Abstract
Past research has concentrated on the stress system and personality in order to explain the variance found in cognitive performance in old age. A growing body of research is starting to focus on genetic polymorphism as an individual difference factor to explain the observed heterogeneity in cognitive function. While the functional mechanism is still under investigation, polymorphism of the 5-HT2A receptor gene (−1438A/G) has been linked to certain behavioral and physiological outcomes, including cortisol secretion, the expression of certain personality traits, and memory performance. It was the goal of the present study to investigate the association between the −1438A/G polymorphism and stress hormone secretion, stress-related psychological measures, and cognitive performance in a group of adults between the ages of 50 and 65. To examine these associations, 101 middle-aged adults were recruited, completed a battery of psychological questionnaires and were administered a battery of cognitive tasks that assess frontal lobe and hippocampal function. Basal and stress-reactive salivary cortisol levels were collected, at home and in the laboratory. Analyses on psychological measures showed that participants with the GG genotype reported significantly higher levels of neuroticism compared to the AG group and higher levels of depression and more emotion-based coping strategies compared to both the AG and AA group. In terms of cortisol secretion, the AA genotype was related to a significantly higher awakening cortisol response (ACR) compared to the AG and GG group and the GG genotype group displayed a greater increase in cortisol secretion following a psychosocial stressor compared to the two other groups. On measures of cognitive performance, the AA genotype group performed significantly better on a test of declarative memory and selective attention compared to the other two groups. Together, these results suggest that carriers of the GG genotype are more susceptible to low mood and display a greater potential for an overactive stress system, which may influence cognitive function in later years.
Keywords: serotonin, 5-HTR2A polymorphism, cortisol, cognition
Introduction
With an aging population on the rise, researchers have attempted to understand what makes an individual vulnerable or resilient to cognitive decline with age. Indeed, numerous studies have attempted to explain the heterogeneity observed in cognitive function within the aging population. Thus far, two factors have received considerable attention in explaining the variance in cognitive performance, namely the stress system and personality traits. Specifically, studies have shown that overactivity of the hypothalamic–pituitary–adrenal (HPA) axis, evidenced by elevated levels of daily glucocorticoid (GC) secretion, is linked to poorer cognitive performance in both animals and in humans (Issa et al., 1990; Landfield et al., 1978; Lupien et al., 1994). In addition, personality factors, including low self-esteem and high neuroticism have been linked to poorer cognitive performance in older adults (Crowe et al., 2006; Pruessner et al., 2004; Wilson et al., 2005). While these two individual difference factors may provide insight into cognitive variability in old age, recent studies have started to examine candidate genes of specific biological systems that may not only explain variability in cognitive function, but further underlie variance in HPA activity and personality. One biological system under investigation is the serotonergic system (5-HT).
The 5-HT system is a widely investigated neurotransmitter system that has been found to play an important role in various sensory, motor, and cognitive processes. The 5-HT system has not only been linked to the expression of stress-related disorders and personality traits (Moresco, 2002; Walderhaug et al., 2002), but has also been found to play a role in the regulation of the HPA axis (Dinan, 1996; Zhang et al., 2002). Overall, studies have shown that depletion of 5-HT is related to increased HPA activity (Hood et al., 2006; Pruessner et al., 2004) and the expression of certain personality traits, including lower impulse control, increased aggression-hostility, and increased neuroticism (Cleare and Bond, 1997; Depue, 1995; Flory et al., 2004; Hennig et al., 2005; Manuck et al., 1998). Within the 5-HT system, the 5-HT2A receptor has been widely investigated.
Studies have reported that 5-HT2A receptors are located on neuroendocrine cells in the hypothalamus and modulate the neuroendocrine response and reactivity to novelty (Greyer, 1996; Rittenhouse et al., 1994; Zhang et al., 2002). It has been shown that stimulation of 5-HT2A receptors induces HPA activity, evidenced by increased GC secretion (Bagdy, 1996; Zhang et al., 2002). Also, lower 5-HT2A binding has been implicated in certain personality traits, namely harm avoidance and hopelessness (Moresco et al., 2002; van Heeringen et al., 2003). Thus, studies have shown that 5-HT2A receptors are implicated in the individual difference factors that have been investigated in explaining cognitive variability in old age.
Apart from their role in personality and HPA activity, 5-HT2A receptors have been implicated in cognitive processing and pathological cognitive function (Hirano and Fibiger, 1995; Lai et al., 2005; Versijpt et al., 2003). Specifically, 5-HT2A receptors are important for cognitive function as these receptors are found to facilitate cholinergic release (Buhot, 1997). Furthermore, 5-HT2A receptors are located in the prefrontal cortex and in the hippocampus, two important brain regions for learning and memory. In addition, studies have shown that 5-HT2A receptor binding decreases in a variety of brain regions with age, reflecting the loss of specific 5-HT receptors (Rosier et al., 1996; Sheline et al., 2002). In fact, a marked decrease in 5-HT2A receptors has been found in patients suffering from dementia (Versijpt et al., 2003). Thus, it appears that the 5-HT2A receptor not only plays an important role in personality and HPA activity, but also underlies cognitive function. From this, it may be postulated that individual differences in the 5-HT2A system may explain the expression of certain personality traits as well as individual differences in HPA activity and cognitive performance in later years.
