Abstract
Dendritic cells (DCs) are sentinels of the immune system and represent a heterogeneous cell population. The existence of distinct DC subsets is due to their inherent plasticity and to the changing microenvironment modulating their immunological properties. Numerous signalling pathways have impacts on DCs. It appears that besides cytokines/chemokines, lipid mediators also have profound effects on the immunogenicity of DCs. Some of these lipid mediators exert an effect through nuclear hormone receptors. Interestingly, more recent findings suggest that DCs are able to convert precursors to active hormones, ligands for nuclear receptors. Some of these DC-derived lipids, in particular retinoic acid (RA), have a central function in shaping T-cell development and effector functions. In this review, we summarize and highlight the function of a set of nuclear receptors (PPARγ, RA receptor, vitamin D receptor and glucocorticoid receptor) in DC biology. Defining the contribution of nuclear hormone receptor signalling in DCs can help one to understand the regulatory logic of lipid signalling and allow the exploitation of their potential for therapeutic intervention in various immunological diseases.
Keywords: dendritic cells, immunity, lipid signalling, nuclear receptors, PPARγ
Immunological functions of dendritic cells serve the regulation of the adaptive immune response
Antigen presentation is a key step in engaging the adaptive immune response. Traditionally, macrophages and B cells were thought to be the only cell types able to present antigens to T cells and thus elicit immune response. The pioneering work of Steinman and Cohn showed that a third kind of antigen-presenting cell types, dendritic cells (DCs), are indispensable for the initiation of the adaptive immune response (Steinman and Cohn, 1973; Banchereau and Steinman, 1998). The migratory, tissue-resident DCs work at the interface of peripheral tissues and lymphoid organs. These cells are sentinels of the immune system, and they sense and translate environmental cues by sampling and processing extracellular and intracellular antigens (Figure 1). DCs are able to pick up antigens using various uptake mechanisms (Norbury, 2006; Savina and Amigorena, 2007). A key defining feature of this cell type is that after antigen uptake and processing, DCs migrate to lymph nodes, where they present antigens and stimulate T cells (Figure 1, insert). DCs are armed with numerous receptors that detect ‘danger' signals in the surrounding environment. These signals, associated with an ongoing infection, such as pathogen-associated molecular patterns or pro-inflammatory cytokines, cause DCs to undergo phenotypic changes (maturation, activation) that maximize their ability to elicit proliferation of T cells. Mature DCs have an extraordinary capacity to activate naive T cells owing to the high expression of various co-stimulatory molecules (Ni and O'Neill, 1997). However, besides eliciting immune response, DCs could also provoke immunological tolerance by inducing deletion or anergy; thus, DCs contribute to limiting autoimmunity (Cools et al, 2007). There are several mechanisms for the maintenance of peripheral tolerance, one of which is when external stimuli reprogramme DCs towards a less activated tolerogenic cell type.
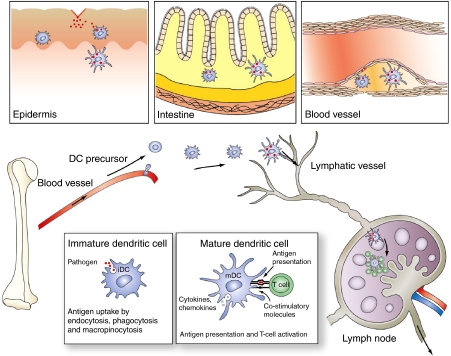
Figure 1.
The immunological function of DCs. Differentiation and migration of tissue-resident DCs. DC precursors are bone marrow-derived cells that leave the blood vessel and reside in various tissues in the periphery (for example, epidermis, intestine or the vessel wall or the atherogenic plaque) as immature DCs. These cells are well armed for sampling antigens and can receive various cues from the surrounding tissues. Antigen uptake associated with ‘danger signals' leads to DC maturation and migration of draining lymph nodes. In the lymph nodes, mature DCs present antigens to naive T cells and activate them.
DCs form a heterogeneous cell population, which could be classified as plasmacytoid or conventional DCs (Shortman and Naik, 2007). DCs could also be subdivided into migratory DCs, which reside in peripheral tissues, or lymphoid organ-resident DCs, which constitute 50% of the lymph node DCs, and all the splenic and thymic DCs (Villadangos and Schnorrer, 2007). The molecular details of the regulation of DC differentiation are still poorly characterized, although it was described and suggested that several cytokines and transcription factors are necessary for DC development (Zenke and Hieronymus, 2006; Wu and Liu, 2007). Flt3 ligand is critical for the development of both conventional and plasmacytoid DC differentiation; in contrast, GM-CSF promotes only conventional DC development (Wu and Liu, 2007). In addition, in humans, treatment with IL-4 and GM-CSF cytokine is used for the ex vivo generation of monocyte-derived DCs (Sallusto and Lanzavecchia, 1994). Much less is known about the transcriptional regulation of DC development, and only a few putative ‘master' transcription factors have been identified so far (Zenke and Hieronymus, 2006). A microarray study indicated that some nuclear proteins/transcription factors, including PPARγ and LXRα nuclear hormone receptors, are induced during monocyte-derived DC development (Le Naour et al, 2001). Our global gene expression profiling also showed that 20 out of 48 nuclear receptors are present in human monocyte-derived DCs (L Nagy et al, unpublished results). These findings indicate that nuclear receptors are likely to have functions in the differentiation and function of this cell type.
Nuclear hormone receptors are ligand-activated transcription factors that modulate gene expression through binding to specific hormone response elements. It is well established that ligands/hormones (i.e. retinoids (metabolites of vitamin A), vitamin D, glucocorticoids (GCs)) of nuclear receptors, besides regulating development and metabolism, also have an impact on the immune system. It was assumed that the main immune targets of these ligands are lymphocytes. Now this picture is being altered by findings that activators of these receptors can also modulate DC differentiation and function. In the first part of this review, we will provide an introduction to the function of lipids in DC biology, and then summarize the potential function of nuclear hormone receptors in DC differentiation and function. We will focus on RXR heterodimeric receptors (PPAR, RAR, LXR and vitamin D receptor (VDR)) and also discuss the potential function of GC receptors in DC function. In the second part, we will provide an overview on how endogenous ligand production for these receptors is taking place in DCs.
DCs are exposed to lipids
In the body, there are several tissue compartments in which DCs are likely to be exposed to large amounts of lipids. An obvious one is the gut-associated lymphoid tissue (GALT) where diet-derived lipids, fatty acids, retinoids and cholesterol are abundant (Figure 1). In the small intestine, the lamina propria is important to maintain peripheral tolerance towards commensal bacterial flora (Nagler-Anderson and Shi, 2001), and the dietary lipid mediators might contribute to this type of tolerance. It is well established that the atherosclerotic plaques contain macrophages and T cells; however, DCs were also detected in the plaques (Bobryshev and Lord, 1995; Angeli et al, 2004). In this microenvironment, cells are encountering oxidized low-density lipoproteins (oxLDL). In addition, several other active lipid mediators might be present, which could modulate the migratory and immunological properties of the cells (Figure 1).
