Abstract
We analyzed the mechanism of action for perifosine (D-21266) a new synthetic alkylphospholipid Akt inhibitor using LNCaP and PC-3 prostate cancer cells. Perifosine treatment of PC-3 cells resulted in cytostatic and cytotoxic effects. Cytostatic effects were characterized by cell growth arrest, cell cycle block and morphological changes, such as a cell enlargement and granulation, hallmarks of differentiating PC-3 cells. Specific differentiation markers including prostasomal, secretory and plasma membrane proteins, and keratins were induced by perifosine. Among them, we detected strong induction and secretion of CEACAM5 protein. In contrast, perifosine strongly reduced caveolin-1 RNA levels. Cytotoxic effects included para-apoptosis, apoptosis, and necrosis. To pursue the mechanisms responsible for these activities we focused on signaling pathways that lie downstream of Akt. Perifosine-triggered GSK-3β activation in PC-3 and LNCaP cells resulted in the expression of GSK-3β-related differentiation markers. This expression was reduced in the presence of specific siRNA for GSK-3β or for its target CREB protein. The use of the GSK-3β inhibitor lithium chloride indicated that GSK-3β partially protects prostate cancer cells from the cytotoxic effects of perifosine. Together, these findings indicate that perifosine induces GSK-3β-related differentiation and caspase-independent cell death in prostate cancer PC-3 cells. In addition our results identify specific biomarkers for perifosine therapy.
Keywords: perifosine, prostate cancer, PC-3, LNCaP
1. Introduction
Prostate cancer remains the most commonly diagnosed malignancy in men and second only to lung cancer as a leading cause of tumor deaths in males [1]. Currently there is no cure for locally advanced or metastatic prostate cancer. Prostate cancer progression to an androgen-insensitive state is associated with the development of resistance to cell death. Therefore, it is essential to understand the molecular pathways responsible for the survival of androgen-independent prostate cancer cells. This understanding will enable development of effective, targeted therapies for this disease.
Recent studies indicate that phosphatidylinositol 3-kinase (PI3K)-Akt pathway plays an important role in regulating cell proliferation and cell survival in many malignances including prostate cancer [2]. The kinase activity of Akt is stimulated by a variety of extracellular stimuli, such as growth factors, cytokines, chemokines, integrin engagement and T-cell receptor. We previously demonstrated that caveolin-1 overexpression leads to stimulation of Akt through the inhibition of serine/threonine protein phosphatase PP1 and PP2A [3]. The activation of Akt leads to the phosphorylation and regulation of a wide spectrum of its substrates involved in multiple cellular processes such as cell survival, cell growth, cell differentiation, cell cycle progression, cell proliferation and cellular metabolism [4].
Phosphatase and tensin homologue deleted on chromosome 10 (PTEN) is an important negative regulator of Akt activation as it antagonizes the actions of PI3K. However, PTEN is mutated or deleted in a substantial number of cancers including prostate, breast, ovarian cancers, and glioblastomas. Cells with PTEN mutation or deletion often exhibit a constitutive PI3K activity [2].
Perifosine (D-21266), a new synthetic alkylphospholipid, was shown to be a potent Akt inhibitor in prostate cancer PC-3 cells [5] and activator of the stress-activated protein kinase/c-jun-NH2-kinase (JNK) pathways in human leukemia cells [6].
GSK-3β is a characterized physiological substrate of Akt [7]. GSK-3β is an evolutionary conserved, ubiquitous serine/threonine kinase whose activity is inhibited by Akt phosphorylation on Ser9 in response to growth factor stimulation. There is evidence of the diverse functions of GSK-3β including regulation of cellular activity, structure, and survival [8]. GSK-3β is a critical negative regulator of both PI3K and Wnt cell signaling [9,10]. The ability of GSK-3β to phosphorylate the regulatory domain of β-catenin, a ligand-dependent co-activator of androgen receptor, places it in the category of a tumor suppressor gene. Activated GSK-3β was also shown to phopshorylate CREB on Ser129 in prostate cancer PC-3 cells [11]. Phoshorylated CREB on Ser129 and Ser133 was also found in the nuclei of low grade (well differentiated) prostate cancer but decreased in high grade (poorly differentiated) prostate cancer.
In the present study, we demonstrated that perifosine treatment of PC-3 cells resulted in the induction of differentiation which was characterized by morphologic changes, and induction of genes coding for prostasomal, secretory proteins, and keratins among others. We demonstrated that perifosine-induced Akt inhibition results in the activation of GSK-3β and its translocation to the nucleus of differentiating cells. We demonstrated that perifosine activates GSK-3β/CREB pathway in LNCaP cells. We also demonstrated that GSK-3β protects differentiated cells from cell death and contributes to the expression of multiple differentiation markers.
2. Material and Methods
2.1. Cell cultures and chemicals
PC-3 cells were obtained from ATCC (American Type Culture Collection, Manassas, VA). Cells were cultured in 5% CO2 at 37°C in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Logan, UT), 2 mM L-glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin (Invitrogen). Perifosine (AOI Pharmaceuticals, Memphis, TN) was dissolved in PBS to prepare a fresh 10 µM stock solution. To determine cell numbers, PC-3 cells were plated in 60 mm plates at 3 × 105 per plate or in 100 mm plates at 1–2 × 106 per plate a day prior treatment. Attached cells were harvested with trypsin and counted using a Beckman Coulter cell counter (Fullerton, CA). To obtain photographs of control and treated cells, cells were washed three times with PBS and stained with a Giemsa solution (Sigma, St. Louis, MO) for 1 minute. Images of stained cells were captured with a conventional Zeiss inverted microscope using transmitted light and phase contrast imaging. Cell viability was measured using an MTS assay according to the manufacturer’s protocol (Promega, Madison, WI) or by means of flow cytometry using calcein-AM (Sigma) staining. For calcein-AM staining, concentration of harvested cells was adjusted to 1 × 105 cells/ml. Cells at 1 × 105 were incubated with 100 nM calcein-AM for 15 minutes and analyzed by flow cytometry using Beckman Coulter Epics XL (Beckman). Usually 10,000 cells were analyzed in the appropriate channel. Percentages of viable control cells were considered to be 100% for re-calculation of viability of treated cells. Cell cycle analysis was performed as described previously [12]. Annexin V/propidium iodide (Sigma) staining was performed as described previously [13] and analyzed by flow cytometry.
2.2. Real-time PCR
Real-time PCR and ΔCt calculation was performed as previously described [14]. To test for the expression levels of studied genes, we used primers from the PrimerBank database [15]: KRT15 (24430190a1), KRT20 (27894337a3), KRT6isr4 (28173552a1), S100P (5174663a1), DUSP1 (4758204a1), MIG6 (10047086a1), NDRG1 (5174657a1); other primers were used as previously described [14] and synthesized by MWG-Biotech (High Point, NC). Reactions were monitored by real time PCR system ABI Prism 700 Sequence Detection System (Applied Biosystems, Foster City, CA) and relative changes in gene expression were calculated. Data were normalized to the expression levels of RPS25. The melting curve analysis was performed for each pair of primers.
