Abstract
This study used Ward’s minimum variance hierarchical cluster analysis to identify homogeneous subgroups of rheumatoid arthritis patients suffering from chronic pain who exhibited similar pain behavior patterns during a videotaped behavior sample. Ninety-two rheumatoid arthritis patients were divided into two samples. Six motor pain behaviors were examined: guarding, bracing, active rubbing, rigidity, grimacing, and sighing. The cluster analysis procedure identified four similar subgroups in Sample 1 and Sample 2. The first subgroup exhibited low levels of all pain behaviors. The second subgroup exhibited a high level of guarding and low levels of other pain behaviors. The third subgroup exhibited high levels of guarding and rigidity and low levels of other pain behaviors. The fourth subgroup exhibited high levels of guarding and active rubbing and low levels of other pain behaviors. Sample 1 contained a fifth subgroup that exhibited a high level of active rubbing and low levels of other pain measures. The results of this study suggest that there are homogeneous subgroups within rheumatoid arthritis patient populations who differ in the motor pain behaviors they exhibit.
Keywords: Pain behavior subgroups, observable pain behaviors, self-report pain levels, psychological distress, rheumatoid arthritis
Introduction
Pain behaviors include both verbal behaviors (e.g., crying, verbal descriptions of pain) and non-verbal behaviors (e.g., guarded movement, pain-related facial expressions) that serve to communicate pain to others (1). Pain behaviors are increasingly being viewed as important by pain theorists, researchers, and clinicians. The newly developed evolutionary theory of pain (2), for example, highlights the adaptive role pain behaviors play in survival of the human species since these behaviors can effectively signal distress and elicit help from others. The communication model of pain, likewise, emphasizes the value of pain behavior in fostering interpersonal communication about the perceived severity and impact of pain (3). Observational measures of pain behavior, long used in animal studies of pain, are now being more frequently incorporated into clinical research studies to supplement and provide comparisons to ratings of pain gathered using self-report (4). Improvements in pain behaviors (e.g., increases in activity level, decreases in pain medication intake) are also viewed as key indices of outcome in clinical settings (5,6).
Over the past decade, one of the most consistent findings emerging from studies of pain behavior in persons having persistent pain is the variability in observed in pain behavior. Put simply, some persons display high levels of pain behavior, while others display few or no pain behaviors. Although numerous studies have examined how these variations relate to biological, psychological, and social factors (4,7–9), few studies have addressed the possibility that these variations in pain behavior may be due to the fact that the samples of patients studied are quite heterogeneous and that, within these samples there may be subgroups of patients who are homogeneous with regard to the types of pain behavior they display. In fact, to our knowledge, only one study of chronic low back pain patients has used sophisticated multivariate cluster analytic methods to identify homogenous pain behavior subgroups (10).
Rheumatoid arthritis (RA) provides a particularly good model in which to study variations in pain behavior. RA is painful, debilitating, and is associated with a range of pain behaviors that can be coded in a reliable and valid fashion (11). Furthermore, there is evidence that patients with RA vary considerably in how they express pain behaviorally (12). These variations are not only important not only from a clinical perspective but also because they can make it difficult for family members, friends, and others to accurately identify and respond to the patient’s pain experience.
The present study represents a preliminary step in identifying pain behavior subgroups in persons having RA. The aims of the study were twofold: 1) to use cluster analysis to identify homogenous pain behavior subgroups among persons having RA; and, 2) to determine if the subgroups identified differed on measures of pain, self-efficacy, and psychological distress.
Methods
Participants
Participants for the study were 104 RA patients (81.5% women) recruited from the Rheumatology Clinics of Duke University Medical Center. All patients were participants in a treatment outcome study which examined the efficacy of psychosocial intervention for managing pain in rheumatoid arthritis patients. The findings reported in this paper are based on data collected at a pre-treatment evaluation that occurred prior to the onset of any treatment. Eighty percent of the patients in the study sample were White and 17% were African American. The mean age of the patients was 56.67 years (SD ± 13.0) with average disease duration of 13.34 years (SD ± 10.7).
Measures
Pain Behavior
Following the protocol previously established for recording pain behavior in persons having RA (12), a videotaped sample of pain behaviors was obtained from each participant. During the session, the patient was asked by a research assistant to: a) stand for both a one-minute and two-minute period; b) sit for both a one-minute and two-minute period; c) recline for two separate one-minute periods; and, d) walk for two separate one-minute periods. The total recording time was 10 minutes. The order of these activities was randomly varied across subjects to minimize order effects. No interaction with the patients occurred during these activities except when the research assistant directed the patient to change position.