Tissue-resident DCs sense and translate environmental cues by sampling and processing protein antigens; however, these cells can also present lipid/glycolipid antigens through CD1 cell surface molecules (Brigl and Brenner, 2004). CD1s molecules are critical for the presentation of various bacterial glycolipid/peptidolipid antigens (Willcox et al, 2007), but the ways in which endogenous lipids are recognized and presented are still poorly characterized (Tsuji, 2006). An interesting aspect of lipid antigen presentation is that extracellular lipid particles facilitate the uptake of pathogen-derived CD1 ligands. For example, apoptotic bodies from mycobacterium-infected macrophages are efficient vehicles for lipid uptake of uninfected DCs (Schaible et al, 2003); in addition, a CD1d ligand precursor is delivered to DCs by apolipoproteins (van den Elzen et al, 2005). These results underscore the notion that the local lipid environment has the ability to modify the lipid presentation capacity of DCs. In addition, lipid-derived mediators also elicit intracellular signalling, which alters the maturation and immunogenicity of the antigen-presenting cells. Human monocyte-derived DC maturation is induced by oxLDL exposure and this effect is mediated by lysophosphatidyl choline through the activation of a G-protein-coupled receptor (Coutant et al, 2002). DCs also sense and integrate lipid signals through PG receptors; for example, DC migration and cytokine production could be modulated by PGE2 and LTB4. PGD is also an important modulator of DC function (Harizi and Gualde, 2005). As we have briefly highlighted, DCs sense the lipid environment by various cell membrane receptors, and now there is an increasing amount of evidence to suggest that DCs could also survey the lipid environment by lipid sensing nuclear hormone receptors. In the following section, we summarize the potential function of these lipid-activated nuclear receptors in DCs.
The function of lipid-activated nuclear hormone receptors PPARs and LXRs in DCs
Candidates for sensing the lipid environment in DCs are the PPAR receptors. PPARs are fatty acid sensors that regulate various facets of lipid metabolism. There are three isotypes of PPARs, α, γ and δ/β. These receptors have key physiological functions; for example, PPARα promotes fatty acid oxidation in the liver (Lefebvre et al, 2006), PPARγ is indispensable for adipocyte differentiation and generally stimulates lipid storage (Willson et al, 2001), and PPARδ is an important regulator of skeletal muscle lipid oxidation (Barish et al, 2006). Recent findings indicated that these receptors, especially PPARγ, have important functions in DC biology (Figure 2). Initially, a comprehensive microarray study indicated that in humans, monocyte-derived DCs have an elevated expression of PPARγ (Le Naour et al, 2001). This finding was consistent with a previous observation indicating that IL-4 is a positive regulator of PPARγ in human and murine monocytes/macrophages (Huang et al, 1999). Later, it was confirmed by several laboratories that both the mRNA and also the protein of PPARγ are induced in human monocyte-derived DCs (Gosset et al, 2001; Nencioni et al, 2002; Szatmari et al, 2004). In addition, we found that the bona fide PPARγ target (FABP4/aP2) is highly upregulated upon PPARγ ligand treatment in developing human DCs. Moreover, ex vivo cultured blood-derived conventional (CD1c+) DCs also express PPARγ. Finally, in human lymphoid tissues (tonsils), several PPARγ and S100 double-positive cells were detected, suggesting that at least a sub-population of lymphoid tissue DCs express this receptor (Szatmari et al, 2004). Murine splenic CD11c+ DCs also express PPARγ (Faveeuw et al, 2000) and this nuclear receptor was detected in bone marrow-derived murine DCs (Hammad et al, 2004). The changes in PPARγ expression during various stages of DC development are still poorly characterized; however, it was described that the CD1a-negative population of monocyte-derived DCs express more PPARγ than the CD1a-positive counterparts (Gogolak et al, 2007).
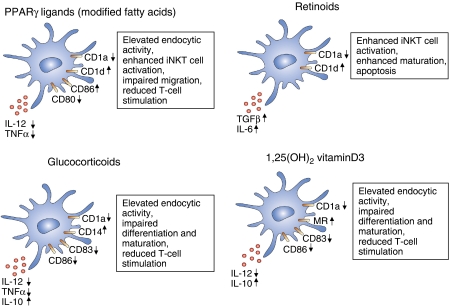
Figure 2.
Effects of nuclear receptor agonists on the immunophenotype of DCs. Characteristic changes of cell surface markers and cytokine production on DCs on nuclear receptor activation. Here, we summarize the effects of PPARγ activators, retinoids, glucocorticoids and the active form of vitamin D on monocyte-derived DCs.