2.3. Western Blot Analysis
Western blot analysis for performed as described previously [14] using following primary antibodies: cyclin B1 (1:200, Santa Cruz Biotechnology, Santa Cruz, CA), CEACAM5 (1:500, NeoMarkers, Fremont, CA), MIG6 (1:200, Santa Cruz Biotechnology), NDRG1 (1:200, Santa Cruz), p21Cip1 (1:500, Santa Cruz), GAPDH (1:1,000, Santa Cruz), caspase-3 p19/17 (1:500, Cell Signaling, Danvers, MA), caspase-9 p37 (1:500, Cell Signaling), and PARP p85 (1:500, Cell Signaling). Usually 30 µg of proteins was loaded per lane. After the membrane was washed three times with M/P/T at room temperature, it was incubated with appropriate secondary antibody (goat anti-mouse or anti-rabbit, 1:1,000, Pierce, Rockford, IL) or donkey anti-goat (1:5,000; Santa Cruz) labeled with horseradish peroxidase for 1 h at room temperature. Chemiluminescence was obtained by SuperSignal West Dura Extented Duration Substrate or West Femto kits (Pierce, Rockford, IL).
2.4. Isolation of cytosolic and nuclear fractions and western blot analysis
Nuclei from mock- or perifosine-treated PC-3 cells were prepared as previously described [14] to detect levels of P-GSK-3β(Ser9), GSK-3β, and Histone H3. Cytosolic fractions were collected to analyze levels of P-Akt(Ser473), P-GSK-3β(Ser9), GSK-3β, and tubulin. Western blot analysis was performed as described above using following antibodies: P-Akt(Ser473) (1:500, Cell Signaling), P-GSK-3β(Ser9) (1:500, Cell Signaling), and GSK-3β (1:500, Cell Signaling). Antibodies for tubulin (1:2,000, Sigma) or Histone H3 (1:200, Santa Cruz) were used to monitor equal protein loading. The secondary antibody (goat anti-mouse or goat anti-rabbit labeled with horse radish peroxidase, Pierce, Rockford, IL) was used at dilution 1:1,000 for 1 h at room temperature. Membranes were developed as described above.
2.5. Transfection of siRNA oligonucleotides
Transfection of siRNA oligonucleotides was performed as described previously [14]. Here we used 50 nM of siRNA for GSK-3β (Cell Signaling), and CREB (Santa Cruz). Two days after transfection, cells were treated with 5 µM perifosine for indicated time periods. At the end of treatment, cells were harvested, washed in cold PBS, and resuspended in 900 µl of PBS. Cells in 300 µl of PBS were used for Western blotting as described above and the rest of the sample was used for RNA isolation in order to perform a real-time PCR assay as described above. Western blots were analyzed using the Gel Expert software from NucleoTech (San Francisco, CA). Reduction in the gene expression was calculated according following formula: reduction (%)=100-(CtRNAi/perifosine/CtNSi/perifosine)*100, RNAi=GSKi or CREBi.
3. Results
3.1. Perifosine induces cell growth inhibition, cell cycle arrest, and morphological changes in PC-3 cells
Biological activity of perifosine, a novel alkylphospholipid, was initially studied in prostate cancer PC-3 cells [5]. Perifosine was shown to strongly inhibit Akt and induce cell growth arrest and cell death. To extend these studies, we initially confirmed cytostatic/cytotoxic activities of perifosine in PC-3 cells.
Treatment of PC-3 cells with increasing concentration of perifosine (1–20 µM) for 2 and 4 days resulted in cell growth inhibition, which was dose dependent (Fig. 1A). We used an MTS assay to test the viability of attached cells treated with increasing concentrations of perifosine (1–20 µM) for 2 and 4 days. Cell viability decreased with increasing perifosine concentrations. Perifosine treatment of PC-3 cells for 2 and 4 days resulted in 45% and 35% cell survival, respectively, while treatment with 20 µM perifosine for 2 and 4 days resulted in 28% and 20% cell survival, respectively (Fig. 1A). Based on our survival data and reported plasma levels of perifosine during clinical trials, which were 5–25 µM depending on a dosing schedule [16,17], we chose to use perifosine at 5 µM concentration for subsequent studies.
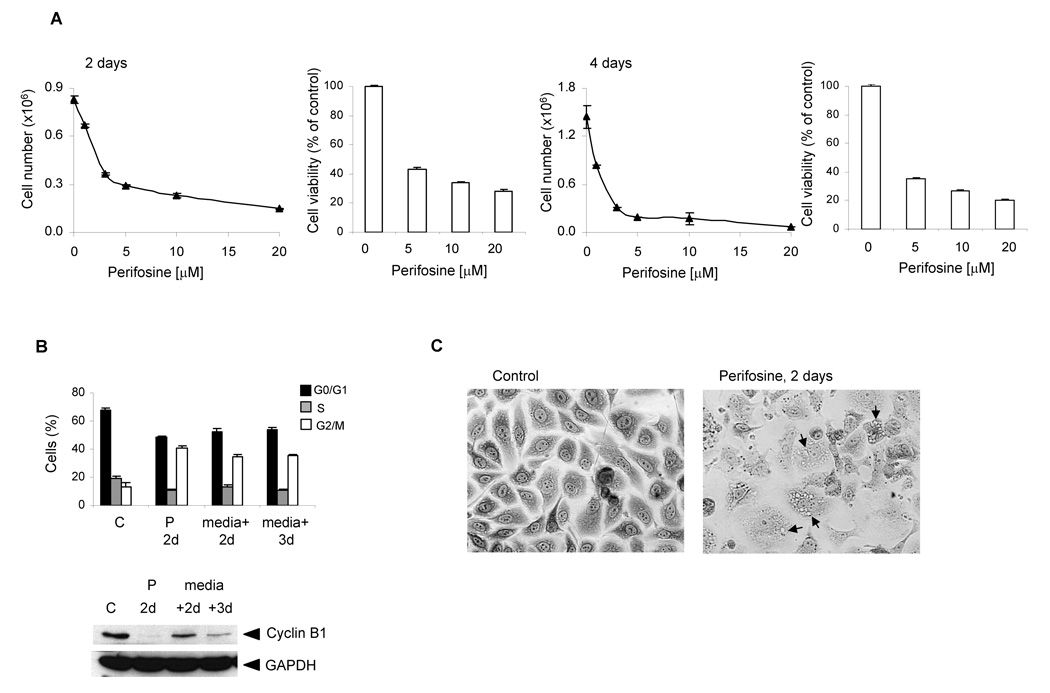
Figure 1. Perifosine-induced cell growth arrest and reduced viability (A), cell cycle block (B) and morphological changes (C).