A common polymorphism of the 5-HT2A receptor gene (5-HTR2A) that has been identified is the −1438A/G gene in the promoter region on chromosome 13q14-q21. Studies have shown that variations in the −1438 SNP modulates 5-HTR2A promoter activity, with the AA genotype associated with higher 5-HT2A gene expression in cell lines that endogenously express 5-HT2A (Parsons et al., 2004). This genotype effect on 5-HTR2A expression may differentially facilitate or modulate other interacting biological systems such as the neuroendocrine system. Indeed, a study investigating the impact of the −1438A/G polymorphism on the stress system reported that carriers of the G allele were more likely to exhibit cortisol non-suppression following the dexamethasone test (Rosmond et al., 2002). Non-suppression of cortisol following dexamethasone treatment has been observed in depressed patient populations and may be an indication of chronic stress or an overactive HPA axis (Rush et al., 1996; Westrin and Nimeus, 2003). While this study showed potential for the −1438A/G gene to influence HPA activity, no studies have directly assessed the association between this polymorphism and basal diurnal cortisol secretion, or reactive cortisol levels following a psychosocial stressor, two measures that are found to correlate with cognitive performance in old age (Lupien et al., 1994, 1997).
A number of association studies have also reported a relationship between the 5-HTR2A promoter polymorphism and stress-related psychological measures. Coinciding with the aforementioned HPA findings, presence of the G allele of the −1438A/G gene has been linked to depressive symptomatology (Chee et al., 2001; Choi et al., 2004). Also, the GG genotype has been related to reports of greater reward dependence, more social introversion, fewer friends, and a tendency for higher neuroticism (Golimbet et al., 2004; Rybakowski et al., 2006; Tochigi et al., 2005). In a sample of anorexic females, it was reported that the AA genotype group displayed lower reward dependence than the GG group and lower harm avoidance than the AG group (Rybakowski et al., 2006). Interestingly, reward dependence and harm avoidance have both been found to be greater in patients with mood disorders than healthy controls (Ampollini et al., 1999). Thus, genetic variance in this gene appears to be associated with personality traits and behaviors that are related to stress-induced negative affect.
To date, only one study has assessed this polymorphism in relation to cognitive function. Reynolds et al. (2006) examined the relationship between the −1438A/G polymorphism and cognitive performance in a 13-year longitudinal study. While no group differences were found for tests that assessed immediate and delay recall of names and faces, working memory, and attention, it was reported that the GG genotype group outperformed the two other groups on a picture memory task. This group difference was observed at baseline and at the 13-year testing period. While a genotype effect was observed, the outcome is somewhat surprising given the aforementioned studies suggesting that the G allele is associated with HPA overactivity and personality traits that would commonly be associated with poorer cognitive performance. Indeed, additional studies are required to assess the influence of this genetic variant on cognitive function.
Given the established role of the 5-HT2A receptor in HPA activity, personality, and cognition, and the potential underlying role of the −1438A/G polymorphism on these parameters, it was the goal of the present study to evaluate the relationship between the −1438A/G polymorphism and cognitive function, and the relationship between this genetic variant and stress-related indices that are associated with cognitive function, including cortisol secretion and personality in healthy middle-aged adults. We hypothesized that genetic variation in the 5-HT2A receptor affects HPA activity, estimated by basal salivary cortisol secretion and reactive salivary cortisol levels following a psychosocial stressor. It was also postulated that this polymorphism is associated with stress-related personality traits and cognitive performance, both of which are related to HPA activity.
Materials and Method
Participants
One hundred and one male and female middle-aged adults were recruited for the present study. The mean age of the sample was 57.91 (SE = 0.37) years, 77% of the sample was female, 88% were Caucasian, and 43% attained an education level above college (or Cegep in Quebec). The sample was cognitively intact as measured by the mini-mental state exam (M = 29.49, SE = 0.10).
Procedure
Prior to commencing the study, all participants were screened on the telephone for diagnosed Axis I disorders and medications that may alter the HPA axis (e.g., GCs and antidepressants). Also, individuals who smoked more than ten cigarettes a day, which may alter stress hormone secretion, were excluded from the present study.
Participants who passed the telephone screening visited the Douglas Mental Health University Institute on two separate days and were asked to sample their saliva at home for three consecutive days between the two visits. Before beginning the study, all participants read and signed an informed consent for their participation in the study. During the first visit, participants gave blood for genotype analyses and medical analyses to ensure that the participant was in good health and to ensure the absence of a thyroid dysfunction, which may influence HPA activity. Following the blood draw, participants were seen by a medical doctor to ensure overall good health. Participants were then administered a battery of neurocognitive tasks and were asked to complete a booklet of questionnaires.
At home, participants were asked to sample their saliva five times a day for three consecutive days. Samples were taken at the following five time points: upon awakening, 30 minutes after awakening, 14:00, 16:00, and before bedtime. Participants were asked to store samples in their freezer until their second visit to the Research Institute.
During the second visit, participants were exposed to the Trier Social Stress Test (TSST), which is a validated psychosocial stressor that entails giving a five-minute speech to an audience, followed by a five-minute mental arithmetic task (Kirschbaum et al., 1993). Saliva samples were collected pre- and post-TSST, at 10-minute intervals (baseline, pre-anticipation, pre-TSST, post-TSST, 5, 15, 25, 35, and 45 minute post-TSST).
Protocol for this study was approved by the Ethics Board of the Douglas Mental Health University Institute (#03/40).
Cognition and psychological questionnaires
The neurocognitive battery consisted of tests that assess hippocampal and frontal lobe function, including
Word-pair task: A declarative memory task that required participants to recall related and non-related word pairs immediately after word-pair presentation (immediate recall) and then 20 minutes later (delay recall). Total immediate and total delay recall scores for related and non-related word pairs were calculated for analyses.
Digit span task: Participants were asked to repeat a series of digits in forward and reversed sequence. Total scores were calculated for forward span and reverse span for analyses.