The videotaped behavior sample was then viewed and coded by a trained observer. The observers noted the occurrence of six different pain behaviors in ongoing intervals containing 20 seconds of observation and 10 seconds of recording. These pain behaviors have been previously described and investigated in studies in both our lab and others (11,12). The definitions of the six motor pain behavior categories are as follows:
Guarding – abnormally stiff, interrupted, or rigid movement while changing positions.
Bracing – a stationary position in which a fully extended limb supports and maintains an abnormal distribution of weight for at least three seconds.
Active rubbing – Rubbing/massaging affected pain area for at least three seconds using either another body part or object (e.g., chair).
Grimacing – an obvious facial expression of pain which may include a furrowed brow, narrowed eyes, tightened lips, corners of mouth pulled back and clenched teeth.
Sighing – an obvious exaggerated exhalation of breath usually accompanied by shoulders first rising and then falling; cheeks may be expanded.
Rigidity – the affected body part held stationary in an unnaturally stiff or awkward position for at least three seconds.
A composite score, total pain behavior, was also calculated by summing the total occurrences of all six motor pain behaviors observed in one session. Percent agreement of 0.85 was found between observers on coding of pain behaviors.
Pain Ratings
Pain was assessed using the McGill Pain Questionnaire (MPQ) (13). The MPQ consists of a series of 20 sets of adjective pain descriptors. Patients were asked to check the one adjective in each category that best described their pain. The adjectives are grouped into three subscales, sensory, affective, and evaluative, that measure different aspects of the pain experience. Pain intensity was assessed using the Pain Rating MPQ subscale score. Previous research has provided strong support for the reliability and validity of the MPQ (14).
Arthritis Self-Efficacy Scale
The Arthritis Self-Efficacy Scale (ASES) (15) was used to assess patients’ beliefs in their ability to control arthritis pain, perform routine physical functions, and control other arthritis symptoms (e.g., fatigue and frustration). The ASES is a 20-item scale on which patients rate how confident they are that they can perform a particular task using a scale ranging from 10 (very uncertain) to 100 (very certain). The ASES has been found to have good validity, reliability and internal consistency (15) and has been used in a number of studies with rheumatoid arthritis patients (16–18).
Psychological Distress
The Symptom Checklist-90 Revised (SCL-90-R) (19) was used to measure psychological distress. Patients were asked to rate the extent to which they had been bothered by each symptom during the past week using a scale ranging form 0 (not at all) to 4 (extremely). The 90-item, self-report inventory measured overall psychological distress based on nine symptom dimensions: somatization, obsessive-compulsiveness, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, and psychoticism. The reliability and validity of the SCL-90-R has been widely established (19).
Analysis of Data
Patients were randomly divided into two samples (Sample 1 and Sample 2), each containing 52 subjects. Agglomerative hierarchical cluster analysis using Wards Minimum Variance method was performed on the observed pain behaviors of Sample 1 in an attempt to identify homogeneous subgroups. The Wards Minimum Variance method sums clusters together to form new clusters in a manner that minimizes the increase in the squared distance between variables (20). Although empirical studies have shown that Wards minimum variance method consistently works well in recovering structures from subject data (i.e., non-hypothetical data) with average linkage appearing to have near equal structure recovery (20), outliers have been shown to have a significantly detrimental effect on this method. Therefore, to reduce outlier distortion and to optimize the Ward clustering technique, 10 percent of both samples were trimmed (in accordance with the SAS/STAT User Guide (21)). This trimming procedure resulted in removing six patients for each of the study samples. The cubic clustering criterion, which measured the increase in the within-group sums of squares, was used as an aid in determining the optimal number of subgroups or clusters. Observing a sharp increase would suggest that accuracy had been lost by reducing the amount of clusters (22). The same clustering procedure was applied to the Sample 2 data. For purposes of describing the subgroups identified, low frequency behaviors were identified as occurring from zero to two times per session; moderate frequency behaviors as more than two and less than six times per session; and high frequency behaviors as occurring six or more times per session.