The effects of PPARγ activators on the immunological function and phenotype of ex vivo cultured DCs were investigated in detail. PPARγ-activated immature DCs have an enhanced phagocytic activity; in addition, these cells possess a diminished migratory capacity (Angeli et al, 2003; Szatmari et al, 2004; Appel et al, 2005). PPARγ-activated human DCs produce less IL-12 and TNFα; moreover, these cells secrete lower amounts of MCP2, IP-10 and RANTES chemokines (Gosset et al, 2001; Nencioni et al, 2002) (Figure 2). Furthermore, PPARγ-instructed DCs have an altered cell surface expression pattern of co-stimulatory molecules; they express less CD80 but increased CD86 (Gosset et al, 2001; Nencioni et al, 2002; Szatmari et al, 2004). These observations appeared to be consistent with a model in which PPARγ-activated DCs skew the differentiation to a special DC subset that has a reduced Th1 activation capacity but an enhanced Th2 activation propensity (Faveeuw et al, 2000; Gosset et al, 2001). Others suggested that the activation of the PPARγ pathway generally reduces/inhibits the immunogenicity of developing human DCs (Nencioni et al, 2002; Appel et al, 2005). Recently, in murine DCs, a similar effect was observed, suggesting that PPARγ activation has a negative effect on the stimulatory capacity of DCs. More importantly, in the same report, PPARγ-deficient DCs were used and the authors found that PPARγ's effects on the stimulatory capacity of the cells are receptor dependent (Klotz et al, 2007). However, the molecular mechanism of this negative regulation is not clear. By analogy, the anti-inflammatory properties of PPARγ ligands are better characterized in murine macrophages. One suggested mechanism for this activity is the sumoylation of the receptor and recruitment of co-repressors on the promoter region of NF-κB-binding sites of various inflammatory genes (Pascual et al, 2005). In DCs, it was suggested that the PPARγ ligand treatment interfered with the activation of the NF-κB and the MAP kinase pathways (Appel et al, 2005). Interestingly, besides these essentially inhibitory activities, PPARγ appears to possess positive regulatory activities in immune cells as well. Our recent global gene expression profiling study revealed that on acute PPARγ ligand treatment, more than 100 genes were immediately induced and most of these early activated genes fall into the lipid metabolism category (Szatmari et al, 2007). In contrast, immune response-related genes were induced only after 24 h or later, suggesting that PPARγ activation directly regulates lipid metabolism of these cells but rather indirectly modifies the immune phenotype of DCs. As we have described in the previous section, DCs are able to present lipid antigens by CD1 antigen-presenting molecules. Our results indicated that the PPARγ ligand coordinately regulates the CD1 gene family expression in human monocyte-derived DCs. PPARγ-activated DCs express less CD1a (Nencioni et al, 2002; Szatmari et al, 2004), but have an elevated expression of CD1d. More importantly, the elevated expression of CD1d was coupled to the enhanced capacity to activate a CD1d-dependent cell type, the iNKT (invariant natural killer T) cells (Szatmari et al, 2004). The lack of iNKT cell activation has been implicated in the development of autoimmune conditions, suggesting that iNKT cells are intimately linked to sustaining immunological tolerance (Hammond and Kronenberg, 2003). We also defined the possible mechanism for this regulation: PPARγ indirectly stimulated the expression of CD1d through the production of all-trans retinoic acid (ATRA), the natural ligand of RA receptors (RARs) (Szatmari et al, 2006a). Confirming this, it was reported recently that the human CD1d promoter contains a retinoid response element (Chen and Ross, 2007). We also described that PPARγ-activated DCs have an elevated expression of ABCG2, a xenobiotic transporter. These results suggested that the PPARγ activation modifies the xenobiotic and drug resistance of human DCs (Szatmari et al, 2006b).
The functions of the other two PPAR receptors are poorly characterized in DCs. We and others described that DCs express very low levels of PPARα. Consistent with this finding, PPARα ligand treatment has only a marginal effect on monocyte-derived DC differentiation and function (Gosset et al, 2001; Szatmari et al, 2004). In contrast, it was shown that PPARα is present in human epidermal DCs (Langerhans cells) and that the activation of the PPARα pathway blocks the activation and migration of these skin DCs (Dubrac et al, 2007). Curiously, DCs also express PPARδ (Szatmari et al, 2004), but the potential role of these receptors in DC function is not yet characterized. Another metabolite receptor, the liver X receptors (LXRs), the sensor of oxidized forms of cholesterol (Zelcer and Tontonoz, 2006), were reported to be upregulated during monocyte-derived DC development; moreover, LXR ligand-treated DCs have a reduced T-cell activation capacity (Geyeregger et al, 2007). These nuclear hormone receptors are metabolic sensors. The natural ligands of these receptors are poorly characterized (polyunsatured fatty acids, oxidized fatty acids or cholesterol). Therefore, much remains to be discovered how the receptor's endogenous activation takes place. Nevertheless, high-affinity synthetic ligands for these receptors are available to probe their function in DCs ex vivo and potentially in vivo. In the next sections, we describe the potential function of two receptors having a high-affinity hormonal ligand in DC biology.
Vitamin D and GCs are modulators of DC differentiation and activation
Activators of GC and VDRs have profound immunosuppressive effects. It was assumed that lymphocytes are the main target of these compounds. However, several in vitro and some in vivo observations suggested that GCs and vitamin D also diminished the immunogenicity of DCs and that these effects might contribute to the impaired immune response and to the anti-inflammatory effects. There are a few recent reviews that provide an overview on the immunosuppressive effects of GCs and vitamin D on DCs (Abe and Thomson, 2003; Hackstein and Thomson, 2004; van Etten and Mathieu, 2005), and thus we only briefly discuss the effects of these ligands on DC biology.
The immunosuppressive effects of GCs have been well documented and several studies have suggested that, among other cell types, DCs are also affected. GCs decrease the co-stimulatory molecule expression in murine DCs, and therefore these cells have a poor T-cell stimulatory capacity in vitro and in vivo (Moser et al, 1995). In humans, GC-treated monocyte-derived DCs have a reduced T-cell activation capacity; moreover, GCs interfere with the differentiation of DCs from monocytes (Piemonti et al, 1999; Rea et al, 2000) (Figure 2). The anti-inflammatory effects of GCs are well characterized, but the molecular mechanisms are still poorly defined (Hackstein and Thomson, 2004). It has been shown that GCs regulate the immune function of T cells and DCs parallelly by the coordinated activation of the GITR ligand in T cells and the induction of GITR receptors in plasmacytoid DCs. This signalling in DCs leads to a non-canonical NF-κB-dependent induction of indolamine 2,3-dioxygenase (IDO), a key enzyme that catalyses the initial and rate-limiting step in the degradation of tryptophan and a negative modulator of lymphocyte proliferation (Grohmann et al, 2007). In addition, GCs exert an IDO-dependent protection in a model of allergic airway inflammation. These observations clearly establish DCs as valid targets of GC action.
The active form of vitamin D also has immunosuppressive effects, and numerous studies have shown that the regulation of DC immunofunctions is an important part of this profound suppressive activity. 1α,25 dihydroxy vitamin D3 highly suppresses the activation/maturation of DCs; moreover, these cells have diminished T-cell activation capacity (Penna and Adorini, 2000; Piemonti et al, 2000) (Figure 2). An in vivo murine model has also confirmed that the administration of VDR agonist negatively modulates the stimulatory capacity of DCs. Consistent with this finding, VDR-deficient mice have hypertrophy of subcutaneous lymph nodes and an elevated number of mature DCs (Griffin et al, 2001). The ways in which VDR carries out its functions, whether it interferes with positive signalling or induces suppressor molecules directly or simply inhibits DC differentiation and/or maturation is not clear. It was suggested that VDR transcriptionally represses the expression of one of the components of NF-κB (RelB), thus blocking the activation of DCs (Dong et al, 2003) (Figure 2). The extent of this mechanism is not known.
In summary, the data obtained to date suggest that the activation of GC or VDR has profound inhibitory or immunosuppressive/tolerogenic effects in DCs' immunophenotype and that these pathways are potentially amenable to therapeutic exploitation. In the next section, we discuss the function of retinoids, which show a much more complex behaviour having both repressive and activator functions in DCs.
