Abstract
A new practical method for the synthesis of N-fused heterocycles via the transition metal-catalyzed cycloisomerization of heterocyles possessing a propagyl group has been developed. This very mild, base- and ligand-free method allows for the synthesis of diverse fused heterocyclic cores in good to excellent yields.
Heteroaromatic molecules containing N-fused bicyclic fragments and their partially or completely reduced analogues are pharmaceutically important scaffolds, widely found in naturally occurring, as well as synthetic biologically active, molecules.1 For instance, it was shown that molecules containing indolizine and other closely related cores exhibit strong anti-inflammatory,2 anti-HIV,3 and anti-leukemia activities.4 Although few routes toward substituted fused pyrrolo-heterocycles exist,5 new methods allowing for the efficient construction of these heterocycles with different substitution patterns are of high demand.
We have recently developed two complimentary protocols for the synthesis of C-3 substituted6 and C-1 - C-2 disubstituted7 fused and non-fused pyrrole-containing heterocycles (Scheme 1). The first approach which ates via a copper-assisted cycloisomerization of conjugated alkynyl imines into pyrrole ring (eq 1) was demonstrated to be very general and efficient, though requires excess base and elevated temperatures, and is limited to C-3 monosubstituted products.6 Another protocol is based on a gold-catalyzed cascade alkyne-vinylidene isomerization/1,2-metalloid migration in non-conjugated propargyic systems (eq 2).7 This method does not require base and can be efficiently used at 60 °C to construct C-1 - C-2 bifunctional scaffolds. Herein, we wish to report a room temperature, base- and additive-free transition metal-catalyzed cycloisomerization of propagyl heterocycles leading to the formation of C-1 - C-3 disubstituted N-fused heterocycles in good to excellent yields (eq 3).8
Scheme 1.

Formation of differently substituted pyrrole ring by transition metal-catalyzed cycloizomerizations
We hypothesized that a π-philic metal would coordinate to the propargylic moiety of the heterocycle rendering its triple bond electrophilic, thus provoking cyclization via an intramolecular nucleophilic attack of heterocyclic nitrogen (Scheme 1, eq 3). To test this hypothesis, we first subjected easily available9 propargyl-containing pyridine 1a to the copper-catalyzed cycloisomerization conditions.6,10 It was found that 1a, indeed, underwent the desired cycloizomerization, affording indolizine 2a in good yield. After brief optimization, we were pleased to find that this reaction can be performed equally well at room temperature, the base can be omitted, and DMA can be substituted with easier to handle dichloromethane.
Next, catalyst optimization for this transformation performed. Thus, it was found that dramatic decrease of the catalyst load to 3 mol % of CuI or CuCl had virtually no effect on the reaction course, producing indolizine 2a in 77% and 83% yields respectively (Table 1). Similarly, gold catalysts were found to be efficient in this transformation: AuCl3 afforded 71% yield, while AuI gave 95%, though the reaction was slower. In contrast, employment of Al, Sn, In, Mg, Pt, and Pd catalysts under these conditions resulted in moderate yields only, and the reactions were generally much more sluggish. Gratifyingly, switching to AgBF4 and AgPF6 led to nearly quantitative yields of 2a (entries 12, 13). In order to verify whether an eventual proton can serve as a catalyst,11 we tested this reaction in the presence of triflic acid. However, it was found that only small amounts of 2a were produced under Brønsted catalysis (entry 15).
Table 1.
Catalyst optimization 
| no. | catalyst | reaction time | yield, %b |
|---|---|---|---|
| 1 | CuI | 3 h | 77% |
| 2 | CuCl | 30 min | 83% |
| 3 | AuCl3 | 30 min | 71% |
| 4 | AuI | 3 h | 95% |
| 5 | AlCl3 | 48 h | 64%c |
| 6 | Sn(OTf)2 | 48 h | 31% |
| 7 | Mg(OTf)2 | 48 h | 52% |
| 8 | In(OTf)2 | 48 h | 33% |
| 9 | PtCl2 | 48 h | 41% |
| 10 | PdCl2(PPh3)2 | 30 min | 61% |
| 11 | Pd(OAc)2 | 30 min | 74% |
| 12 | AgBF4 | 30 min | >99% |
| 13 | AgPF6 | 30 min | >99% |
| 14 | AgSbF6 | 30 min | 52% |
| 15 | HOTf | 30 min | 9% |
Reactions were run in the presence of 3 mol % of catalyst in DCM (0.25 M) at room temperature. Most reactions work equally well in toluene.
GC-MS yields.
10 mol % of catalyst were used.