A, A day after cells were seeded at 3 × 105 per well in 6-well plate in triplicates, they were incubated for 2 and 4 days with 1, 3, 5, 10 and 20 µM perifosine. To analyze cell viability, cells were seeded at 5 × 104 per well in 12-well plate in triplicates. Cell viability was measured by MTS proliferation assay as described in Material and Methods at indicated times. Bars, SD. B, For the cell cycle analysis, cells were seeded at 1 × 106 per 10 cm plate in triplicates and incubated with 5 µM perifosine for 2 days. Treated cells were further incubated for 2 and 3 days without the presence of perifosine. Columns, percentage of cells; bars, SD. The protein levels of cyclin B1 were analyzed by western blotting as described in Material and Methods. The experiments were repeated three times and typical results are presented. P 2d, perifosine treatment for 2 days; (medium + 2d), cells without perifosine for 2 days; (medium + 3d), cells without perifosine for 3 days. C, A day after seeding PC-3 cells at 3 × 105 per well in 6-well, they were treated with 5 µM perifosine for 2 days. At the end of treatment, cells stained with Giemsa solution and photographed (x10 magnification). Arrows indicate cytoplasmic vacuoles.
Cell cycle analysis revealed that perifosine-treatment resulted in the accumulation of treated cells in the G2/M phase (Fig. 1B). Proliferating control PC-3 cells yielded 67% cells in the G0/G1 phase, 18% cells in the S phase, and 15% cells in G2/M phase of cell cycle two days after plating. Treatment of PC-3 cells with 5 µM perifosine for 2 days increased the percentage of cells in the G2/M phase to 40% and reduced the percentage of the cells in the S-and G0/G1-phase. The subdiploid population of cells accounted for about 2%. We next determined whether the cell cycle arrest induced by perifosine was reversible. PC-3 cells were cultured in medium containing 5 µM perifosine for 2 days. Then the cells were washed twice with PBS, replenished with the fresh medium without perifosine, and recultured for additional 2 and 3 days. Results of DNA cell cycle analysis are shown in Fig. 1B. The percentages of cells in the G2/M phase remain relatively unchanged after the perifosine withdraw suggesting that cell cycle arrest is not reversible within 3 days.
We also investigated whether perifosine induces G2/M arrest through alterations in cyclin B1 protein levels. Western blot analysis was performed in the cell extracts prepared from perifosine-treated PC-3 cells. Our results showed that perifosine treatment for 2 days significantly reduced the levels cyclin B1. Withdraw of perifosine resulted in the expression of cyclin B1, although the levels were reduced compare to control cells (Fig. 1B). Interestingly, perifosine also induced morphological changes in PC-3 cells, such as cell enlargement and production of cytoplasmic vacuoles after 2 days (Fig. 1C, right panel).
3.2. Perifosine induces differentiation markers in PC-3 cells
Cell growth inhibition, morphological changes and cell cycle block are traits of differentiating PC-3 cells [12,14]. Floryk et al [14] used a microarray approach to analyze gene expression in differentiating PC-3 cells after treatment with inosine 5’-monophosphate dehydrogenase and histone deacetylase inhbitors mycophenolic acid and tributyrin, respectively. We suggested that these genes may be used to test whether a compound of interest could induce differentiation in prostate cancer PC-3 cells. To verify this hypothesis, we treated PC-3 cells with 5 µM perifosine for 72 hours and measured RNA levels for the genes listed in Table 1 by real-time PCR. These genes code for prostasomal, plasma and secretory proteins, keratins, and other proteins. We detected increased expression of CD46, CD55, and zinc-α-2-glycoprotein (AZGP) (prostasomal proteins), carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5), TNF ligand superfamily, member 9 (TNFSF9), integrin β4 (ITGB4), Cyr61, perlecan (HSPG2), galectin 3 (LGALS3), galanin (GAL), tryptase-ε/serine protease 22 (PRSS22), and growth differentiation factor 15 (GDF15) (plasma membrane and secretory proteins); keratins 20, 15, 19, 4 and 6irs4 (KRT20, KRT15, KRT19, KRT4, KRT6irs4, keratins); calcium binding protein S100 P (S100P), p21Cip1 (p21, cell cycle), dual specificity phosphatase 1 (DUSP1, signal transduction), and cyclooxygenase-2 (COX-2, inflammation, apoptosis), mitogen-induced gene 6 (MIG6/ERRFI1, response to stress, differentiation), and N-myc downstream regulated gene (NDRG1, differentiation). Interestingly, perifofine dramatically reduced CAV1 levels after 3 days of treatment (Table 1).
Table 1.
Changes in the expression of genes coding for differentiation markers in PC-3 cells treated with 5 µM perifosine for 72 hours
| Gene | 72h |
|---|---|
| CD55 | 4.9 ± 0.2 |
| CD46 | 3.8 ± 0.5 |
| AZGP | 2.5 ± 0.2 |
| CEACAM5 | 23.8 ± 7.4 |
| TNFSF9 | 6.1 ± 0.1 |
| RPSS22 | 4.4 ± 0.1 |
| GDF15 | 2.6 ± 0.4 |
| Cyr61 | 3.0 ± 1.0 |
| GAL | 2.4 ± 0.1 |
| S100P | 9.1 ± 4.6 |
| ITGB4 | 4.0 ± 1.7 |
| LGALS3 | 2.7 ± 0.8 |
| HSPG2 | 2.5 ± 0.7 |
| KRT15 | 8.2 ± 3.7 |
| KRT19 | 2.1 ± 0.1 |
| KRT4 | 2.8 ± 2.1 |
| KRT20 | 7.0 ± 3.5 |
| KRT6irs4 | 2.7 ± 1.4 |
| p21 | 16.2 ± 2.0 |
| DUSP1 | 2.5 ± 0.8 |
| MIG6 | 2.7 ± 0.2 |
| COX-2 | 3.1 ± 1.3 |
| NDRG1 | 3.3 ± 0.1 |
| CAV1 | −22.5 ± 3.5 |
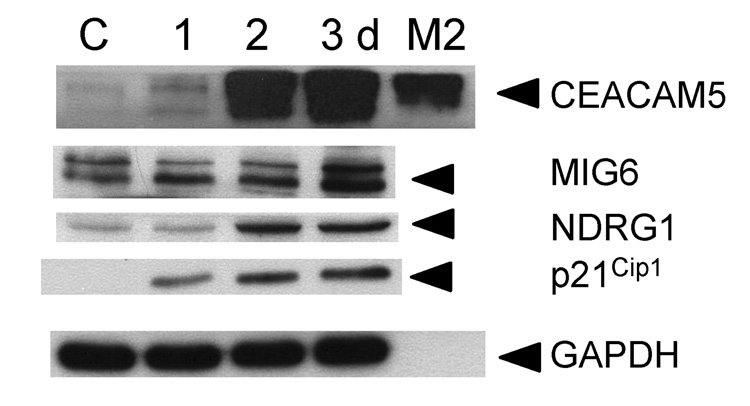
We used western blotting to confirm increased expression of CEACAM5, MIG6, NDRG1, and p21Cip1 proteins in perifosine-treated PC-3 cells (Fig. 2). Interestingly, we also detected CEACAM5 protein in the culture media suggesting that perifosine may induce the expression of secretory protein(s) which may be used as potential biomarker(s) to monitor perifosine activities in prostate cancer cells using serum samples from patients undergoing perifosine treatment.