Regulation of DCs by retinoids
It is well known that retinoids (derivatives of vitamin A and ligands of RAR and RXR receptors) exert a modulatory effect on the immune system. Vitamin A deficiency causes immune dysfunction and increases the susceptibility of an individual to infectious diseases, thus contributing to elevated child morbidity and mortality (Underwood and Arthur, 1996). Retinoids modulate the differentiation and functions of both lymphocytes and DCs. There are conflicting results on whether retinoids enhance or repress the immunogenicity of DCs. It was reported that mouse splenic DCs are less stimulatory (Bedford and Knight, 1989) on RA treatment; however, retinoid-treated Langerhans cells have an enhanced T-cell activation capacity (Meunier et al, 1994). Moreover, RA promotes the differentiation and maturation of monocytes to DC-like cells (Mohty et al, 2003) and these compounds enhance the DNA-binding activity of NK-κB, thus promoting the maturation of DCs (Geissmann et al, 2003) (Figure 2). It should be noted that retinoids also provoke apoptosis in developing DCs through RARα–RXR heterodimers (Geissmann et al, 2003). It was also described that RA pretreated DCs, if injected into tumours in mice, showed an increased accumulation in draining lymph nodes. It was concluded that the enhanced DC migration was due to the elevated matrix metalloproteinase production and the concurrently diminished expression of inhibitors of metalloproteinase (Darmanin et al, 2007). Moreover, RA-treated monocyte-derived DCs produce an elevated level of TGFβ and IL-6 and these RA-instructed DCs acquire several attributes characteristic of mucosal DCs (Saurer et al, 2007). Our laboratory also investigated the effects of activation of the RARs using synthetic retinoids on human monocyte-derived DC differentiation and found that these cells have an enhanced iNKT cell activation capacity as a consequence of the induction of CD1d (Szatmari et al, 2006a); moreover, our gene expression profiling indicates that a large number of genes are regulated by retinoids leading to the activation of multiple pathways. All-trans RA is an agonist of RAR; however, an isomeric form, 9-cis-RA, can activate both RAR and RXR (Mangelsdorf and Evans, 1995); therefore, 9-cis-RA has pleiotropic effects and can modulate the activity of several nuclear receptors in principle. Interestingly, 9-cis-RA exerts a suppressive effect on human monocyte-derived DC differentiation, probably through the activation of the PPARγ–RXR heterodimer (Zapata-Gonzalez et al, 2007). We also tested the effects of a synthetic and specific activator of RXR (LG268) on DC differentiation and obtained complex phenotypic changes, suggesting that probably multiple nuclear receptor heterodimers are affected (I Szatmari and L Nagy, unpublished results). Exposure to retinoids has a complex phenotype in DCs, and as we will demonstrate in the next section, RA has a profound effect on lymphocyte homing and activation as well.
DC as a factory of ligands for nuclear receptors
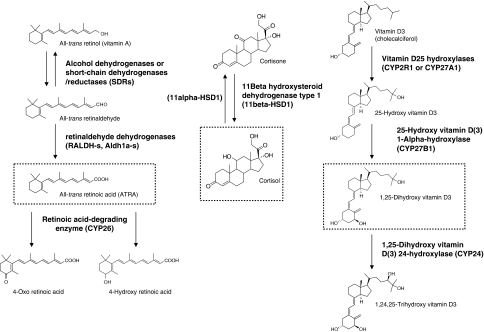
In this review, we have systematically analysed the potential function of the activation of various nuclear receptors in DC development and function. It should be emphasized that most studies used high-affinity synthetic ligands for the activation of the nuclear hormone receptors and for probing their biological functions. Two important issues need to be considered. One is whether the natural activators of these receptors have similar effects on DCs. The other is whether DCs are actively participating in the production of nuclear hormone receptor ligands or simply get those through endocrine or paracrine mechanisms. It should be noted that in the serum, a large number of compounds are present as biologically inactive precursors. However, on activation (oxidation/hydroxylation), the inactive compounds can be converted to the active forms. For example, vitamin D3 must be hydroxylated to 1,25 dihydroxy vitamin D3, which is a high-affinity ligand of VDR (Okuda et al, 1995), and vitamin A (retinol) first gets oxidized to retinaldehyde; thereafter, this compound is converted to RA, which is an agonist of RARs (Duester, 2000). Similarly, cortisone is converted to cortizole, a ligand of GR (Seckl and Walker, 2001) (Figure 3). Additionally, hydroxylation of cholesterol at the position of 27 leads to 27-hydroxy-cholesterol, a potent activator of the LXR receptors (Fu et al, 2001), and finally polyunsaturated fatty acids are oxidized by lipoxygenases and these oxidized compounds are relatively potent ligands of the PPARγ receptor (Nagy et al, 1998; Huang et al, 1999). The organic and cellular localization of these steps and the enzymes that catalyse these reactions are well characterized for some cell types. Most of these chemical transformations occur in the liver and the kidney but, significantly, some of these steps can also be found in DCs.
Figure 3.
The chemical structures and conversion steps of three nuclear hormone receptor ligand precursors (vitamin A, cortisone and vitamin D) to the active compounds. Most of these chemical reactions can occur in DCs.