Next, the scope of this cycloisomerization was examined under the optimized conditions (Table 2). To our delight, acetyloxy, diethylphosphatyloxy, and O-TBS-protected propargylic substrates 1a-k bearing alkyl (entries 2, 8, 10), aryl (entries 1, 5, 9), heteroaryl (entry 11), and alkenyl (entries 3, 7) substituents at the triple bond, as well as those possessing terminal alkyne moiety (entries 4, 6), underwent very smooth cycloizomerization to give corresponding heterocycles 2a-k in good to excellent yields. In contrast, the reaction of diyne-containing substrate 1l gave a very low yield of pyrrolothiazole 2l (entry 12).12 It deserves mentioning that this cycloisomerization protocol appeared to be general with regard to the heterocyclic core: C-1 - C-3 disubstituted indolizines (entries 1-6), pyrrolo-quinoxalines (entries 7, 8), and pyrrolothiazoles (entries 9-11) can efficiently be synthesized via this method from readily available precursors.9
Table 2.
Scope of cycloizomerization 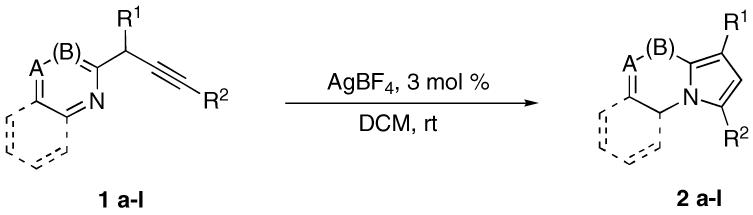
| no. | substrate | product | yield, %a |
|---|---|---|---|
| 1 |  |
 |
95 |
| 2 |  |
 |
76 |
| 3 |  |
 |
83 |
| 4 |  |
 |
64 |
| 5 |  |
 |
94 |
| 6 |  |
 |
87 |
| 7 |  |
 |
52 |
| 8 |  |
 |
72 |
| 9 |  |
 |
82 |
| 10 |  |
 |
85c |
| 11 |  |
 |
89b,c |
| 12 |  |
 |
< 10c,d |
Isolated yields.
10 mol % of catalyst was used.
Reaction was performed at 40 °C.
NMR yield.
We propose the following mechanistic rationale for the transition-metal catalyzed cycloizomerization of propargyl-heterocycles 1 (Scheme 2). π-Philic metal activates the triple bond toward an intramolecular nucleophilic attack of the heterocyclic nitrogen,13 leading to the formation of a bicyclic zwitterionic adduct 3. The latter can rearomatize into product 2 via two different ways: through a deprotonation-protonation sequence (Path A), or via 1,2- hor 1,5-hydride shift (Path B). In the former scenario, heterocyclic nitrogen of 1 serves as a base, as there is no other base present in the reaction mixture.
Scheme 2.

Proposed mechanisms for cycloisomerization
Thus, we hypothesized that if deprotonation-protonation event indeed takes place (Path A), a substantial deuterium scrambling would be observed.14 On the other hand, if the rearomatization proceeds via a hydride shift (Path B), deuterium would cleanly end up at the C-2 position of the cyclized product 2.15 To test the above idea, we performed deuterium-labeling experiment employing isotopically pure propargyl pyridine 5 (Scheme 3). It was found that a severe proton-deuterium exchange took place upon cycloizomerization, thus, strongly supporting the deprotonation-protonation pathway A, and ruling out the possibility of a clean hydride shift (Path B).
Scheme 3.

Deuterium labeling experiment
In summary, we have developed an exceptionally mild, practical, and efficient method en route to C-1 - C-3 disubstituted N-fused heterocycles, including indolizines, pyrroloquinoxalines, and pyrrolothiazoles. This approach is complimentary to our previously developed methods6,7 as it allows for the synthesis of heterocycles with different substitution patterns.
Supplementary Material
Acknowledgment
The support of the National Institutes of Health (Grant GM-64444) is gratefully acknowledged.
Footnotes
vlad@uic.edu
References
- (1).For review, see:Michael JP. Nat. Prod. Rep. 1999;16:675. doi: 10.1039/a809408j.
- (2).For studies on sPLA2 inhibition activity, see:Hagishita S, Yamada M, Shirahase K, Okada T, Murakami Y, Ito Y, Matsuura T, Wada M, Kato T, Ueno M, Chikazawa Y, Yamada K, Ono T, Teshirogi I, Ohtani M. J. Med. Chem. 1996;39:3636. doi: 10.1021/jm960395q.