Figure 2. Expression of selected differentiation markers in perifosine treated PC-3 cells.
PC-3 cells were treated with 5 µM perifosine for indicated periods of time. Attached cells were harvested, washed in PBS, and lysed as described in Material and Methods. Conditioned media (M2) was collected and concentrated to analyze secretion of CEACAM5 two days after perifosine treatment. Total proteins at 30 µg were separated in a 10% polyacrylamide gel and transferred to a PVDF membrane. Membranes were incubated with an anti-CEACAM5, MIG6, NDRG1, and p21Cip1 antibodies. Appropriate secondary antibodies labeled with horse peroxidase were used. GAPDH was used to monitor equal protein loading. Membranes were developed as described in Materials and Methods. Experiments were repeated three times and representative blots are presented.
3.3. Perifosine induces GSK-3β nuclear translocation and GSK-3β-dependent expression of differentiation markers in PC-3 cells
It was recently reported that overexpression of the GSK-3β active form in PC-3 cells resulted in cell growth inhibition and phosphorylation of pre-phoshorylated CREB(Ser133) protein at Ser129 which resulted in increased CRE activity [11]. We hypothesized that perifosine-induced inhibition of Akt and subsequent activation of GSK-3β would result in GSK-3β-dependent expression of differentiation markers.
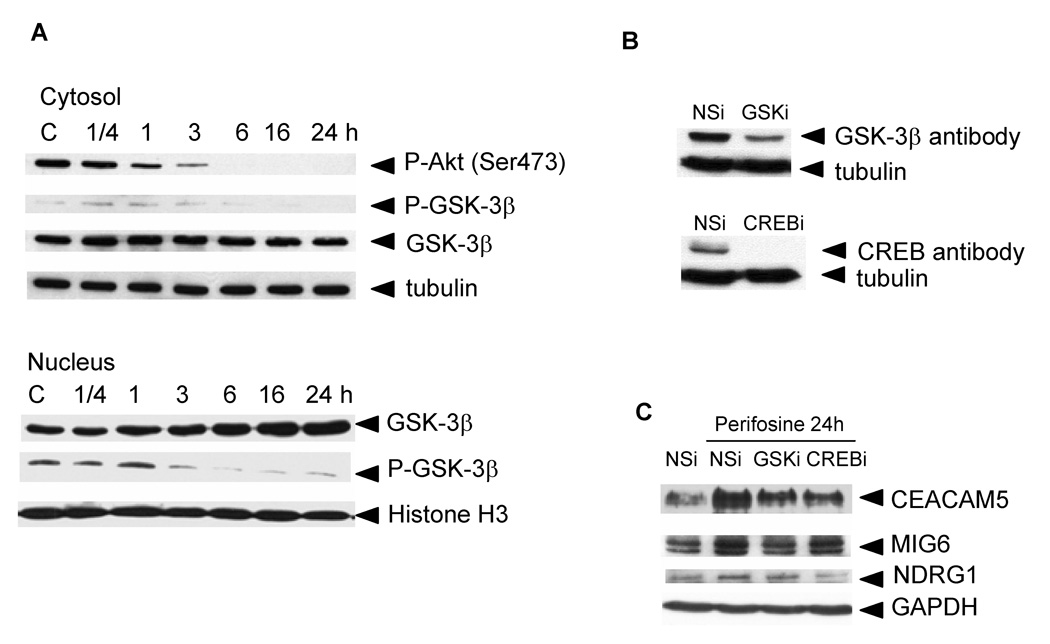
We prepared cytosolic and nuclear extracts from control PC-3 cells and PC-3 cells treated with 5 µM perifosine for 0.25–24 hours to monitor the expression levels of total GSK-3β and phosphorylated GSK-3β on Ser9 in treated cells. Our results confirmed that perifosine-induced inhibition of Akt resulted in GSK-3β activation as demonstrated by decreasing levels of phosphorylated GSK-3β in the cytosol of treated PC-3 cells (Fig. 3A). Phosphorylated form of GSK-β (Ser9) in the cytosol diminished within 24 h of treatment. We also detected increased levels of total GSK-3β in the nuclei of treated PC-3 cells after 16 h of treatment. Phosphorylated GSK-3β levels are reduced 3 hours after perifosine treatment (Fig. 3A). These results suggest that perifosine induces translocation of active GSK-3β to the nuclei of PC-3 cells.
Figure 3. Perifosine-induced Akt inhibition, GSK-3β activation and translocation to the nuclei (A), GSK-3β and CREB protein levels in siRNA-transfected PC-3 cells (B), and GSK-3β and CREB-dependent proteins expression (C).
A, PC-3 cells were treated for indicated periods of time with 5 µM perifosine. Cytosolic and nuclear extracts were prepared as described in Material and Methods. Cytosolic and nuclear proteins were loaded at 5 µg per lane. Western blotting was performed as described in Material and Methods. Membranes were incubated with an anti-P-Akt (Ser473), P-GSK-3β (Ser9), and GSK-3β antibodies. Appropriate secondary antibodies labeled with horse peroxidase were used. Tubulin was used to monitor equal loading of cytosolic proteins and Histone H3 for nuclear proteins. Membranes were developed as described in Materials and Methods. Experiments were repeated three times and representative blots are shown. B, PC-3 cells transfected with non-specific (NSi) or specific siRNA (GSKi, CREBi) duplexes were treated with 5 µM perifosine for 24 h as described in Material and Methods. Cell extracts were prepared to evaluate protein levels of GSK-3β and CREB proteins in transfected cells by western blotting as described in Material and Methods. Experiments were repeated three times with similar results. C, Cell extracts collected at B were used to monitor changes in protein levels of CEACAM5, NDRG1, MIG6, and p21Cip1. GAPDH was used to monitor equal protein loading. Experiments were repeated three times and typical results are presented.
We used GSK-3β siRNA (GSKi) to analyze the GSK-3β-dependent expression of differentiation markers. PC-3 cells were transfected with 50 µM non-specific siRNA or GSKi for 2 days and subsequently treated with 5 µM perifosine for 1 day. Harvested cells were used to perform western blotting to determine GSK-3β protein levels (Fig. 3B) and real-time PCR to analyze RNA levels of genes coding for differentiation markers (Table 2). The results indicated that GSK-3β levels were reduced by 70% in the presence of GSKi. Real-time PCR analysis revealed that reduction in the GSK-3β expression caused 50%–84% reduction in the expression of 12 out of 23 tested genes to be associated with differentiation of PC-3 cells when compared to perifosine-treated control cells.
Table 2.