Verbal fluency: Participants were required to name as many words beginning with a certain letter (phonetic fluency) and as many objects from a given category (category fluency). Total score on phonetic and category fluency were calculated for analyses.
Selective attention: Participants were required to respond to the presence or absence of a target stimulus, which was or was not presented among 2–10 distracters. Reaction time was calculated and slope of reaction time was calculated for stimulus present and stimulus absent conditions.
Psychological questionnaires: The administered psychological questionnaires included the neuroticism subscale of the NEO-Five Factor Inventory (McCrae and Costa, 1986), the Rosenberg Self-Esteem Scale (Rosenberg, 1965), the Geriatric Depression Scale (Yesavage et al., 1982), and the Ways of Coping Scale (Vitaliano et al., 1985). For analyses, a total score was calculated for each subtest of the Ways of Coping Scale, and for each of the remaining questionnaires.
Genotyping
Genomic DNA was extracted from 200 μL of white blood cells using the QIAamp blood Mini Kit according to manufacturer's instructions (QIAGEN Inc., Valencia, CA, USA). The Taq DNA Polymerase Kit was used to perform PCR (QIAGEN Inc.). A 50 μL of reaction mixture was used, containing 0.5 μM of each primer, 2.5 units of Taq polymerase, 20 mM dNTP mix, 25 mM MgCl2, and 250 μg of template DNA. PCR conditions included a denaturation step of 95°C for 10 minute, followed by 95°C for 1.5 minute, 58°C for 2.5 minute, 72°C for 3 minute and a final extension at 72°C for 10 minute. The primers for the PCR yielded an amplification of 53 bp.
For the development of pyrosequencing assay, the sequencing primer was designed using the PSQ assay design 1.06 software (Biotage, Uppsala, Sweden). The primers are listed in Table 1. Following the aforementioned PCR procedure, the PCR product was immobilized onto streptavidin-coated beads by mixing 25–30 μL of amplification product, 3 μL of streptavidin-coated Sepharose HP beads (GE, Healthcare), 40 μL of binding buffer, and 20 μL of Milli-Q water. The mixture was then agitated for 10 minute at 14 000 rpm on a Labnet Orbit P2 digital shaker (ICS BioExpress). The beads were then captured by the Vacuum Prep Tool (Biotage) and single-stranded DNA was generated by passing the captured beads through three steps: 70% ethanol, 0.2 M NaOH, and then a washing buffer. The beds were then released in a PSQ plate pre-filled with 0.3 μM sequencing primer in 40 μL of annealing buffer. The plate was then incubated at 80°C for 2 minute using a Dry Analog Heat Block (VWR), and then left to cool at room temperature to allow for annealing of the sequencing primer. Sequencing was done using a PSQ96 Reagent Kit and a PSQ 96MA system (Biotage). Results were analyzed using the PSQ96MA software v.1. (Biotage)
Table 1.
Primer sequences for genotyping of the −1438 A/G polymorphism by pyrosequencing.
| Primer sequencing 5′− 3 | Annealing temperature (° C) |
|---|---|
| F: GTCAGGTGCTGGAACACCTAGCC | 60 |
| R: Biotin-TAGGTCTTGTGGTTCAGAACG | 60 |
| Sequencing primer: TCATGACTTCCCCCATGC |
Cortisol measures
For assessment of salivary cortisol levels, Salivettes (Sarstedt, Ville St. Laurent) were utilized, which consists of placing a cotton swab in the mouth for 2 minute until fully saturated and then placing the cotton into a tube. All salivary cortisol samples were maintained at −20°C until cortisol determinations. Salivary cortisol concentrations were assayed in Dr. Dominique Walker's laboratory at the Douglas Mental Health University Institute by radioimmunoassay using a kit from DSL (Diagnostic System Laboratories, Inc, Texas, USA) with small modifications. Total binding and non-specific binding typically range between 47–63 and 0.5–1.5%, respectively. The intra- and inter-assay coefficient of variation for these analyses are 4.6 and 5%, respectively. The limit of detection of the assay is 0.01 dl, and all samples were assayed in duplicates.
For cortisol collected at home, each time point of sampling was averaged over the 3 days. Awakening cortisol response (ACR) was calculated to assess the morning rise in cortisol within the first 30 minute of awakening. The ACR is commonly noted by a 50% increase in cortisol levels after awakening and is considered a reliable biomarker of HPA activity (Pruessner et al., 1997; Wust et al., 2000). For cortisol collected during the TSST, area under the curve (AUC) was calculated for total cortisol secreted during the TSST protocol, and percentage change in cortisol was calculated to estimate the change in cortisol from pre-TSST to 15 minute post-TSST to estimate the rise in cortisol specific to stress exposure.
Results
For cognitive and psychological (i.e., personality and mood) scores, independent analyses of co-variance (ANCOVA) were performed on all outcome measures, controlling for age, gender, education, and language.
For each sample of basal and reactive cortisol levels, repeated measures analyses were conducted controlling for age, gender, education, and language. ANCOVAs were performed for ACR and for percentage change in cortisol pre-to-post TSST. All results were considered significant at an alpha level of 0.05.
Demographics
Within the sample, 35 were GG carriers, 51 were AG carriers, and 15 were AA carriers. The observed frequencies for the three 5-HTR2A genotypes obeyed the Hardy–Weinberg equilibrium, χ2 (1) =0.67, p = 0.72. A significant group difference was found between the GG group and the AG group for age. Specifically, the AG (M = 56.89, SE = 0.58) group was found to be significantly younger than the GG group (M = 59.19, SE = 0.64) but not significantly younger than the AA group (M = 58.00, SE = 0.95) (Table 2).