- (3).For studies on biological activity of Lamellarin family, see:Facompre M, Tardy C, Bal-Mahieu C, Colson P, Perez C, Manzanares I, Cuevas C, Bailly C. Cancer Res. 2003;63:7392.Reddy MV, Rao MR, Rhodes D, Hansen MS, Rubins K, Bushman FD, Venkateswarlu Y, Faulkner DJ. J. Med. Chem. 1999;42:1901. doi: 10.1021/jm9806650.Østby OB, Dalhus B, Gundersen L-L, Rise F, Bast A, Haenen GRMM. Eur. J. Org. Chem. 2000:3763.
- (4)(a).Anderson WK, Heider AR, Raju N, Yucht JA. J. Med. Chem. 1988;31:2097. doi: 10.1021/jm00119a008. [DOI] [PubMed] [Google Scholar]; (b) Anderson WK, DeRuiter J, Heider AR. J. Org. Chem. 1985;50:722. [Google Scholar]
- (5).For a general review, see:Behnisch A, Behnisch P, Eggenweiler M, Wallenhorst T. Indolizine, In Houben-Weyl. 1994;E6b/1(2a):323–450.See also:Marchalin S, Baumlova B, Baran P, Oulyadi H, Daich A. J. Org. Chem. 2006;71:9114. doi: 10.1021/jo0615044.Kaloko J, Jr., Hayford A. Org. Lett. 2005;7:4305. doi: 10.1021/ol051860t.
- (6).Kel’in AV, Sromek AW, Gevorgyan V. J. Am. Chem. Soc. 2001;123:2074. doi: 10.1021/ja0058684. [DOI] [PubMed] [Google Scholar]
- (7).Seregin IV, Gevorgyan V. J. Am. Chem. Soc. 2006;128:12050. doi: 10.1021/ja063278l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).When this project was underway, a report on a related Pt-catalyzed 1,2-migration/cyclization toward indolizine core appeared; see:Smith CR, Bunnelle EM, Rhodes AJ, Sarpong R. Org. Lett. 2007;9:1169. doi: 10.1021/ol0701971.
- (9).See Supporting Information for detailed preparative procedures.
- (10).The reactions were performed in N,N-dimethylacetamide (DMA) at 130 °C in the presence of 30 mol % CuI.
- (11).For recent discussions on the role of Brønsted acids in transition metal-catalyzed transformations, see:Hashmi ASK. Catal. Today. 2007;122:211.Li Z, Zhang J, Brouwer C, Yang C-G, Reich NW, He C. Org. Lett. 2006;8:4175. doi: 10.1021/ol0610035.Rosenfeld DC, Shekhar S, Takemiya A, Utsunomiya M, Hartwig JF. Org. Lett. 2006;8:4179. doi: 10.1021/ol061174+.Rhee JU, Krische MJ. Org. Lett. 2005;7:2493. doi: 10.1021/ol050838x.
- (12).Alkynyl N-fused heterocycles can be alternatively accessed via our recently developed direct C-H alkynylation approach:Seregin IV, Ryabova V, Gevorgyan V. J. Am. Chem. Soc. 2007;129:7742. doi: 10.1021/ja072718l.
- (13).For selected examples on nucleophilic attack of heteroatom at alkyne activated by Au and Ag complexes, see:Gorin DJ, Davis NR, Toste FD. J. Am. Chem. Soc. 2005;127:11260. doi: 10.1021/ja053804t.Dubé P, Toste FD. J. Am. Chem. Soc. 2006;128:12062. doi: 10.1021/ja064209+.Shapiro ND, Toste ND. J. Am. Chem. Soc. 2007;129:4160. doi: 10.1021/ja070789e.Hashmi ASK, Rudolph M, Schymura S, Visus J, Frey W. Eur. J. Org. Chem. 2006:4905.Hashmi ASK, Weyrauch JP, Frey W, Bats JW. Org. Lett. 2004;6:4391. doi: 10.1021/ol0480067.Belting V, Krause N. Org. Lett. 2006;8:4489. doi: 10.1021/ol061751u.Sun J, Kozmin SA. Angew. Chem. Int. Ed. 2006;45:4991. doi: 10.1002/anie.200601276.Wang S, Zhang L. J. Am. Chem. Soc. 2006;128:14274. doi: 10.1021/ja066220f.McDonald FE, Gleason MM. J. Am. Chem. Soc. 1996;118:6648.McDonald FE, Burova SA, Huffman LG. Synthesis. 2000:970.
- (14).For an example of a base-assisted substantial deuterium scrambling upon cycloisomerization in conjugated propargylic systems, see Ref. 6.
- (15).For an example of a clean 1,2-deuterium shift upon cycloisomerization, see:Sromek AW, Rubina M, Gevorgyan V. J. Am. Chem. Soc. 2005;127:10500. doi: 10.1021/ja053290y.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


