Neuroradiol. 2006;27:656–60. [PMC free article] [PubMed] [Google Scholar]
- 41.Ragin AB, Wu Y, Storey P, et al. Diffusion tensor imaging of subcortical brain injury in patients infected with human immunodeficiency virus. J Neurovirol. 2005;11:292–298. doi: 10.1080/13550280590953799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cloak CC, Chang L, Ernst T. Increased frontal white matter diffusion is associated with glial metabolites and psychomotor slowing in HIV. J Neuroimmunol. 2004;157:147–152. doi: 10.1016/j.jneuroim.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 43.Ragin AB, Storey P, Cohen BA, et al. Disease burden in HIV-associated cognitive impairment: a study of whole-brain imaging measures. Neurology. 2004;63:2293–2297. doi: 10.1212/01.wnl.0000147477.44791.bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ragin AB, Storey P, Cohen BA, et al. Whole brain diffusion tensor imaging in HIV-associated cognitive impairment. AJNR Am J Neuroradiol. 2004;25:195–200. [PMC free article] [PubMed] [Google Scholar]
- 45.Thurnher MM, Schindler EG, Thurnher SA, et al. Highly Active Antiretroviral Therapy for Patients with AIDS Dementia Complex: Effect on MR Imaging Findings and Clinical Course. AJNR. 2000;21:670–678. [PMC free article] [PubMed] [Google Scholar]
- 46.Bartzokis G, Lu PH, Geschwind DH, et al. Apolipoprotein E genotype and age-related myelin breakdown in healthy individuals: implications for cognitive decline and dementia. Arch Gen Psychiatry. 2006;63:63–72. doi: 10.1001/archpsyc.63.1.63. [DOI] [PubMed] [Google Scholar]