Results
Pain Behavior Subgroups
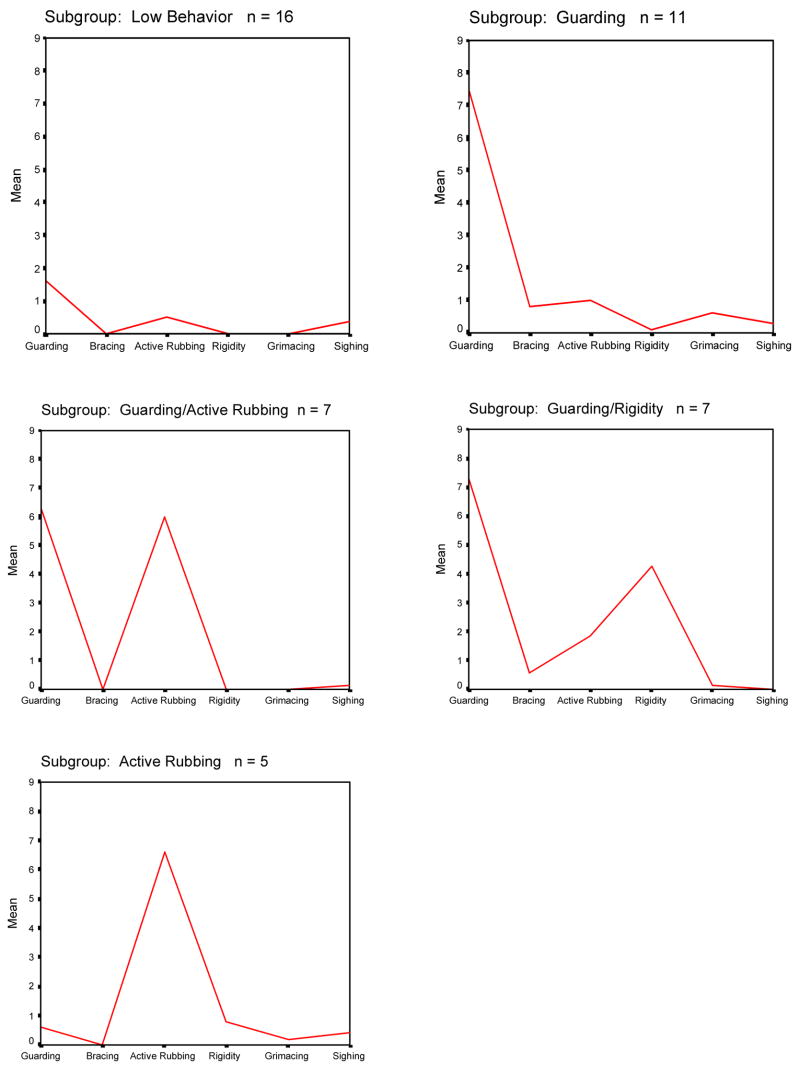
The cluster analysis procedure identified five pain behavior subgroups for patients in Sample 1 (Figure 1). These subgroups were: 1) a low pain behavior subgroup (n=16; 35% of sample) – patients in this subgroup displayed low levels of all pain behavior; 2) a high guarding subgroup (n=11; 24% of sample) – patients in this subgroup exhibited high levels of guarding but low levels of all other pain behaviors; 3) a high guarding/active rubbing subgroup (n=7; 15% of sample) – patients in this subgroup exhibited high levels of guarding and active rubbing and low levels of the other pain behaviors; 4) a high guarding/rigidity subgroup (n=7; 15% of sample) – patients in this subgroup exhibited high levels of guarding and rigidity and low levels of the other pain behaviors; and, 5) a high active rubbing subgroup (n=5; 11% of sample) –patients in this subgroup displayed high levels of active rubbing and low levels of the other pain behaviors.
Fig. 1.
Mean frequency of occurrence of pain behaviors for each of the five subgroups identified through cluster analysis of the pain behavior data of Sample 1.