Reduction in the gene expression in perifosine treated PC-3 and LNCaP cells with down-regulated GSK-3β or CREB proteins
| Gene | GSKi (perifosine) | CREBi (perifosine) | ||
|---|---|---|---|---|
| % reduction | % reduction | |||
| PC-3 | LNCaP | PC-3 | LNCaP | |
| AZGP | 70 ± 6 | 60 ± 1 | ||
| CEACAM5 | 50 ± 5 | 44 ± 1 | ||
| COX-2 | 65 ± 2 | |||
| GDF15 | 79 ± 2 | 51 ± 10 | 60 ± 8 | |
| ITGB4 | 58 ± 3 | |||
| KRT15 | 62 ± 2 | |||
| KRT19 | 52 ± 3 | |||
| KRT4 | 73 ± 5 | 82 ± 2 | 84 ± 2 | |
| KRT6irs4 | 80 ± 5 | 75 ± 1 | 70 ± 2 | |
| MIG6 | 70 ± 1 | |||
| NDRG1 | 51 ± 7 | |||
| S100P | 84 ± 1 | 57 ± 9 | 48 ± 3 | 60 ± 5 |
| TNFAIP2 | 54 ± 2 | |||
3.4. Perifosine induces expression of CREB target genes
We also tested whether activated GSK-3β resulted in the increased expression of CREB target genes. We used CREB siRNA (CREBi) to analyze the expression of studied differentiation markers. Cells were transfected with NSi and CREBi and treated with 5 µM perifosine for 24 hours 2 days after transfection. At the end of treatment, cells were harvested and used to determine CREB protein levels by western blotting (Fig. 3B) and RNA levels of genes coding for tested differentiation markers by real-time PCR (Table 2). Transfection of PC-3 cells with CREBi resulted in the suppression of CREB protein to undetectable levels. Real-time PCR analysis revealed that suppression of CREB protein resulted in 44%–84% reduction in the expression of CEACAM5, S100P, NDRG1, KRT6irs4, and KRT4 when compared to perifosine-treated control cells.
We used western blotting (Fig. 3C) to confirm real-time PCR results and demonstrated that the expression of CEACAM5, and MIG6 proteins was reduced in perifosine-treated PC-3 cells with reduced GSK-3β expression; the expression of CEACAM5, and NDRG1 was reduced in perifosine-treated PC-3 cells with eliminated CREB protein.
Taken together, our data demonstrates that perifosine-induced GSK-3β activity significantly contributes to the expression of differentiation markers in PC-3 cells.
3.5. Perifosine induces expression of GSK-3β and CREB-target genes in androgen-sensitive prostate cancer LNCaP cells
We used LNCaP cells to demonstrate that perifosine-induced GSK-3β/CREB pathway is activated in androgen-sensitive prostate cancer cells. Perifosine at 10 µM reduced viability of LNCaP cells by 26 ± 3% after 2 days as measured by the MTS assay suggesting that LNCaP cells are not as sensitive to perifosine as PC-3 cells. However, treatment of LNCaP with 10 µM perifosine for 1 day inhibited Akt and activated GSK-3β as assessed by western blotting (data not shown). Perifosine induced the expression of GDF15 (3x), KRT4 (4x), KRT6irs4 (2x) and S100P (5x) genes in LNCaP cells after 2 days as measured by real-time PCR. We subjected LNCaP cells to GSK-3β and CREBspecific siRNA. Perifosine treatment of LNCaP cells with reduced GSK-3β protein levels by 70% (data now shown) resulted in the down-regulation of GDF15, S100P, AZGP, KRT6irs4 and KRT4 by 57–82%. Transfection of LNCaP cells with CREBi reduced CREB protein levels by 80% (data not shown) and resulted in the down-regulation of GDF15 and S100P genes by 60% as measured by real-time PCR (Table 2). These data demonstrate that perifosine-induced GSK-3β activity leads to the expression of differentiation markers in androgen-sensitive cells.
3.6. Differentiating PC-3 cells express apoptotic markers
Our initial data suggests that perifosine has both cytostatic and cytotoxic effects in PC-3 cells. We used calcein-AM to assess percentages of viable cells in the culture after 5 µM perifosine treatment for 2 days. We collected both floating and attached cells and detected 40 to 45% of cells surviving perifosine treatment at this time point by means of flow cytometry. Analysis of cells stained with propidium iodide and annexin V revealed that approximately 5% of collected cells were double positive (necrosis/late apoptosis), and about 15% stained with annexin V (apoptosis) (data not shown).
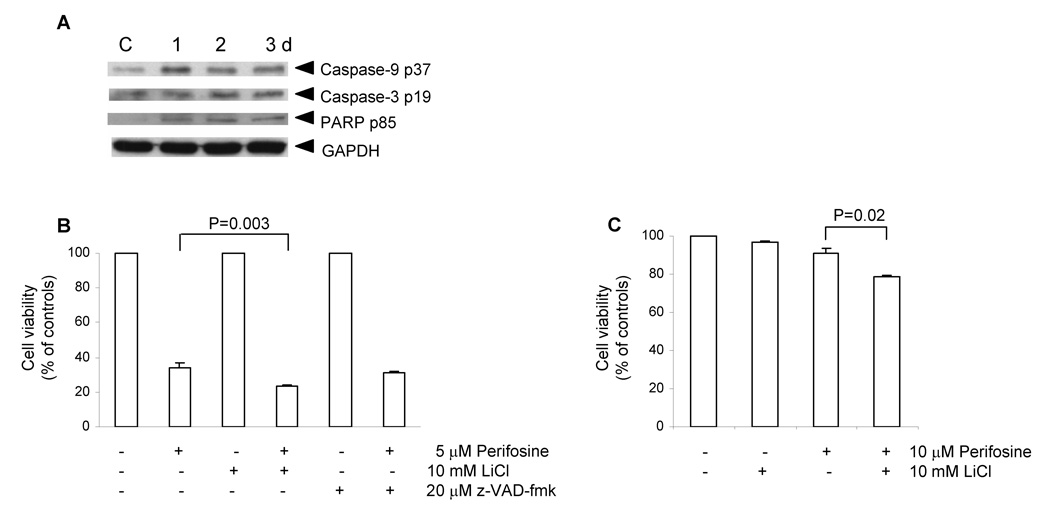
We used western blotting to test whether perifosine treated PC-3 cells express typical apoptotic markers. Analysis of extracts prepared from cells treated with 5 µM perifosine for 1–3 days revealed that attached cells contained low levels of cleaved caspases −9, and −3, and cleaved PARP (Fig. 4A).
Figure 4. Expression of apoptotic markers by differentiating cells (A), cell viability of PC-3 cells treated with perifosine together with GSK-3β LiCl or pan-caspase z-VAD-fmk inhibitors (B), and viability of LNCaP cells treated with perifosine together with GSK-3β inhibitor LiCl (C).
A, Cell lysates prepared as described in Figure 2 were used to detect protein levels of typical apoptotic markers, such as cleaved caspase-9 p37, caspase-3 p17, and PARP p85 in PC-3 cells treated with 5 µM perifosine for indicated periods of time by means of western blotting as described in Materials and Methods. GAPDH was used to detect equal protein loading, C, control. Experiments were repeated three times and typical results are presented. B, PC-3 cells were seeded at 3 × 105 per well in 6-well plate in triplicates and pre-treated with 10 mM lithium chloride or 20 µM z-VAD-fmk for 1 h prior perifosine treatment at 5 µM for 2 days. Floating and attached cells were collected at the end of treatment, stained with calcein-AM and analyzed by flow cytometry as described in Material and Methods. Bars, SD. C, LNCaP cells were seeded at 2 × 105 per well in 6-well plate in triplicates and pre-treated with lithium chloride for 1 h prior treatment with 10 µM perifosine for 3 days. Floating and attached cells were collected at the end of treatment, stained with calcein-AM and analyzed by flow cytometry as described in Material and Methods. Bars, SD.