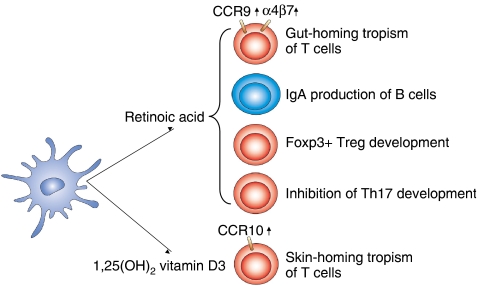
The most studied nuclear receptor ligand in the context of DCs is RA, the active form of vitamin A. In a breakthrough study, Iwata et al (2004) discovered that murine intestinal DCs produce and release RA, and this DC-derived RA instructs the gut-homing tropism of T cells by the induction of the expression of α4β7 integrin and CCR9 receptors. This study established that a DC-derived nuclear receptor ligand is able to reprogramme the local T cells and imprint T-cell tropism. Interestingly, several recent publications suggest that RA, in combination with other immunological mediators, modulates the differentiation of various lymphocyte populations in the GALT. In the intestine, RA blocks the IL-6- and TGFβ-driven induction of the pro-inflammatory IL-17-producing T (Th17) cells, but promotes the differentiation of the tolerogenic regulatory T (Treg) cells (Mucida et al, 2007). These findings were confirmed by others: intestinal DC-produced RA enhances the TGFβ-dependent conversion of peripheral T cells to Foxp3-positive Treg cells, suggesting that RA works as a mediator of gut/oral tolerance in vivo (Benson et al, 2007; Coombes et al, 2007; Sun et al, 2007). Gut-associated DCs also promote B cell gut tropism by the upregulation of the aforementioned receptors; additionally, they enhance the IL-6-dependent IgA secretion of B cells (Mora et al, 2006). Collectively, these findings underscore that DC-derived RA has a prominent function in the regulation of gut-associated immune processes. It should be mentioned that RA itself could induce the expression of α4β7 and CCR9 on T cells and thus imprint gut tropism, but for the study of the other effects of RA on lymphocytes, in most cases DC-T cell co-cultures were used. Therefore, formally, it is possible that RA also affects the immunophenotype of the DCs and that these ‘reprogrammed' DCs promote Treg development or B-cell IgA production. Another interesting point is to address which factors regulate RA production and which enzymes are responsible for the unique capacity of the intestinal DCs to produce RA. There is a remarkable selectivity of this phenotype. In vivo, only intestinal DCs of the Peyer patches and mesenteric lymph node DCs produced RA. In contrast, non-gut lymphoid tissue DCs are unable to generate RA (Iwata et al, 2004). Intestinal DCs express several enzymes that might participate in RA synthesis, the most important ones being the retinaldehyde dehydrogenases (RALDHs), which catalyse the retinaldehyde oxidation to RA (Figure 2). DCs of Peyer's patches mostly express RALDH1 (Aldh1a1); in contrast, mesenteric lymph node DCs mostly express RALDH2 (Aldh1a2) (Iwata et al, 2004). A specialized sub-population of mesenteric lymph node DCs, CD103+ cells, promote the generation of Treg cells and these DCs show an elevated expression of RALDH2 (Coombes et al, 2007). Interestingly, we observed that PPARγ-instructed human monocyte-derived DCs also produce RA; moreover, RALDH2 and RDH10 (retinol dehydrogenase 10) enzymes were upregulated in these cells (Szatmari et al, 2006a). It is well established that RALDH2 is indispensable for embryonic RA production (Niederreither et al, 1999). Significantly, a recent report suggested that RDH10 might catalyse the first oxidation step of retinol, a critical step for embryonic RA generation (Sandell et al, 2007). Further investigations are needed to define what kind of mechanisms regulate RA production in DCs and which enzyme/s is/are rate limiting for RA synthesis. Once these issues are clarified, one would be able to define the in vivo contribution of DC-derived RA signalling in the various immunological processes described earlier and also beyond those.
DCs are also able to activate other vitamins: the inactive form of vitamin D, 25-hydroxy vitamin D3, is converted to 1alpha,25 dihydroxy vitamin D3 during the ex vivo differentiation of human DCs. This conversion is catalysed by 25(OH)D3-1 alpha hydroxylase, which is present in developing DCs; in addition, this enzyme is upregulated on maturation of DCs (Fritsche et al, 2003; Hewison et al, 2003). Consistent with these findings, the production of the VDR ligand is claimed to negatively regulate the early differentiation of monocyte-derived DCs. Of note, dermal DCs are able to directly convert vitamin D3 to 1,25 dihydroxy vitamin D3 in the skin, and this active form of vitamin D instructed the local T cells to express CCR10, thus enabling them to migrate to the epidermis (Sigmundsdottir et al, 2007). Remarkably, these results suggested that similar to RA, which elicits gut-homing specificity of T cells, the active form of vitamin D imprints skin-homing specificity of T cells (Figure 4).
Figure 4.
DC-produced nuclear receptor ligands modulate T-cell development and homing. Intestinal DCs produce the active form of vitamin A (retinoic acid). In contrast, skin DCs could secrete the active form of vitamin D (1,25 dihydroxy vitamin D3). Retinoic acid (RA) imprints gut-homing tropism of lymphocytes by inducing α4β7 integrin and CCR9 receptor; in addition, RA contributes to B-cell IgA production; moreover, this compound promotes FoxP3+ Treg cell differentiation but blocks Th17 T-cell development. 1,25 Dihydroxy vitamin D3 primes skin-homing tropism of T cells by inducing CCR10.
Human monocyte-derived DCs are also able to convert the inactive cortisone to the high-affinity GR ligand, cortisole as a result of the upregulation of 11beta hydroxysteroid reductase. In addition, DC differentiation is inhibited by the administration of physiological concentration of cortisone, suggesting that this pathway enhances the GC-dependent negative modulation of DC development (Freeman et al, 2005). Finally, as far as the PPARγ ligand is concerned, we observed that during monocyte-derived DC development the bona fide PPARγ targeting the FABP4/aP2 gene is upregulated, especially when we culture the cells in human serum instead of FBS (Szatmari et al, 2004). We extended this finding and observed that most of these genes whose expression was upregulated on synthetic PPARγ ligand treatment also showed an elevated expression if DCs were cultured in human serum-containing medium (Szatmari et al, 2007). These results suggested that in the presence of human serum, the PPARγ ligands are generated/accumulated in DCs. Further experiments are warranted to identify the PPARγ activators. Indeed, a recent paper suggested that lysophosphatidic acid and cardiolipin are abundant components of the human serum and that these lipid species might contribute to the activation of PPARγ (Leslie et al, 2008).
These results underscore the notion that, remarkably, DCs are actively participating in the production of several ligands of nuclear receptors. The production of ligands appears to be tightly controlled, however. Moreover, some of these ligands are likely to be released and are able to locally modulate the function of other immune cell types.
Concluding remarks: DCs as potential therapeutic targets
The functional characterization of DC is an intensively investigated area in immunology. DCs are indispensable for the initiation of primary immune response, but now it is well established that these cells are able to orchestrate the entire immune response, including the regulation of peripheral immune tolerance. Therefore, it is of importance to identify and characterize the regulatory processes underpinning these changes. It is becoming increasingly evident that members of the nuclear hormone receptor superfamily are involved in the regulation of DC biology. This group of transcription factors also represents a gateway to manipulating DCs for therapeutic use. In clinical practice, tumour antigen-loaded DCs are intensively investigated tools to elicit antitumour immune response. Ex vivo differentiated DCs may be loaded with tumour antigens and injected back to patients, or they can be used for ex vivo expansion of antitumour lymphocytes. Improving anticancer immunotherapies appear to largely depend on how DC immunogenicity, survival and migration are regulated, and thus it is very important to find ways to modulate antigen presenting, migratory capacity and viability of DCs (Nencioni et al, 2008). There are several ways to manipulate DCs ex vivo; administration of cytokine cocktails or other signal molecules can modify the DC function and activation state. As we have demonstrated in this review, natural or synthetic ligands of nuclear hormone receptors can also modulate DC functions. In the future, it will be important to explore and exploit the benefit of ligand treatment to modulate DC immunogenicity in vivo. An inverse relationship should also be considered. The metabolic state of the patient might affect his/her immune responses. DCs express several lipid sensor nuclear hormone receptors (i.e. PPARγ LXRα). Thus, in patients with an altered lipid profile, these receptors might be overactivated and this can adversely modify the immune phenotype of their DCs. As an example, we have described that serum lipoproteins skewed DC development towards a CD1a− DC phenotype, which has a distinct cytokine and chemokine production profile (Gogolak et al, 2007).