Table 2.
Demographic factors across genotype group.
| Demographics | AA | AG | GG |
|---|---|---|---|
| N | 15 | 51 | 35 |
| Age (mean, SE) | 58.00 (0.95) | 56.89 (0.58)* | 59.19 (0.64) |
| Gender (female:male) | 12:4 | 41:12 | 28:8 |
| Language (english:french) | 10:6 | 41:12 | 25:11 |
| Retired (% ) | 40% | 37% | 45% |
| MMSE (mean, SE) | 29.31 (0.23) | 29.50 (0.16) | 29.57 (0.16) |
*p = 0.03 significant group difference between AG and GG.
Psychological measures
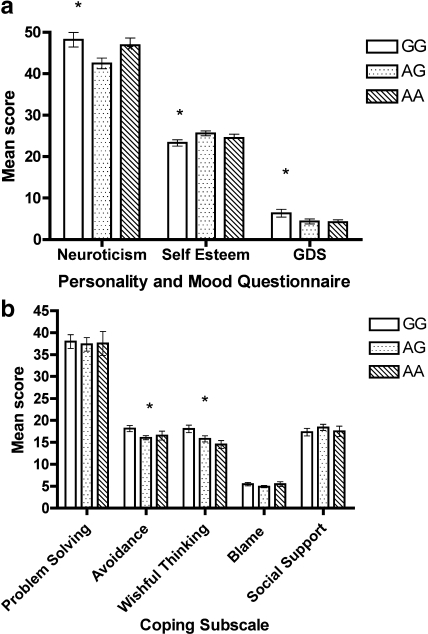
ANCOVA found a significant group difference for neuroticism, F (2, 97) =3.75, p = 0.03, self-esteem, F (2, 98) =3.72, p = 0.03, and depression, F (2, 98) =3.72, p = 0.04. Specifically, the GG group reported higher levels of neuroticism and lower levels of self-esteem compared to the AG group, and reported higher levels of depression than the AA group (Figure 1a).
Figure 1.
Mean score (SE) on psychological measures across genotype group.
In terms of coping strategies, no significant differences were found for problem solving, blame, or social support. However, a significant group difference was found for avoidance coping, F (2, 98) =3.51, p = 0.03, and wishful thinking, F (2, 98) =3.75, p = 0.03. The GG group reported more avoidance coping than the AG group and more wishful thinking than both the AG and AA group, ps < 0.03 (Figure 1b).
Cognitive performance
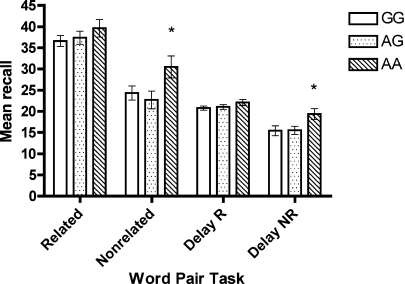
ANCOVA found no effect of genotype on digit span or verbal fluency performance. However, a significant difference was found for performance on the declarative word-pair task, for immediate recall of non-related word pairs, F(2, 96) =3.18, p = 0.048, and delay recall of non-related word pairs, F(2, 94) =4.21, p = 0.02. For both immediate and delayed recall, the AA genotype group performed significantly better than the other two groups, ps < 0.03 (Figure 2).
Figure 2.
Mean score (SE) on word-pair task across genotype.
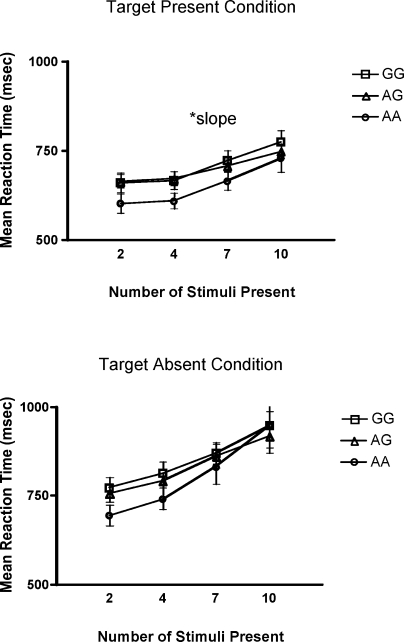
In addition, a significant group difference was found for performance on the selective attention task when the target stimulus was present. Specifically, the AA group displayed a steeper slope in reaction time than the other two groups, F(2, 97) =5.90, p = 0.01. A steeper positive slope in the AA group signifies a quicker time to respond to the target stimulus when fewer distracter stimuli were present on the computer screen, compared to the GG or AG group. As the number of distracter stimuli reached a maximum (i.e., 10 distracter stimuli), the AA group displayed the same reaction time as the other groups. No differences were found, however, when target stimulus was absent (Figure 3).
Figure 3.
Mean reaction time (SE) on the selective attention task for stimulus absent and stimulus present conditions across genotype group.