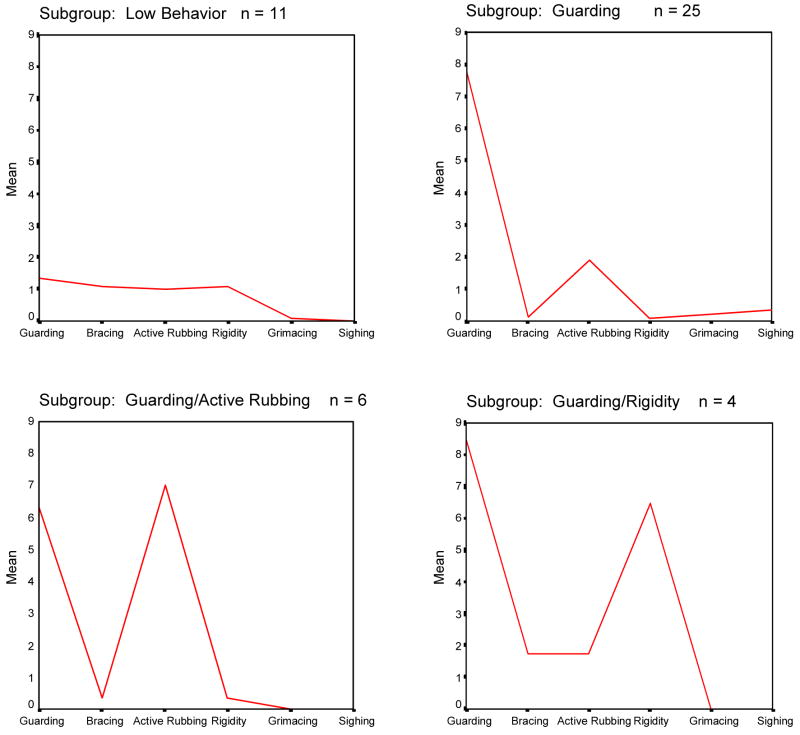
The cluster analysis procedure identified four pain behavior subgroups for patients in Sample 2 (Figure 2). These subgroups were: 1) a low pain behavior subgroup (n=11; 24% of sample) – patients in this subgroup displayed low levels of all pain behavior; 2) a high guarding subgroup (n=25; 54% of sample) – patients in this subgroup exhibited high levels of guarding but low levels of all other pain behaviors; 3) a high guarding/active rubbing subgroup (n=6; 13% of sample) – patients in this subgroup exhibited high levels of guarding and active rubbing and low levels of the other pain behaviors; 4) a high guarding/rigidity subgroup (n=4; 9 % of sample – patients in this subgroup exhibited high levels of guarding and rigidity and low levels of the other pain behaviors. The fifth subgroup identified in Sample 1 (high active rubbing) was not identified in Sample 2.
Fig. 2.
Mean frequency of occurrence of pain behaviors for each of the four subgroups identified through cluster analysis of the pain behavior data of Sample 2.
Demographic Characteristics of Pain Behavior Subgroups
Table 1 provides demographic information for patients in each of the subgroups identified in Sample 1 and Sample 2. A series of one-way analyses of variance (ANOVA) and Chi-squared tests of independence identified no statistically significant differences between the subgroups in age, disease duration, race, and sex.
Table 1.
Demographics of Pain Behavior Subgroups for Sample 1 and Sample 2
| Pain Subgroups
|
|||||
|---|---|---|---|---|---|
| Low Behavior | High Guarding | High Guarding/Active Rubbing | High Guarding/Rigidity | High Active Rubbing | |
| Sample 1 | |||||
| n = | 16 | 11 | 7 | 7 | 5 |
| Disease Duration | |||||
| Mean Years (SD) | 9.7(12.4) | 15.3(7.4) | 15.3(13.1) | 17.1(8.1) | 10.1(8.1) |
| Age | |||||
| Mean Years (SD) | 52.1(15.7) | 59.5(14.0) | 64.9(13.3) | 60.1(5.9) | 48.8(10.1) |
| Sex | |||||
| Female | 88% | 73% | 71% | 86% | 100% |
| Race | |||||
| White | 94% | 73% | 71% | 71% | 100% |
| African American | 6% | 27% | 14% | 29% | |
| Sample 2 | |||||
| n = | 11 | 25 | 6 | 4 | |
| Disease Duration | |||||
| Mean Years (SD) | 11.4(7.8) | 13.9(12.2) | 12.5(8.8) | 17.83.7(6.3) | |
| Age | |||||
| Mean Years (SD) | 51.4(12.9) | 56.8(13.6) | 61.0(15.9) | 54.8(13.3) | |
| Sex | |||||
| Female | 82% | 80% | 83% | 75% | |
| Race | |||||
| White | 82% | 72% | 83% | 100% | |
| African American | 9% | 28% | 17% | ||
SD = standard deviation.