Until now, most of the studies on nuclear receptors used ex vivo DC models and only a few reports investigated tissue-specific nuclear receptor knockout models (Griffin and Kumar, 2003; Klotz et al, 2007). In the future, it will be of great importance to generate DC-specific nuclear receptor deletion models. These models will almost certainly prove to be helpful to define the receptor specificity of the effects of receptor agonists. In addition, these systems will help to define the in vivo function of these receptors in DCs. The in vivo approaches might also clarify some of the controversies that still exist in the field. However, as we have emphasized concerning human immunotherapy, ex vivo DC models are also used. Thus, it is important to characterize the in vitro effects of ligands of nuclear hormone receptors, not in small part, because there are major differences between mice and humans regarding DC functions, and murine models might not always be predictive of human responses. DCs are a very heterogeneous cell population (Villadangos and Schnorrer, 2007), and until now most data were derived from human monocyte-derived DCs. In the future, it would also be important to define the function of nuclear receptors/ligands on the various subtypes of DCs and DC precursors. And, finally, activators of nuclear receptors are widely used in clinical practice, for example, ligands of the PPARγ (rosiglitazone and pioglitazone) are applied to treat type II diabetes (Willson et al, 2001); in addition, GCs are frequently used as immunosuppressive agents. Defining the exact functions of these activators in various immune cell types could help in exploiting their potential for therapeutic intervention against various immunological diseases.
Acknowledgments
The study in the author's laboratory is supported by a grant from the National Research and Technology Office RET-06/2004 to LN and another grant from the Hungarian Scientific Research Fund (OTKA no. NK72730). LN is an International Scholar of the Howard Hughes Medical Institute and holds a Wellcome Trust Senior Research Fellowship in Biomedical Sciences in Central Europe no. 074021.
References
- Abe M, Thomson AW (2003) Influence of immunosuppressive drugs on dendritic cells. Transpl Immunol 11: 357–365 [DOI] [PubMed] [Google Scholar]
- Angeli V, Hammad H, Staels B, Capron M, Lambrecht BN, Trottein F (2003) Peroxisome proliferator-activated receptor gamma inhibits the migration of dendritic cells: consequences for the immune response. J Immunol 170: 5295–5301 [DOI] [PubMed] [Google Scholar]
- Angeli V, Llodra J, Rong JX, Satoh K, Ishii S, Shimizu T, Fisher EA, Randolph GJ (2004) Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity 21: 561–574 [DOI] [PubMed] [Google Scholar]
- Appel S, Mirakaj V, Bringmann A, Weck MM, Grunebach F, Brossart P (2005) PPAR-gamma agonists inhibit toll-like receptor mediated activation of dendritic cells via the MAP kinase and NF-kappaB pathways. Blood 106: 3888–3894 [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392: 245–252 [DOI] [PubMed] [Google Scholar]
- Barish GD, Narkar VA, Evans RM (2006) PPAR delta: a dagger in the heart of the metabolic syndrome. J Clin Invest 116: 590–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford PA, Knight SC (1989) The effect of retinoids on dendritic cell function. Clin Exp Immunol 75: 481–486 [PMC free article] [PubMed] [Google Scholar]
- Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ (2007) All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med 204: 1765–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobryshev YV, Lord RS (1995) S-100 positive cells in human arterial intima and in atherosclerotic lesions. Cardiovasc Res 29: 689–696 [PubMed] [Google Scholar]
- Brigl M, Brenner MB (2004) CD1: antigen presentation and T cell function. Annu Rev Immunol 22: 817–890 [DOI] [PubMed] [Google Scholar]
- Chen Q, Ross AC (2007) Retinoic acid regulates CD1d gene expression at the transcriptional level in human and rodent monocytic cells. Exp Biol Med (Maywood) 232: 488–494 [PMC free article] [PubMed] [Google Scholar]
- Cools N, Ponsaerts P, Van Tendeloo VF, Berneman ZN (2007) Balancing between immunity and tolerance: an interplay between dendritic cells, regulatory T cells, and effector T cells. J Leukoc Biol 82: 1365–1374 [DOI] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F (2007) A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-{beta} and reti. J Exp Med 204: 1757–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutant F, Perrin-Cocon L, Agaugue S, Delair T, Andre P, Lotteau V (2002) Mature dendritic cell generation promoted by lysophosphatidylcholine. J Immunol 169: 1688–1695 [DOI] [PubMed] [Google Scholar]
- Darmanin S, Chen J, Zhao S, Cui H, Shirkoohi R, Kubo N, Kuge Y, Tamaki N, Nakagawa K, Hamada J, Moriuchi T, Kobayashi M (2007) All-trans retinoic acid enhances murine dendritic cell migration to draining lymph nodes via the balance of matrix metalloproteinases and their inhibitors. J Immunol 179: 4616–4625 [DOI] [PubMed] [Google Scholar]
- Dong X, Craig T, Xing N, Bachman LA, Paya CV, Weih F, McKean DJ, Kumar R, Griffin MD (2003) Direct transcriptional regulation of RelB by 1alpha,25-dihydroxyvitamin D3 and its analogs: physiologic and therapeutic implications for dendritic cell function. J Biol Chem 278: 49378–49385 [DOI] [PubMed] [Google Scholar]
- Dubrac S, Stoitzner P, Pirkebner D, Elentner A, Schoonjans K, Auwerx J, Saeland S, Hengster P, Fritsch P, Romani N, Schmuth M (2007) Peroxisome proliferator-activated receptor-alpha activation inhibits Langerhans cell function. J Immunol 178: 4362–4372 [DOI] [PubMed] [Google Scholar]
- Duester G (2000) Families of retinoid dehydrogenases regulating vitamin A function: production of visual pigment and retinoic acid. Eur J Biochem 267: 4315–4324 [DOI] [PubMed] [Google Scholar]
- Faveeuw C, Fougeray S, Angeli V, Fontaine J, Chinetti G, Gosset P, Delerive P, Maliszewski C, Capron M, Staels B, Moser M, Trottein F (2000) Peroxisome proliferator-activated receptor gamma activators inhibit interleukin-12 production in murine dendritic cells. FEBS Lett 486: 261–266 [DOI] [PubMed] [Google Scholar]
- Freeman L, Hewison M, Hughes SV, Evans KN, Hardie D, Means TK, Chakraverty R (2005) Expression of 11beta-hydroxysteroid dehydrogenase type 1 permits regulation of glucocorticoid bioavailability by human dendritic cells. Blood 106: 2042–2049 [DOI] [PubMed] [Google Scholar]
- Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M (2003) Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood 102: 3314–3316 [DOI] [PubMed] [Google Scholar]
- Fu X, Menke JG, Chen Y, Zhou G, MacNaul KL, Wright SD, Sparrow CP, Lund EG (2001) 27-Hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J Biol Chem 276: 38378–38387 [DOI] [PubMed] [Google Scholar]
- Geissmann F, Revy P, Brousse N, Lepelletier Y, Folli C, Durandy A, Chambon P, Dy M (2003) Retinoids regulate survival and antigen presentation by immature dendritic cells. J Exp Med 198: 623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyeregger R, Zeyda M, Bauer W, Kriehuber E, Saemann MD, Zlabinger GJ, Maurer D, Stulnig TM (2007) Liver X receptors regulate dendritic cell phenotype and function through blocked induction of the actin-bundling protein fascin. Blood 109: 4288–4295 [DOI] [PubMed] [Google Scholar]
- Gogolak P, Rethi B, Szatmari I, Lanyi A, Dezso B, Nagy L, Rajnavolgyi E (2007) Differentiation of CD1a− and CD1a+ monocyte-derived dendritic cells is biased by lipid environment and PPARgamma. Blood 109: 643–652 [DOI] [PubMed] [Google Scholar]
- Gosset P, Charbonnier AS, Delerive P, Fontaine J, Staels B, Pestel J, Tonnel AB, Trottein F (2001) Peroxisome proliferator-activated receptor gamma activators affect the maturation of human monocyte-derived dendritic cells. Eur J Immunol 31: 2857–2865 [DOI] [PubMed] [Google Scholar]
- Griffin MD, Kumar R (2003) Effects of 1alpha,25(OH)2D3 and its analogs on dendritic cell function. J Cell Biochem 88: 323–326 [DOI] [PubMed] [Google Scholar]
- Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R (2001) Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci USA 98: 6800–6805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohmann U, Volpi C, Fallarino F, Bozza S, Bianchi R, Vacca C, Orabona C, Belladonna ML, Ayroldi E, Nocentini G, Boon L, Bistoni F, Fioretti MC, Romani L, Riccardi C, Puccetti P (2007) Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat Med 13: 579–586 [DOI] [PubMed] [Google Scholar]
- Hackstein H, Thomson AW (2004) Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol 4: 24–34 [DOI] [PubMed] [Google Scholar]
- Hammad H, de Heer HJ, Soullie T, Angeli V, Trottein F, Hoogsteden HC, Lambrecht BN (2004) Activation of peroxisome proliferator-activated receptor-gamma in dendritic cells inhibits the development of eosinophilic airway inflammation in a mouse model of asthma. Am J Pathol 164: 263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond KJ, Kronenberg M (2003) Natural killer T cells: natural or unnatural regulators of autoimmunity? Curr Opin Immunol 15: 683–689 [DOI] [PubMed] [Google Scholar]
- Harizi H, Gualde N (2005) The impact of eicosanoids on the crosstalk between innate and adaptive immunity: the key roles of dendritic cells. Tissue Antigens 65: 507–514 [DOI] [PubMed] [Google Scholar]
- Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG, Kilby MD, Moss PA, Chakraverty R (2003) Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol 170: 5382–5390 [DOI] [PubMed] [Google Scholar]
- Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, Witztum JL, Funk CD, Conrad D, Glass CK (1999) Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature 400: 378–382 [DOI] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY (2004) Retinoic acid imprints gut-homing specificity on T cells. Immunity 21: 527–538 [DOI] [PubMed] [Google Scholar]
- Klotz L, Dani I, Edenhofer F, Nolden L, Evert B, Paul B, Kolanus W, Klockgether T, Knolle P, Diehl L (2007) Peroxisome proliferator-activated receptor gamma control of dendritic cell function contributes to development of CD4+ T cell anergy. J Immunol 178: 2122–2131 [DOI] [PubMed] [Google Scholar]
- Le Naour F, Hohenkirk L, Grolleau A, Misek DE, Lescure P, Geiger JD, Hanash S, Beretta L (2001) Profiling changes in gene expression during differentiation and maturation of monocyte-derived dendritic cells using both oligonucleotide microarrays and proteomics. J Biol Chem 276: 17920–17931 [DOI] [PubMed] [Google Scholar]
- Lefebvre P, Chinetti G, Fruchart JC, Staels B (2006) Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest 116: 571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie DS, Dascher CC, Cembrola K, Townes MA, Hava DL, Hugendubler LC, Mueller E, Fox L, Roura-Mir C, Moody DB, Vincent MS, Gumperz JE, Illarionov PA, Besra GS, Reynolds CG, Brenner MB (2008) Serum lipids regulate dendritic cell CD1 expression and function. Immunology (doi:10.1111/j.1365-2567.2008.02842.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Evans RM (1995) The RXR heterodimers and orphan receptors. Cell 83: 841–850 [DOI] [PubMed] [Google Scholar]
- Meunier L, Bohjanen K, Voorhees JJ, Cooper KD (1994) Retinoic acid upregulates human Langerhans cell antigen presentation and surface expression of HLA-DR and CD11c, a beta 2 integrin critically involved in T-cell activation. J Invest Dermatol 103: 775–779 [DOI] [PubMed] [Google Scholar]
- Mohty M, Morbelli S, Isnardon D, Sainty D, Arnoulet C, Gaugler B, Olive D (2003) All-trans retinoic acid skews monocyte differentiation into interleukin-12-secreting dendritic-like cells. Br J Haematol 122: 829–836 [DOI] [PubMed] [Google Scholar]
- Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH (2006) Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314: 1157–1160 [DOI] [PubMed] [Google Scholar]
- Moser M, De Smedt T, Sornasse T, Tielemans F, Chentoufi AA, Muraille E, Van Mechelen M, Urbain J, Leo O (1995) Glucocorticoids down-regulate dendritic cell function in vitro and in vivo. Eur J Immunol 25: 2818–2824 [DOI] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H (2007) Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317: 256–260 [DOI] [PubMed] [Google Scholar]
- Nagler-Anderson C, Shi HN (2001) Peripheral nonresponsiveness to orally administered soluble protein antigens. Crit Rev Immunol 21: 121–131 [PubMed] [Google Scholar]
- Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM (1998) Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 93: 229–240 [DOI] [PubMed] [Google Scholar]
- Nencioni A, Grunebach F, Schmidt SM, Muller MR, Boy D, Patrone F, Ballestrero A, Brossart P (2008) The use of dendritic cells in cancer immunotherapy. Crit Rev Oncol Hematol 65: 191–199 [DOI] [PubMed] [Google Scholar]
- Nencioni A, Grunebach F, Zobywlaski A, Denzlinger C, Brugger W, Brossart P (2002) Dendritic cell immunogenicity is regulated by peroxisome proliferator-activated receptor gamma. J Immunol 169: 1228–1235 [DOI] [PubMed] [Google Scholar]