Cortisol measures
Basal cortisol
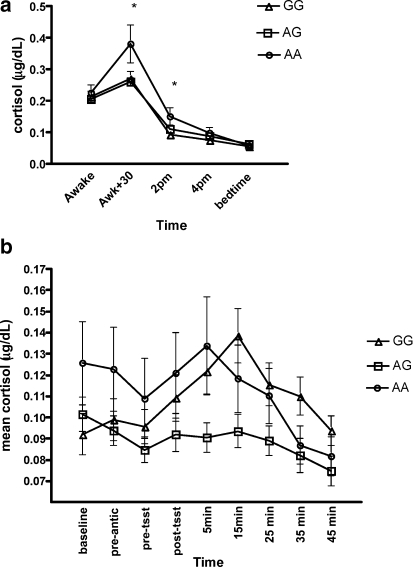
Repeated measures analyses found a significant time × group difference over the five sampling times (Figure 4a). There was also a trend for a group difference (p = 0.09). Univariate analyses found group differences at the 30 minutes after awakening point, F(2, 103) =4.68, p = 0.01, and at the 14:00 point, F(2, 103) =4.05, p = 0.02. Specifically, the AA group secreted higher levels of cortisol at the 30-minute mark compared to the AG and GG group and secreted higher levels than the GG group at the 14:00 point (ps < 0.05).
Figure 4.
(a) Mean (SE) diurnal cortisol secretion across genotype group. (b) Mean (SE) stress-reactive cortisol secretion across genotype group.
ANCOVA found a significant group difference in ACR, F(2, 93) =3.17, p = 0.04. The AA group exhibited a significantly higher ACR (M = 0.67, SE = 0.08) than the AG group (M = 0.34, SE = 0.15), p < 0.05. Although the AA group exhibited a larger ACR than the GG group (M = 0.33, SE = 0.11), comparison tests failed to reach significance (p > 0.05).
Reactive cortisol
Repeated measures analyses found a significant time × group difference, F(2, 93) =5.01, p = 0.01. Univariate analyses found a significant between group difference for overall AUC cortisol during the TSST, F(2, 93) =4.06, p = 0.02 (Figure 4b), with the GG group displayed higher overall AUC (MAUC = 0.14, SEAUC = 0.08), compared to the AG group (MAUC = 0.02, SEAUC = 0.09), p < 0.05. A trend was found for the GG group to display higher AUC compared to the AA group (M = −0.10, SE = 0.09), p = 0.06. A significant effect was also found for percentage change from baseline to 15 minutes post-TSST, F(2, 91) =7.53, p = 0.001. Specifically, the GG group displayed greater percentage change (M = 0.72, SE = 0.17) compared to the AG group (M = −0.10, SE = 0.04) and the AA group (M = 0.15, SE = 0.19), ps < 0.05.
Discussion
The present study investigated the association between the −1438A/G 5-HT2A receptor polymorphism and cognition in a sample of middle-aged adults. This polymorphism was also assessed in relation to basal and reactive cortisol levels and personality, two individual difference factors commonly associated with cognitive function. This is the first study to assess the −1438A/G polymorphism in healthy middle-aged adults and, to the best of our knowledge it is the first study to directly assess the association of this polymorphism with basal and stress-reactive cortisol secretion.
In terms of psychological measurements, our findings suggest that homozygote carriers of the G allele have a tendency to report greater depressive symptomatology, poorer self-esteem, and higher reports of neurotic traits compared to individuals who are not homozygote carriers of the G allele. These findings are in line with past association studies that have found a link between the GG genotype and risk for depression (Chee et al., 2001; Choi et al., 2004), and are in line with findings that GG carriers tend to report greater social introversion and fewer number of friends (Golimbet et al., 2004). Also, high reward dependence and harm avoidance have been found in individual carriers of the GG genotype, which are traits correlated with depression (Pelissolo and Corruble, 2002). We further found greater use of emotion-based coping strategies in the GG carriers compared to other genotype groups, including avoidance and wishful thinking, both of which have been associated with low mood (Sigmon et al., 2007; Vollman et al., 2007). Overall, it appears that the GG genotype is associated with psychological factors that denote a tendency for low affect, which has further been found to correlate with dysregulation of the HPA system.
Assessment of basal cortisol levels in the morning showed that the AA group displayed a significantly steeper ACR compared to the AG genotype group. Also, although not significant, the AA carriers tended to display a higher ACR than the GG group. Indeed, a close look at the graph shows that the GG and AG genotype groups failed to show the common 50% increase in cortisol secretion after awakening. Interestingly, a flat ACR has been linked to depression and other stress-related disorders (Kuehner et al., 2007). Thus, the flat ACR finding not only mirrors past research but also corresponds to the present psychological findings. Based on our results, it may be speculated that carrying the G allele may lead to a flatter awakening response and may increase the risk of presenting low affect.
To date, one other study has assessed the HPA system in relation to the −1438A/G polymorphism using the dexamethasone test. Following administration of 0.25 mg of dexamethasone, it was reported that homozygotic carriers of the G allele exhibited less suppression of cortisol (Rosmond et al., 2002), which has also been reported in stress-related pathology (Rush et al., 1996; Westrin and Nimeus, 2003). In the present study, it was found that compared to AA and AG carriers, the GG genotype displayed a greater percentage increase in cortisol levels in response to the TSST, a widely used laboratory psychosocial stressor. This finding is in agreement with the dexamethasone study by Rosmond et al (Rosmond et al., 2002), suggesting an overactive HPA response to perturbations in homeostasis of the individual. While this is the first study to assess stress reactivity in this polymorphism, this finding, in combination with the personality outcomes, is in agreement with what has been reported in the literature thus far. Studies have shown that low self-esteem, which was characteristic of our GG sample, is related to higher reactivity to the TSST (Pruessner et al., 1999). In terms of high neuroticism, also characteristic of our GG sample, the findings are less clear, with some reports of enhanced reactivity (Houtman and Bakker, 1991), some reports of blunted reactivity (Burke et al., 2005; Phillips et al., 2005), and some reports of negative findings (Schommer et al., 1999). Nonetheless, the present findings suggest an influence of the −1438A/G polymorphism on the HPA system, for both basal and stress-reactive activity.