Differences in Total Pain Behavior by Pain Behavior Subgroup
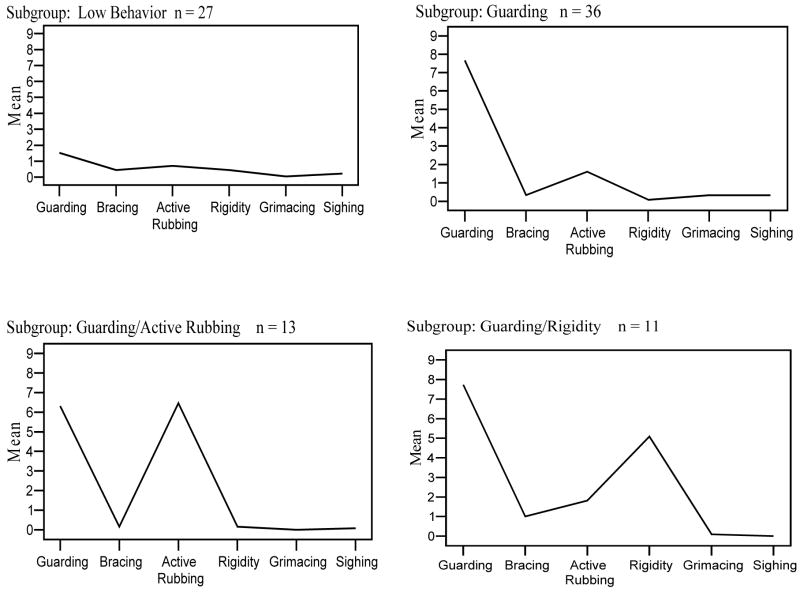
To examine differences in total pain behavior as a function of pain behavior subgroup membership, the patient data from the subgroups in Sample 1 and Sample 2 were pooled and the mean total pain behavior values for the combined subgroups were compared (see Figure 3). The results of a one-way ANOVA identified statistically significant subgroup differences in total pain behaviors displayed, F(4,90) = 33.01, P<0.001. An overall η2 = 0.16 indicated a large effect size (23). Post-hoc analyses using Bonferroni adjustment (0.05/5 = 0.01) indicated that patients in the high guarding/rigidity subgroup exhibited the highest total pain behavior, with approximately 16 pain behaviors observed per session. The total pain behavior level observed for the high guarding/rigidity group was significantly greater than that exhibited by the high guarding, high active rubbing, and low pain behavior subgroups. The total pain behavior level observed for the low pain behavior subgroup was significantly lower than that exhibited by all of the other pain behavior subgroups.
Fig. 3.
Mean frequency of occurrence of pain behaviors for each of the four subgroups of the combined Sample 1 and Sample 2 pain behavior data.
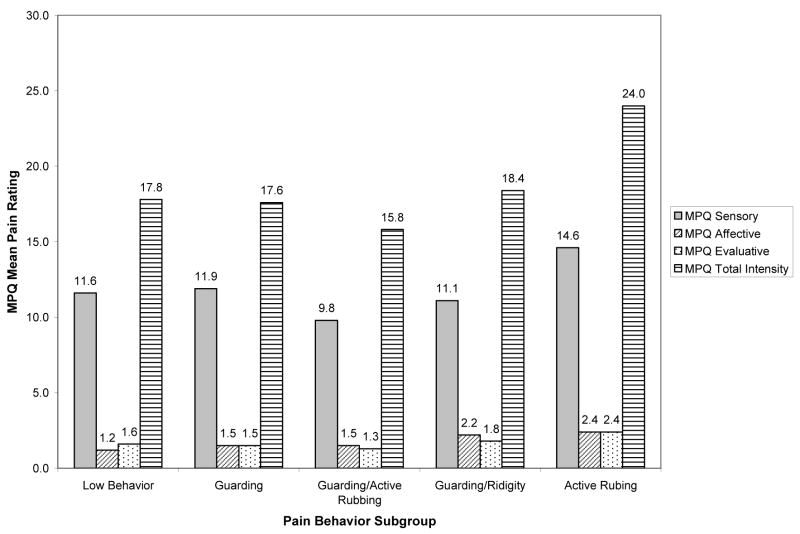
Differences in Total Pain Ratings by Pain Behavior Subgroup
Average self-report ratings of pain for the five pain behavior subgroups are presented in Figure 4. A one-way analysis of variance (ANOVA) found no statistically significant differences in the amount of reported pain among the five pain behavior subgroups, F(4, 87) = .565, ns, η2=.01. Moreover, one-way analyses of variance (ANOVA) found no statistically significant pain behavior group differences in the sensory, F(4, 87) = 0.555, η2=0.02; affective, F(4, 87) = 0.898, η2=0.02; or evaluative, F(4, 87) = 0.747, η2=0.01, pain subscales. It should be noted, however, that 1%–2% of the variance (viz., small effect sizes (23)) in overall pain scores and scores on the three pain subscales could be explained by differences in pain behavior subgroups.