To assess whether perifosine toxicity in PC-3 cells is mediated by activated caspases, we pre-treated PC-3 cells with pan-caspase inhibitor z-VAD-fmk and used calcein-AM staining to asses percentages of viable cells by flow cytometry. Z-VAD-fmk treatment did not protect PC-3 cells from perifosine-induced cell death, suggesting that perifosine also induces caspase-independent cell death (Fig. 4B).
Next we tested whether GSK-3β activity contributes to perifosine-induced toxicity. We pre-treated PC-3 and LNCaP cells with the well-known GSK-3β inhibitor lithium chloride at 10 mM prior to perifosine treatment. At the end of the treatment, we stained cells with calcein-AM to assess percentages of viable cells. We detected reduced viability of PC-3 (Fig. 4B) and LNCaP (Fig. 4C) cells treated with lithium and perifosine when compared with cells treated only with perifosine. This analysis revealed that inhibition of GSK-3β by lithium ions enhances perifosine-induced cytotoxicity.
In summary, perifosine-differentiated cells express apoptotic markers, although perifosine induces caspase-independent cell death in PC-3 cells. Importantly, the inhibition of GSK-3β activity enhances perifosine-induced cytotoxicity.
4. Discussion
Perifosine is a novel alkylphospholipid which displayed significant antiproliferative activity in prostate cancer PC-3 cells. This antiproliferative activity was described as a result of induced cell growth inhibition and cell death [5]. Our initial studies confirmed these published data, however we further demonstrated that treatment of PC-3 cells with 5 µM perifosine results in a 40–45% survival under our experimental conditions using MTS or calcein-AM assays. We also noted significant morphological changes in PC-3 cells, similar to those induced by inosine 5’-monophosphate dehydrogenase (IMPDH) or histone deacetylase (HDAC) inhibitors as recently described [12,14], after perifosine treatment. We speculated that perifosine induces differentiation of PC-3 cells.
We continued our studies to test whether perifosine induces differentiation of PC-3 cells. Perifosine treatment of PC-3 cells resulted in the accumulation of cells in the G2/M phase of cell cycle. Previously published resulted showed that treatment of PC-3 cells with IMPDH inhibitors results in the accumulation of cells in the S phase [12] while treatment with HDAC inhibitor causes the accumulation of cells in the G0/G1 phase of cell cycle [14]. These results suggest that the differentiation process in PC-3 cells is initiated independently of a specific cell cycle phase block.
Floryk et al [14] used microarray approach to detect changes in the transcriptome of differentiating PC-3 cells after treatment with IMPDH or HDAC inhibitors mycophenolic acid (MPA) or tributyrin, respectively. We speculated that common genes induced or reduced by both inhibitors could be used as markers for PC-3 differentiation independently of pathways leading to their induction. Thus, we used real-time PCR to test induction of selected markers in perifosine treated PC-3 cells. We detected increased expression of significant numbers of differentiation markers including prostasomal, extracellular and plasma membrane proteins, and keratins. We also used western blotting to confirm data obtained by real-time PCR. Among them, we detected a strong induction of CEACAM5 protein after 2 days of treatment. This protein was also released from differentiating cells into culture media. We suggest that CEACAM5 can be used as a predictive biomarker of perifosine activity in prostate cancer during clinical trials. It will be of interest to characterize a subset of prostate cancers that express this marker as this subset can be more likely responsive to perifosine treatment. It’s noteworthy that CEACAM5 has not been detected in normal prostate or prostate cancers [18]. We also confirmed induction of MIG6 and NDRG1 proteins. Interestingly, MIG6 was shown to be expressed in terminally differentiated HL-60 cells [19]. It was suggested by Zhang et al [20] that MIG6 is a tumor suppressor gene. It would be of interest to compare expression patterns of MIG6 in normal prostate and prostate cancers. Similarly, the expression of NDRG1 is associated with differentiation in various cell systems [21,22].
Overexpression of NDRG1 resulted in inhibition of tumor growth and metastasis by induction cell differentiation [23,24] suggesting that NDRG1 is a potential tumor suppressor of which expression is induced by androgens [25], PTEN [26], and p53 [23]. In contrast, we detected reduced levels of caveolin-1 mRNA in perifosine treated PC-3 cells. It’s conceivable that perifosine mediated reduction in caveolin-1 levels contribute to it’s suppression of Akt activities [3]. This observation may lead to the development of new treatment strategies as caveolin-1 has proangiogenic activities in prostate cancer [27].
To understand the mechanism of perifosine-induced differentiation of PC-3 cells, we focused on GSK-3β which is activated as a consequence of Akt inhibition. We detected Akt inhibition 6 hours after perifosine treatment which was followed by GSK-3β activation as detected by reduced GSK-3β Ser9 phosphorylation. Interestingly, we also detected translocation of active GSK-3β to the nuclei of differentiating cells. We suggest that nuclear localization of GSK-3β results in the induced expression of differentiation markers. This experimental system may be useful in characterizing new GSK-3β target proteins.
Our approach using siRNA to down-regulate GSK-3β expression revealed that GSK-3β activation contributes to the induction of a significant number of genes expressed during perifosine-induced PC-3 differentiation. Thus, our data supports the notion that GSK-3β plays an active role during perifosine-induced PC-3 cell differentiation. A requirement of GSK-3β activity during differentiation of 3T3-L1 preadipocytes was described by Tanq et al [28]. In contrast, it was suggested by others that GSK-3β is rather a negative regulator of differentiation in other cell systems [29,30].
Phosphorylated CREB on Ser133 (CREB-Ser133) is present in the nuclei of PC-3 cells. It was recently described by Salas et al [11] that the presence of an active mutant GSK-3βΔ9 resulted in the increase phosphorylation of CREB-Ser133 on Ser129. Double-phosphorylated CREB on Ser133 and Ser129 was detected in low-grade (well differentiated) prostate cancer samples. This observation also supports the idea that active GSK-3β is associated with differentiated phenotype of prostate cells. To characterize CREB target genes in our system, we used an siRNA approach to eliminate CREB protein and used real-time PCR to measure RNA levels for selected genes in control and perifosine treated cells. We detected a reduction in the expression of five genes, among them NDRG1, a recently characterized CREB target [31]. We used western blotting to confirm real-time PCR data and demonstrated that the expression of CEACAM5, MIG6, and NDRG1 was reduced in perifosine-treated PC-3 cells with suppressed GSK-3β expression; the expression of CEACAM5, and NDRG1 was also reduced in perifosine-treated PC-3 cells with undetectable CREB protein. Our real-time PCR and western blotting data support the idea that CREB is activated by GSK-3β in perifosine treated PC-3 cells. We used LNCaP cells to demonstrate that GSK-3β/CREB pathway is activated in perifosine treated androgen-sensitive cells. Our data indicated that LNCaP cells are less sensitive to perifosine compare to PC-3 cells. Thus, we used 10 µM perifosine to test for the expression of differentiation markers. Multiple markers, including GDF15, KRT4, KRT6irs4, and S100P were up-regulated after perifosine treatment for 2 days. Down-regulation of GSK-3β resulted in the reduced expression of GDF15, S100P, AZGP, KRT6irs4 and KRT4, while down-regulation of CREB yielded the reduced expression of GDF15 and S100P genes after perifosine treatment. These data suggest that GSK-3β/CREB pathway is activated by perifosine and may be an alternative pathway involved in the expression of differentiation markers in LNCaP cells.