Apart from greater stress reactivity to the TSST in the GG group, the AG group also displayed an interesting pattern worth noting. The AG genotype group displayed a blunted cortisol response throughout the TSST session. While a heightened response as exhibited by the GG group denotes hyperactivity of the HPA system, a blunted response also denotes dysregulation of the system. Indeed, a blunted cortisol response following a psychosocial stressor has been reported in stress-related psychopathology, including dissociative disorder (Simeon et al., 2007), tinnitus (Hebert and Lupien, 2007), and also in healthy adults who report childhood maltreatment (Carpenter et al., 2007). In a meta-analysis that assessed the relationship between the cortisol response to stress and depression, it was found that individuals with major depressive disorder exhibited a blunted cortisol response to psychosocial stress, especially in aged individuals (Burke et al., 2005a, 2005b). Thus, it may be that presence of the G allele is associated with a risk for abnormal HPA function, which was noted in the present study as a blunted or overactive stress response, and a flat ACR. However, the exact nature of HPA dysregulation among G allele carriers may be dependent on additional interacting factors.
Considering the robust findings linking cortisol to cognitive performance in older adults (Lupien et al., 1994, 2007; Sapolsky, 1994), and the present findings linking the −1438A/G polymorphism with HPA activity, the present findings on cognitive performance are intriguing. Specifically, the AA group performed better on a declarative memory task than other groups and also responded quicker to a selective attention task when fewer distracters were present on the computer screen. This finding contradicts a previous study that reported better performance by the GG genotype on a figural memory task (Reynolds et al., 2006). One explanation for this discrepancy in results is that these two tasks may use different areas of the brain (Ariza et al., 2006). However, both tasks are considered to be tests of episodic memory, which is hippocampal dependent. Unfortunately, there are no additional studies that have assessed the relationship between this polymorphism and cognitive performance and thus more research is required in this area in order to attain a more reliable interpretation of the data. However, it should be noted that the cognitive findings reported herein correspond to the cortisol and personality data in this study.
It should be noted, that although a 5% alpha level was employed throughout the statistical analyses of data, results in the present study are somewhat marginal (e.g., p = 0.03), possibly due to the small sample size. For this reason, additional studies are required to assess these associations and replicate the present findings. A larger sample size would allow for a more reliable interpretation of the data. As well, it would be interesting to assess this polymorphism in relation to cognition, HPA activity and personality in a wider age range, to assess if these associations begin early in life.
However, despite the study limitations, the −1438A/G polymorphism of the 5-HT2A receptor gene appears to be related to cognitive performance as well as cortisol secretion and stress-related personality factors. The reason for these associations remains to be elucidated, as there are few studies that have assessed the functional significance of this polymorphism on the serotonergic system. Studies to date have shown that this polymorphism determines receptor mRNA, with the A allele resulting in greater expression of 5-HT2A receptors in the cortex and greater receptor binding compared to the G allele (Parsons et al., 2004; Polesskaya and Sokolov, 2002). Furthermore, lower 5-HT2A binding has been implicated in poorer cognitive function (Morris et al., 1993). Thus these findings suggest that there is lower binding in individual carriers of the G allele compared to carriers of the A allele of the −1438A/G polymorphism. From this, it may be postulated that greater receptor mRNA may result in greater receptor expression, which may then result in a more efficient signaling system, be it through greater facilitation of cholinergic function or modulation of neuroendocrine activity.
While the polymorphism has not been found to affect 5-HT levels per se, it may be surmised that greater number of receptors may render more efficient synaptic signaling between neurons. This hypothesis corresponds with studies that have shown that blockade of the 5-HT2A receptor results in verbal and spatial impairment, in addition to impairment in sustained attention and motor control (Wingen et al., 2006, 2007). However, this is purely speculative and further analyses are needed in order to understand how the −1438 A/G polymorphism influences the 5-HT system, and in turn influences the stress-related indices reported herein.
Conflict of Interest Statement
None of the authors on this manuscript have any conflict of interest including any financial, personal, or other relationships with other people or organizations that could inappropriately influence (bias) their work.
Acknowledgments
We thank Dr. Dominique Walker from the Douglas Mental Health University Institute, for analyses of all salivary cortisol samples. This study was supported by a grant from the Canadian Institutes of Health Research (Institute of Aging) to SJL (grant no. 1154234). The work of A. J. Fiocco was supported by a CIHR Doctoral Training Award and the work of Dr. Lupien is supported by an Investigator Award from the CIHR Institute of Aging. CIHR had no further involvement in the study.