Fig. 4.
Mean pain ratings for combined Sample 1 and Sample 2 pain behavior subgroups.
Differences in Arthritis Self-Efficacy by Pain Behavior Subgroups
Pain behavior subgroup differences in self-efficacy ratings were identified using one-way ANOVAs. Statistically significant differences in self-efficacy for physical function were identified, F(4,86) = 3.33, P = 0.014, η2 = 0.04. Post-hoc analyses using Bonferroni adjustment (0.05/5 = 0.01) indicated that patients in the low behavior pain behavior subgroup reported significantly higher ratings of self-efficacy for physical function than patients in the high guarding subgroup. No statistically significant differences were found among the subgroups on ratings of self-efficacy for managing arthritis pain, F(4,86) = 0.65, ns, η2 = 0.01, or self-efficacy for managing other arthritis symptoms, F(4,86) = 1.18, ns η2 = 0.03. Overall effect sizes were small (23).
Differences in Psychological Distress by Pain Behavior Subgroups
A one-way ANOVA was performed to examine differences in psychological distress as a function of pain behavior subgroup membership. The results of the analysis revealed statistically significant differences in the level of psychological distress reported by the five pain behavior subgroups, F(4,87) = 3.23, P<0.05, η2 = 0.06. Differences in pain behavior subgroups accounted for 6% of the variance in psychological distress scores, denoting a moderate effect size (23). Post-hoc analyses using Bonferroni adjustment (0.05/5 = 0.01) indicated that patients in the high active rubbing subgroup had higher ratings of psychological distress than patients in the low pain behavior and high guarding subgroups.
Discussion
Using hierarchical cluster analyses, we were able to identify five homogeneous pain behavior subgroups within a population of RA patients (viz., low behavior subgroup, high guarding subgroup, high guarding/active rubbing subgroup, high guarding/rigidity subgroup, and high active rubbing subgroup). Our findings support the results of previous research (10) in suggesting that patients in populations suffering from persistent pain may be classified into homogeneous subgroups based on differences in pain behavior patterns. These pain behavior pattern variations may require different approaches to assessment and treatment among the five pain behavior subgroups. For example, guarding and rigidity are associated with high levels of muscle tension (9), which may increase fatigue and decrease tolerance for daily activities. RA patients in this subgroup may benefit from psychophysiological interventions designed to reduce muscle tension (e.g., applied relaxation therapy) and physical therapy designed to stretch and strengthen muscles. Likewise, cognitive-behavioral interventions designed to reduce depression and anxiety (e.g., cognitive restructuring) (24) may prove beneficial for RA patients who exhibited high levels of active rubbing. For patients who exhibited high levels of both guarding and active rubbing, assessments might include measures of depression and anxiety, as well as using behavioral observation to identify physical activities most closely associated with guarding. Combining the information obtained from a variety of assessments may help clinicians identify which factors have the greatest influence on pain behaviors and determine which intervention(s) should be used.
One of the most interesting findings of this study was that, although the pain behavior subgroups in the study differ in the number of pain behaviors expressed, patients in these subgroups report experiencing similar levels of pain. This finding is consistent with predictions of operant behavioral theory (1), which maintains that non-verbal pain behaviors are not always consistent with verbal pain behaviors (i.e., verbal reports of pain). These findings also fit with the conclusions of a recent meta-analysis of studies reporting correlations between non-verbal pain behaviors and pain report (4). This report found that the pain behavior – pain report association can vary substantially with some studies reporting no association and others finding a highly significant association. One explanation for such variations is that the factors that control non-verbal pain behaviors and verbal pain reports may differ (4). An important implication of these findings is that verbal pain reports cannot be equated with non-verbal pain behaviors or vice versa. A thorough assessment of pain requires consideration of both non-verbal and verbal pain behaviors. A focus on pain behavior (or pain report) alone may lead to inadequate treatment. Patients who report pain yet display few pain behaviors may not receive adequate pain medication because their physicians are less likely to prescribe opioids for them (25). Conversely, in a hospital environment, where communications between patients and medical staff may be limited, adequate medication is often withheld from patients exhibiting what is considered to be excessive pain behaviors (26).