Kondapaka et al [5] tested sensitivity of various cell lines to perifosine, among them PC-3, DU145, and LNCaP cells. They found PC- cells to be the most sensitive to perifosine treatment. Our findings in PC-3 cells are in agreement with these published data. However, we have not observed complete cell death following treatment with 5 µM perifosine for 2 days. Our data suggest that perifosine cytostatic activities are associated with cell growth arrest, cell cycle block, and differentiation. To understand perifosine-triggered cytotoxicity, we tested for the activation of caspases in differentiating cells. We detected activated caspases −9 and −3 in perifosine treated PC-3 cells. Although, treatment with pan-caspase inhibitor z-VAD-fmk did not protect PC-3 cells from perifosine-induced cell death suggesting that the apoptotic pathway is initiated in these cells and perhaps executed in small percentages of cells (up to 15%) as we were able to detect a weak PARP cleavage, however the majority of cells died by non-apoptotic cell death (up to 40%). We used GSK-3β inhibitor lithium chloride (LiCl) to test involvement of GSK-3β in perifosine-induced toxicity as these inhibitors are often associated with apoptotic death [32,33,34]. Our results indicate that GSK-3β has a survival promoting function in perifosine-treated prostate cancer cells. Although the inhibition of GSK-3β by lithium chloride increases toxicity of perifosine by a small margin, this difference may be significant. Our data are supported by Cho et al [35] who demonstrated that inhibition of GSK-3β enhances the in vitro lethality of perifosine in renal cell carcinoma cell lines. Thus, activation of GSK-3β by Akt inhibition may undermine the anti-tumor effects of perifosine. Our study also suggests that clinically relevant GSK-3β inhibitors in combination with perifosine should be tested in cancer patients.
The results of a recent Phase I/II clinical trial of perifosine in prostate cancer failed to show significant therapeutic responses prostate cancer as a single agent [17]. However, Vink et al [36] suggests that alkylphosholipids including perifosine, are attractive candidates for combination treatment with radiotherapy. There are also ongoing Phase II clinical trials testing combinations of perifosine in various malignances, including solid tumors, with one of the chemotherapy agents including gemcitabine, paclitaxel, docetaxel, doxorubicin, capecitabine, pemetrexed, and irinotecan in patients with metastatic disease (information available at http://www.clinicaltrials.gov/ct/show/NCT00398879?order=2). Our present study suggests the possibility for the detection of an extracellular marker(s) to monitor responses to perifosine in patients with prostate cancer. Further studies should be performed to test how the expression of these markers is affected by new perifosine combination treatment.
Acknowledgements
This work was supported by Specialized Program of Research Excellence (SPORE) Grant P50-58204 and in part by RO1 68814 from National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Ittmann MM, Ayala G, Tsai MJ, Amato RJ, Wheeler TM, Miles BJ, Kadmon D, Thompson TC. The emerging role of the PI3-K-Akt pathway in prostate cancer progression. Prostate Cancer Prostatic Dis. 2005;8:108–118. doi: 10.1038/sj.pcan.4500776. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Ren CH, Tahir SA, Ren C, Thompson TC. Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2A. Mol Cell Biol. 2003;23:9389–9404. doi: 10.1128/MCB.23.24.9389-9404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulholland DJ, Dedhar S, Wu H, Nelson CC. PTEN and GSK3beta: key regulators of progression to androgen-independent prostate cancer. Oncogene. 2006;25:329–337. doi: 10.1038/sj.onc.1209020. [DOI] [PubMed] [Google Scholar]
- 5.Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2:1093–1103. [PubMed] [Google Scholar]
- 6.Ruiter GA, Zerp SF, Bartelink H, van Blitterswijk WJ, Verheij M. Alkyllysophospholipids activate the SAPK/JNK pathway and enhance radiation-induced apoptosis. Cancer Res. 1999;59:2457–2463. [PubMed] [Google Scholar]
- 7.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 8.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding VW, Chen RH, McCormick F. Differential regulation of glycogen synthase kinase 3beta by insulin and Wnt signaling. J Biol Chem. 2000;275:32475–32481. doi: 10.1074/jbc.M005342200. [DOI] [PubMed] [Google Scholar]
- 10.Terry S, Yang X, Chen MW, Vacherot F, Buttyan R. Multifaceted interaction between the androgen and Wnt signaling pathways and the implication for prostate cancer. J Cell Biochem. 2006;99:402–410. doi: 10.1002/jcb.20983. [DOI] [PubMed] [Google Scholar]
- 11.Salas TR, Reddy SA, Clifford JL, Davis RJ, Kikuchi A, Lippman SM, Menter DG. Alleviating the suppression of glycogen synthase kinase-3beta by Akt leads to the phosphorylation of cAMP-response element-binding protein and its transactivation in intact cell nuclei. J Biol Chem. 2003;278:41338–41346. doi: 10.1074/jbc.M302972200. [DOI] [PubMed] [Google Scholar]
- 12.Floryk D, Tollaksen SL, Giometti CS, Huberman E. Differentiation of human prostate cancer PC-3 cells induced by inhibitors of inosine 5′-monophosphate dehydrogenase. Cancer Res. 2004;64:9049–9056. doi: 10.1158/0008-5472.CAN-04-1553. [DOI] [PubMed] [Google Scholar]
- 13.Floryk D, Huberman E. Mycophenolic acid-induced replication arrest, differentiation markers and cell death of androgen-independent prostate cancer cells DU145. Cancer Lett. 2006;231:20–29. doi: 10.1016/j.canlet.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Floryk D, Huberman E. Differentiation of androgen-independent prostate cancer PC-3 cells is associated with increased nuclear factor-kappaB activity. Cancer Res. 2005;65:11588–11596. doi: 10.1158/0008-5472.CAN-05-1831. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003;31:e154. doi: 10.1093/nar/gng154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crul M, Rosing H, de Klerk GJ, Dubbelman R, Traiser M, Reichert S, Knebel NG, Schellens JH, Beijnen JH, ten Bokkel Huinink WW. Phase I and pharmacological study of daily oral administration of perifosine (D-21266) in patients with advanced solid tumours. Eur J Cancer. 2002;38:1615–1621. doi: 10.1016/s0959-8049(02)00127-2. [DOI] [PubMed] [Google Scholar]