References
- Ampollini P., Marchesi C., Signifredi R., Ghinaglia E., Scardovi F., Codeluppi S., Maggini C. (1999). Temperament and personality features in patients with major depression, panic disorder and mixed conditions. J. Affect. Disord. 52, 203–207 [DOI] [PubMed] [Google Scholar]
- Ariza M., Pueyo R., Junque C., Mataro M., Poca M. A., Mena M. P., Sahuquillo J. (2006). Differences in visual vs. verbal memory impairments as a result of focal temporal lobe damage in patients with traumatic brain injury. Brain Inj. 20, 1053–1059 [DOI] [PubMed] [Google Scholar]
- Bagdy G. (1996). Role of the hypothalamic paraventricular nucleus in 5-HT1A, 5-HT2A and 5-HT2C receptor-mediated oxytocin, prolactin and ACTH/corticosterone responses. Behav. Brain Res. 73, 277–280 [DOI] [PubMed] [Google Scholar]
- Buhot M. C. (1997). Serotonin receptors in cognitive behaviors. Curr. Opin. Neurobiol. 7, 243–254 [DOI] [PubMed] [Google Scholar]
- Burke H. M., Davis M. C., Otte C., Mohr D. C. (2005a). Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology 30, 846–856 [DOI] [PubMed] [Google Scholar]
- Burke H. M., Fernald L. C., Gertler P. J., Adler N. E. (2005b). Depressive symptoms are associated with blunted cortisol stress responses in very low-income women. Psychosom. Med. 67, 211–216 [DOI] [PubMed] [Google Scholar]
- Carpenter L. L., Carvalho J. P., Tyrka A. R., Wier L. M., Mello A. F., Mello M. F., Anderson G. M., Wilkinson C. W., Price L. H. (2007). Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee I. S., Lee S. W., Kim J. L., Wang S. K., Shin Y. O., Shin S. C., Lee Y. H., Hwang H. M., Lim M. R. (2001). 5-HT2A receptor gene promoter polymorphism −1438A/G and bipolar disorder. Psychiatr. Genet. 11, 111–114 [DOI] [PubMed] [Google Scholar]
- Choi M. J., Lee H. J., Lee H. J., Ham B. J., Cha J. H., Ryu S. H., Lee M. S. (2004). Association between major depressive disorder and the −1438A/G polymorphism of the serotonin 2A receptor gene. Neuropsychobiology 49, 38–41 [DOI] [PubMed] [Google Scholar]
- Cleare A. J., Bond A. J. (1997). Does central serotonergic function correlate inversely with aggression? A study using D-fenfluramine in healthy subjects. Psychiatry Res. 69, 89–95 [DOI] [PubMed] [Google Scholar]
- Crowe M., Andel R., Pedersen N. L., Fratiglioni L., Gatz M. (2006). Personality and risk of cognitive impairment 25 years later. Psychol. Aging 21, 573–580 [DOI] [PubMed] [Google Scholar]
- Depue R. A. (1995). Neurobiological factors in personality and depression. Eur. J. Pers. 9, 413–439 [Google Scholar]
- Dinan T. G. (1996). Serotonin and the regulation of hypothalamic-pituitary-adrenal axis function. Life Sci. 58, 1683–1694 [DOI] [PubMed] [Google Scholar]
- Flory J. D., Manuck S. B., Matthews K. A., Muldoon M. F. (2004). Serotonergic function in the central nervous system is associated with daily ratings of positive mood. Psychiatry Res. 129, 11–19 [DOI] [PubMed] [Google Scholar]
- Golimbet V. E., Alfimova M. V., Mitiushina N. G. (2004). Polymorphism of the serotonin 2A receptor gene (5HTR2A) and personality traits. Mol. Biol. 38, 404–412 [PubMed] [Google Scholar]
- Greyer M. A. (1996). Serotonergic functions in arousal and motor activity. Behav. Brain Res. 73, 31–35 [DOI] [PubMed] [Google Scholar]
- Hebert S., Lupien S. J. (2007). The sound of stress: blunted cortisol reactivity to psychosocial stress in tinnitus sufferers. Neurosci. Lett. 411, 138–142 [DOI] [PubMed] [Google Scholar]
- Hennig J., Reuter M., Netter P., Burk C., Landt O. (2005). Two types of aggression are differentially related to serotonergic activity and the A779C TPH polymorphism. Behav. Neurosci. 119, 16–25 [DOI] [PubMed] [Google Scholar]
- Hirano H. D. J., Fibiger H. C. (1995). Serotonergic regulation of acetylcholine release in rat frontal cortex. J. Neurochem. 65, 1139–1145 [DOI] [PubMed] [Google Scholar]
- Hood S. D., Hince D. A., Robinson H., Cirillo M., Christmas D., Kaye J. M. (2006). Serotonin regulation of the human stress response. Psychoneuroendocrinology 31, 1087–1097 [DOI] [PubMed] [Google Scholar]
- Houtman I. L., Bakker F. C. (1991). Individual differences in reactivity to and coping with the stress of lecturing. J. Psychosom. Res. 35, 11–24 [DOI] [PubMed] [Google Scholar]
- Issa A. M., Rowe W., Gauthier S., Meaney M. J. (1990). Hypothalamic-pituitary-adrenal activity in aged, cognitively impaired and cognitively unimpaired rats. J. Neurosci. 10, 3247–3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C., Pirke K. M., Hellhammer D. H. (1993). The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28, 76–81 [DOI] [PubMed] [Google Scholar]
- Kuehner C., Holzhauer S., Huffziger S. (2007). Decreased cortisol response to awakening is associated with cognitive vulnerability to depression in a nonclinical sample of young adults. Psychoneuroendocrinology 32, 199–209 [DOI] [PubMed] [Google Scholar]
- Lai M. K., Tsang S. W., Alder J. T., Keene J., Hope T., Esiri M. M., Francis P. T., Chen C. P. (2005). Loss of serotonin 5-HT2A receptors in the postmortem temporal cortex correlates with rate of cognitive decline in Alzheimer's disease. Psychopharmacology 179, 673–677 [DOI] [PubMed] [Google Scholar]