This study found some differences among the pain behavior subgroups based on self-efficacy. Specifically, patients in the subgroup that showed high levels of guarding reported significantly lower self-efficacy for controlling physical function than patients in the low pain behavior subgroup. This finding is important because research has shown that self-efficacy is one of the most important factors related to how patients adjust rheumatoid arthritis. Assessment efforts for patients in the high guarding group might focus on pinpointing specific functional tasks that elicit guarding and for which patients report low self-efficacy. Using applied relaxation methods, patients may learn to perform these tasks with much less guarding and, perhaps, enhance their self-efficacy for physical function.
This study contains several limitations. First, the small size of some of the pain behavior subgroups may have masked group differences on some of the variables of interest. However, even with low power, we were able to detect small-to-medium overall effect sizes for pain behavior subgroup differences on overall pain and subscale measures of pain and self-efficacy for pain management and management of other symptoms. Additional studies with larger samples are needed to determine: 1) if pain behavior subgroup sizes remain proportionally the same within a larger sample, and 2) if our results can be replicated.
Another limitation to the present study is the use of patient volunteers participating in a clinical trial within a large medical center, which raises the issue of generalizability. Patients who do not volunteer for research participation or volunteer in other locations may demonstrate pain behaviors differently than the patients in our study. To help other researchers determine the conveyance of our results to their particular setting, we have provided extensive patient demographic information and described our method for identifying and classifying pain behaviors in considerable detail.
Overall, the findings of this study suggest that there are distinct and replicable subgroups of rheumatoid arthritis patients who differ in the motor behaviors they display. Additional research is needed to replicate these findings. Future studies also need to examine other potential psychological correlates (e.g., pain coping, pain-related anxiety, mood, depression, and personality factors) and social correlates (marital satisfaction, spousal responses to pain behavior, work environment) of pain behavior subgroups.
Acknowledgments
This study was supported by a grant to Dr. Keefe from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grant #: AR 42261).
The authors would like to thank an anonymous reviewer for his/her suggestions, which were incorporated into the final paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fordyce WE. Behavioral methods for chronic pain and illness. St. Louis: Mosby; 1976. [Google Scholar]
- 2.Williams AC, de C. Facial expression of pain: an evolutionary account. Behav Brain Sci. 2002;25:439–488. doi: 10.1017/s0140525x02000080. [DOI] [PubMed] [Google Scholar]
- 3.Hadjistavropolous T, Craig KD. A theoretical framework for understanding self-report and observational measures of pain: a communications model. Behav Res Ther. 2002;40:551–570. doi: 10.1016/s0005-7967(01)00072-9. [DOI] [PubMed] [Google Scholar]
- 4.Labus JS, Keefe FJ, Jensen MP. Self-reports of pain intensity and direct observations of pain behavior: when are they correlated? Pain. 2003;102:109–124. doi: 10.1016/s0304-3959(02)00354-8. [DOI] [PubMed] [Google Scholar]
- 5.Hildebrandt J, Pfingsten M, Saur P, Jansen J. Predictions of success from a multidisciplinary treatment program for chronic low back pain. Spine. 1997;22:990–1001. doi: 10.1097/00007632-199705010-00011. [DOI] [PubMed] [Google Scholar]
- 6.Keefe FJ, Block AR, Williams RB, Jr, Surwit RS. Behavioral treatment of chronic low back pain: clinical outcome and individual differences in pain relief. Pain. 1981;11:221–231. doi: 10.1016/0304-3959(81)90007-5. [DOI] [PubMed] [Google Scholar]