- 17.Posadas EM, Gulley J, Arlen PM, Trout A, Parnes HL, Wright J, Lee MJ, Chung EJ, Trepel JB, Sparreboom A, Chen C, Jones E, Steinberg SM, Daniels A, Figg WD, Dahut WL. A phase II study of perifosine in androgen independent prostate cancer. Cancer Biol Ther. 2005;4:1133–1137. doi: 10.4161/cbt.4.10.2064. Epub 2005 Oct 1131. [DOI] [PubMed] [Google Scholar]
- 18.Blumenthal RD, Leon E, Hansen HJ, Goldenberg DM. Expression patterns of CEACAM5 and CEACAM6 in primary and metastatic cancers. BMC Cancer. 2007;7:2. doi: 10.1186/1471-2407-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wick M, Burger C, Funk M, Muller R. Identification of a novel mitogen-inducible gene (mig-6): regulation during G1 progression and differentiation. Exp Cell Res. 1995;219:527–535. doi: 10.1006/excr.1995.1261. [DOI] [PubMed] [Google Scholar]
- 20.Zhang YW, Staal B, Su Y, Swiatek P, Zhao P, Cao B, Resau J, Sigler R, Bronson R, Vande Woude GF. Evidence that MIG-6 is a tumor-suppressor gene. Oncogene. 2007;26:269–276. doi: 10.1038/sj.onc.1209790. [DOI] [PubMed] [Google Scholar]
- 21.Chen B, Nelson DM, Sadovsky Y. N-myc down-regulated gene 1 modulates the response of term human trophoblasts to hypoxic injury. J Biol Chem. 2006;281:2764–2772. doi: 10.1074/jbc.M507330200. [DOI] [PubMed] [Google Scholar]
- 22.Taketomi Y, Sugiki T, Saito T, Ishii S, Hisada M, Suzuki-Nishimura T, Uchida MK, Moon TC, Chang HW, Natori Y, Miyazawa S, Kikuchi-Yanoshita R, Murakami M, Kudo I. Identification of NDRG1 as an early inducible gene during in vitro maturation of cultured mast cells. Biochem Biophys Res Commun. 2003;306:339–346. doi: 10.1016/s0006-291x(03)00942-2. [DOI] [PubMed] [Google Scholar]
- 23.Kurdistani SK, Arizti P, Reimer CL, Sugrue MM, Aaronson SA, Lee SW. Inhibition of tumor cell growth by RTP/rit42 and its responsiveness to p53 and DNA damage. Cancer Res. 1998;58:4439–4444. [PubMed] [Google Scholar]
- 24.van Belzen N, Dinjens WN, Diesveld MP, Groen NA, van der Made AC, Nozawa Y, Vlietstra R, Trapman J, Bosman FT. A novel gene which is up-regulated during colon epithelial cell differentiation and down-regulated in colorectal neoplasms. Lab Invest. 1997;77:85–92. [PubMed] [Google Scholar]
- 25.Segawa T, Nau ME, Xu LL, Chilukuri RN, Makarem M, Zhang W, Petrovics G, Sesterhenn IA, McLeod DG, Moul JW, Vahey M, Srivastava S. Androgen-induced expression of endoplasmic reticulum (ER) stress response genes in prostate cancer cells. Oncogene. 2002;21:8749–8758. doi: 10.1038/sj.onc.1205992. [DOI] [PubMed] [Google Scholar]
- 26.Bandyopadhyay S, Pai SK, Hirota S, Hosobe S, Tsukada T, Miura K, Takano Y, Saito K, Commes T, Piquemal D, Watabe M, Gross S, Wang Y, Huggenvik J, Watabe K. PTEN up-regulates the tumor metastasis suppressor gene Drg-1 in prostate and breast cancer. Cancer Res. 2004;64:7655–7660. doi: 10.1158/0008-5472.CAN-04-1623. [DOI] [PubMed] [Google Scholar]
- 27.Tahir SA, Yang G, Goltsov AA, Watanabe M, Tabata K, Addai J, Fattah el MA, Kadmon D, Thompson TC. Tumor cell-secreted caveolin-1 has proangiogenic activities in prostate cancer. Cancer Res. 2008;68:731–739. doi: 10.1158/0008-5472.CAN-07-2668. [DOI] [PubMed] [Google Scholar]
- 28.Tang QQ, Gronborg M, Huang H, Kim JW, Otto TC, Pandey A, Lane MD. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc Natl Acad Sci U S A. 2005;102:9766–9771. doi: 10.1073/pnas.0503891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munoz-Montano JR, Moreno FJ, Avila J, Diaz-Nido J. Downregulation of glycogen synthase kinase-3beta (GSK-3beta) protein expression during neuroblastoma IMR-32 cell differentiation. J Neurosci Res. 1999;55:278–285. doi: 10.1002/(SICI)1097-4547(19990201)55:3<278::AID-JNR2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 30.Tuhackova Z, Sloncova E, Hlavacek J, Sovova V, Velek J. Activity of glycogen synthase kinase-3beta is down-regulated during transient differentiation of human colon cancer HT-29 cells. Oncol Rep. 1999;6:827–832. doi: 10.3892/or.6.4.827. [DOI] [PubMed] [Google Scholar]
- 31.Wu L, Liu J, Gao P, Nakamura M, Cao Y, Shen H, Griffin JD. Transforming activity of MECT1-MAML2 fusion oncoprotein is mediated by constitutive CREB activation. Embo J. 2005;24:2391–2402. doi: 10.1038/sj.emboj.7600719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alao JP, Stavropoulou AV, Lam EW, Coombes RC. Role of glycogen synthase kinase 3 beta (GSK3beta) in mediating the cytotoxic effects of the histone deacetylase inhibitor trichostatin A (TSA) in MCF-7 breast cancer cells. Mol Cancer. 2006;5:40. doi: 10.1186/1476-4598-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pastorino JG, Hoek JB, Shulga N. Activation of glycogen synthase kinase 3beta disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res. 2005;65:10545–10554. doi: 10.1158/0008-5472.CAN-05-1925. [DOI] [PubMed] [Google Scholar]
- 34.Watcharasit P, Bijur GN, Song L, Zhu J, Chen X, Jope RS. Glycogen synthase kinase-3beta (GSK3beta) binds to and promotes the actions of p53. J Biol Chem. 2003;278:48872–48879. doi: 10.1074/jbc.M305870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho D, Atkins AB, Mier JW. American Association for Cancer Research Annual Meeting: Proceedings. Philadelphia (PA): AACR, Los Angeles; 2007. Inhibition of glycogen synthase kinase 3b (GSK3b) enhances the in vitro activity of the Akt inhibitor perifosine in renal cell carcinoma (RCC) cell lines. [Google Scholar]
- 36.Vink SR, van Blitterswijk WJ, Schellens JH, Verheij M. Rationale and clinical application of alkylphospholipid analogues in combination with radiotherapy. Cancer Treat Rev. 2007;33:191–202. doi: 10.1016/j.ctrv.2006.12.001. [DOI] [PubMed] [Google Scholar]