- Ni K, O'Neill HC (1997) The role of dendritic cells in T cell activation. Immunol Cell Biol 75: 223–230 [DOI] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dolle P, Chambon P (1999) Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet 21: 444–448 [DOI] [PubMed] [Google Scholar]
- Norbury CC (2006) Drinking a lot is good for dendritic cells. Immunology 117: 443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K, Usui E, Ohyama Y (1995) Recent progress in enzymology and molecular biology of enzymes involved in vitamin D metabolism. J Lipid Res 36: 1641–1652 [PubMed] [Google Scholar]
- Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK (2005) A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature 437: 759–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna G, Adorini L (2000) 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol 164: 2405–2411 [DOI] [PubMed] [Google Scholar]
- Piemonti L, Monti P, Allavena P, Sironi M, Soldini L, Leone BE, Socci C, Di Carlo V (1999) Glucocorticoids affect human dendritic cell differentiation and maturation. J Immunol 162: 6473–6481 [PubMed] [Google Scholar]
- Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, Allavena P, Di Carlo V (2000) Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol 164: 4443–4451 [DOI] [PubMed] [Google Scholar]
- Rea D, van Kooten C, van Meijgaarden KE, Ottenhoff TH, Melief CJ, Offringa R (2000) Glucocorticoids transform CD40-triggering of dendritic cells into an alternative activation pathway resulting in antigen-presenting cells that secrete IL-10. Blood 95: 3162–3167 [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A (1994) Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 179: 1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell LL, Sanderson BW, Moiseyev G, Johnson T, Mushegian A, Young K, Rey JP, Ma JX, Staehling-Hampton K, Trainor PA (2007) RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev 21: 1113–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurer L, McCullough KC, Summerfield A (2007) In vitro induction of mucosa-type dendritic cells by all-trans retinoic acid. J Immunol 179: 3504–3514 [DOI] [PubMed] [Google Scholar]
- Savina A, Amigorena S (2007) Phagocytosis and antigen presentation in dendritic cells. Immunol Rev 219: 143–156 [DOI] [PubMed] [Google Scholar]
- Schaible UE, Winau F, Sieling PA, Fischer K, Collins HL, Hagens K, Modlin RL, Brinkmann V, Kaufmann SH (2003) Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat Med 9: 1039–1046 [DOI] [PubMed] [Google Scholar]
- Seckl JR, Walker BR (2001) Minireview: 11beta-hydroxysteroid dehydrogenase type 1––a tissue-specific amplifier of glucocorticoid action. Endocrinology 142: 1371–1376 [DOI] [PubMed] [Google Scholar]
- Shortman K, Naik SH (2007) Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol 7: 19–30 [DOI] [PubMed] [Google Scholar]
- Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC (2007) DCs metabolize sunlight-induced vitamin D3 to ‘program' T cell attraction to the epidermal chemokine CCL27. Nat Immunol 8: 285–293 [DOI] [PubMed] [Google Scholar]
- Steinman R, Cohn Z (1973) Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med 137: 1142–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y (2007) Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 204: 1775–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari I, Gogolak P, Im JS, Dezso B, Rajnavolgyi E, Nagy L (2004) Activation of PPARgamma specifies a dendritic cell subtype capable of enhanced induction of iNKT cell expansion. Immunity 21: 95–106 [DOI] [PubMed] [Google Scholar]
- Szatmari I, Pap A, Ruhl R, Ma JX, Illarionov PA, Besra GS, Rajnavolgyi E, Dezso B, Nagy L (2006a) PPARgamma controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J Exp Med 203: 2351–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari I, Torocsik D, Agostini M, Nagy T, Gurnell M, Barta E, Chatterjee K, Nagy L (2007) PPARgamma regulates the function of human dendritic cells primarily by altering lipid metabolism. Blood 110: 3271–3280 [DOI] [PubMed] [Google Scholar]
- Szatmari I, Vamosi G, Brazda P, Balint BL, Benko S, Szeles L, Jeney V, Ozvegy-Laczka C, Szanto A, Barta E, Balla J, Sarkadi B, Nagy L (2006b) Peroxisome proliferator-activated receptor gamma-regulated ABCG2 expression confers cytoprotection to human dendritic cells. J Biol Chem 281: 23812–23823 [DOI] [PubMed] [Google Scholar]
- Tsuji M (2006) Glycolipids and phospholipids as natural CD1d-binding NKT cell ligands. Cell Mol Life Sci 63: 1889–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood BA, Arthur P (1996) The contribution of vitamin A to public health. FASEB J 10: 1040–1048 [PubMed] [Google Scholar]
- van den Elzen P, Garg S, Leon L, Brigl M, Leadbetter EA, Gumperz JE, Dascher CC, Cheng TY, Sacks FM, Illarionov PA, Besra GS, Kent SC, Moody DB, Brenner MB (2005) Apolipoprotein-mediated pathways of lipid antigen presentation. Nature 437: 906–910 [DOI] [PubMed] [Google Scholar]
- van Etten E, Mathieu C (2005) Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol 97: 93–101 [DOI] [PubMed] [Google Scholar]
- Villadangos JA, Schnorrer P (2007) Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol 7: 543–555 [DOI] [PubMed] [Google Scholar]
- Willcox BE, Willcox CR, Dover LG, Besra G (2007) Structures and functions of microbial lipid antigens presented by CD1. Curr Top Microbiol Immunol 314: 73–110 [DOI] [PubMed] [Google Scholar]
- Willson TM, Lambert MH, Kliewer SA (2001) Peroxisome proliferator-activated receptor gamma and metabolic disease. Annu Rev Biochem 70: 341–367 [DOI] [PubMed] [Google Scholar]
- Wu L, Liu YJ (2007) Development of dendritic-cell lineages. Immunity 26: 741–750 [DOI] [PubMed] [Google Scholar]
- Zapata-Gonzalez F, Rueda F, Petriz J, Domingo P, Villarroya F, de Madariaga A, Domingo JC (2007) 9-cis-Retinoic acid (9cRA), a retinoid X receptor (RXR) ligand, exerts immunosuppressive effects on dendritic cells by RXR-dependent activation: inhibition of peroxisome proliferator-activated receptor gamma blocks some of the 9cRA activities, and precludes them to mature phenotype development. J Immunol 178: 6130–6139 [DOI] [PubMed] [Google Scholar]
- Zelcer N, Tontonoz P (2006) Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest 116: 607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke M, Hieronymus T (2006) Towards an understanding of the transcription factor network of dendritic cell development. Trends Immunol 27: 140–145 [DOI] [PubMed] [Google Scholar]