- 7.Anderson KO, Bradley LA, Turner RA, et al. Observation of pain behavior in rheumatoid arthritis patients during physical examination. Relationship to disease activity and psychological variables. Arthritis Care Res. 1992;5:49–56. doi: 10.1002/art.1790050111. [DOI] [PubMed] [Google Scholar]
- 8.Gil KM, Keefe FJ, Crisson JE, Van Dalfsen PJ. Social support and pain behavior. Pain. 1987;29:209–221. doi: 10.1016/0304-3959(87)91037-2. [DOI] [PubMed] [Google Scholar]
- 9.Keefe FJ, Smith S. The assessment of pain behavior: Implications for applied psychophysiology and future research directions. Appl Psychophysiol Biofeedback. 2002;27:117–127. doi: 10.1023/a:1016240126437. [DOI] [PubMed] [Google Scholar]
- 10.Keefe FJ, Bradley LA, Crisson JE. Behavioral assessment of low back pain: identification of pain behavior subgroups. Pain. 1990;40:153–160. doi: 10.1016/0304-3959(90)90066-M. [DOI] [PubMed] [Google Scholar]
- 11.McDaniel LK, Anderson KO, Bradley LA, et al. Development of an observation method for assessing pain behavior in rheumatoid arthritis patients. Pain. 1986;24:165–184. doi: 10.1016/0304-3959(86)90039-4. [DOI] [PubMed] [Google Scholar]
- 12.Anderson KO, Bradley LA, McDaniel LK, et al. The assessment of pain in rheumatoid arthritis: Validity of a behavioral observation method. Arthritis Rheum. 1987;30:36–43. doi: 10.1002/art.1780300105. [DOI] [PubMed] [Google Scholar]
- 13.Melzack R. The McGill pain questionnaire: major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 14.Melzack R, Katz J. The McGill pain questionnaire: appraisal and current status. In: Turk DC, Melzack R, editors. Handbook of pain assessment. New York: Guilford; 1992. [Google Scholar]
- 15.Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989;32:37–44. doi: 10.1002/anr.1780320107. [DOI] [PubMed] [Google Scholar]
- 16.Brekke M, Hjortdahl P, Kvien TK. Changes in self-efficacy and health status over 5 years: a longitudinal observational study of 306 patients with rheumatoid arthritis. Arthritis Care Res. 2003;49:342–248. doi: 10.1002/art.11112. [DOI] [PubMed] [Google Scholar]
- 17.Keefe FJ, Kashikar-Zuck S, Robinson E, et al. Pain coping strategies that predict patients’ and spouses’ ratings of patients’ self-efficacy. Pain. 1997;73:191–199. doi: 10.1016/S0304-3959(97)00109-7. [DOI] [PubMed] [Google Scholar]
- 18.Lefebvre JC, Keefe FJ, Affleck G, et al. The relationship of arthritis self-efficacy to daily pain, daily mood, and daily pain coping in rheumatoid arthritis patients. Pain. 1999;80:425–435. doi: 10.1016/s0304-3959(98)00242-5. [DOI] [PubMed] [Google Scholar]
- 19.Derogatis LR. SCL-90-R: Administration, scoring, and procedures manual. Minneapolis, MN: National Computer Systems; 1994. [Google Scholar]
- 20.Everitt B, Landau S, Leese M. Cluster analysis. New York: Oxford University Press; 2001. [Google Scholar]
- 21.SAS/STAT User Guide. [Last accessed June 18, 2004]; Available at http://www.id.unizh.ch/software/unix/statmath/sas/sasdoc/stat/chap23/sect4.htm.
- 22.Lorr M. Cluster analysis for social scientists. San Francisco: Jossey-Bass; 1983. [Google Scholar]
- 23.Clark-Carter D. Doing quantitative psychological research: From design to report. East Sussex, UK: Psychology Press, Ltd; 1997. [Google Scholar]
- 24.Waters SJ, Campbell LC, Keefe FJ. Cognitive-behavioral therapy. In: St Clair EW, Pisetsky DS, Haynes BF, editors. Rheumatoid arthritis. Philadelphia: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 25.Turk DC, Okifuji A. What factors affect physicians’ decisions to prescribe opioids for chronic noncancer pain patients? Clin J Pain. 1997;13:330–336. doi: 10.1097/00002508-199712000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Drayer RA, Henderson J, Reidenberg M. Barriers to better pain control in hospitalized patients. J Pain Symptom Manage. 1999;17:434–440. doi: 10.1016/s0885-3924(99)00022-6. [DOI] [PubMed] [Google Scholar]