- Landfield P. W., Waymire J. C., Lynch G. (1978). Hippocampal aging and adrenocorticoids: quantitative correlations. Science (New York) 202, 1098–1102 [DOI] [PubMed] [Google Scholar]
- Lupien S., Lecours A. R., Lussier I., Schwartz G., Nair N. P., Meaney M. J. (1994). Basal cortisol levels and cognitive deficits in human aging. J. Neurosci. 14, 2893–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S. J., Gaudreau S., Tchiteya B. M., Maheu F., Sharma S., Nair N. P., Hauger R. L., McEwen B. S., Meaney M. J. (1997). Stress-induced declarative memory impairment in healthy elderly subjects: relationship to cortisol reactivity. J. Clin. Endocrinol. Metab. 82, 2070–2075 [DOI] [PubMed] [Google Scholar]
- Lupien S. J., Maheu F., Tu M., Fiocco A., Schramek T. E. (2007). The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn. [DOI] [PubMed] [Google Scholar]
- Manuck S. B., Flory J. D., McCaffery J. M., Matthews K. A., Mann J. J., Muldoon M. F. (1998). Aggression, impulsivity, and central nervous system serotonergic responsivity in a nonpatient sample. Neuropsychopharmacology 19, 287–299 [DOI] [PubMed] [Google Scholar]
- McCrae R. R., Costa P. T. (1986). Personality, coping, and coping effectiveness in an adult sample. J. Pers. 54, 385 [Google Scholar]
- Moresco F. M., Dieci M., Vita A., Messa C., Gobbo C., Galli L., Rizzo G., Panzacchi A., De Peri L., Invernizzi G., et al. (2002). In vivo serotonin 5HT(2A) receptor binding and personality traits in healthy subjects: a positron emission tomography study. Neuroimage 17, 1470–1478 [DOI] [PubMed] [Google Scholar]
- Morris P. L., Mayberg H. S., Bolla K., Wong D. F., Dannals R. F., Starkstein S. E., Robinson R. G. (1993). A preliminary study of cortical S2 serotonin receptors and cognitive performance following stroke. J. Neuropsychiatry Clin. Neurosci. 5, 395–400 [DOI] [PubMed] [Google Scholar]
- Parsons M. J., D'Souza U. M., Arranz M. J., Kerwin R. W., Makoff A. J. (2004). The −1438A/G polymorphism in the 5-hydroxytryptamine type 2A receptor gene affects promoter activity. Biol. Psychiatry 56, 406–410 [DOI] [PubMed] [Google Scholar]
- Pelissolo A., Corruble E. (2002). Personality factors in depressive disorders: contribution of the psychobiologic model developed by Cloninger. Encephale 28, 363–373 [PubMed] [Google Scholar]
- Phillips A. C., Carroll D., Burns V. E., Drayson M. (2005). Neuroticism, cortisol reactivity, and antibody response to vaccination. Psychophysiology 42, 232–238 [DOI] [PubMed] [Google Scholar]
- Polesskaya O. O., Sokolov B. P. (2002). Differential expression of the “C” and “T” alleles of the 5-HT2A receptor gene in the temporal cortex of normal individuals and schizophrenics. J. Neurosci. Res. 67, 812–822 [DOI] [PubMed] [Google Scholar]
- Pruessner J. C., Hellhammer D., Kirschbaum C. (1999). Low self-esteem, induced failure and the adrenocortical stress response. Pers. Individ. Dif. 27, 477–489 [Google Scholar]
- Pruessner J. C., Lord C., Meaney M., Lupien S. (2004). Effects of self-esteem on age-related changes in cognition and the regulation of the hypothalamic-pituitary-adrenal axis. Ann. N. Y. Acad. Sci. 1032, 186–190 [DOI] [PubMed] [Google Scholar]
- Pruessner J. C., Wolf O. T., Hellhammer D. H., Buske-Kirschbaum A., von Auer K., Jobst S., Kaspers F., Kirschbaum C. (1997). Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 61, 2539–2549 [DOI] [PubMed] [Google Scholar]
- Reynolds C. A., Jansson M., Gatz M., Pedersen N. L. (2006). Longitudinal change in memory performance associated with HTR2A polymorphism. Neurobiol. Aging 150–154 [DOI] [PubMed] [Google Scholar]
- Rittenhouse P. A., Bakkum E. A., Levy A. D., Li Q., Carnes M., van de Kar L. D. (1994). Evidence that ACTH secretion is regulated by serotonin2A/2C (5-HT2A/2C) receptors. J. Pharmacol. Exp. Ther. 271, 1647–1655 [PubMed] [Google Scholar]
- Rosenberg M. (1965). Society and the Adolescent Self-Image (New Jersey, Princeton University Press; ). [Google Scholar]
- Rosier A., Dupont P., Peuskens J., Bormans G., Vandenberghe R., Maes M., de Groot T., Schiepers C., Verbruggen A., Mortelmans L. (1996). Visualisation of loss of 5-HT2A receptors with age in healthy volunteers using [18F]altanserin and positron emission tomographic imaging. Psychiatry Res. 68, 11–22 [DOI] [PubMed] [Google Scholar]
- Rosmond R., Bouchard C., Bjorntorp P. (2002). 5-HT2A receptor gene promoter polymorphism in relation to abdominal obesity and cortisol. Obes. Res. 10, 585–589 [DOI] [PubMed] [Google Scholar]
- Rush A. J., Giles D. E., Schlesser M. A., Orsulak P. J., Parker C. R., Jr., Weissenburger J. E., Crowley G. T., Khatami M., Vasavada N. (1996). The dexamethasone suppression test in patients with mood disorders. J. Clin. Psychiatry 57, 470–484 [DOI] [PubMed] [Google Scholar]
- Rybakowski F., Slopien A., Dmitrzak-Weglarz M., Czerski P., Rajewski A., Hauser J. (2006). The 5-HT2A −1438 A/G and 5-HTTLPR polymorphisms and personality dimensions in adolescent anorexia nervosa: association study. Neuropsychobiology 53, 33–39 [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M. (1994). The physiological relevance of glucocorticoid endangerment of the hippocampus. Ann. N. Y. Acad. Sci. 746, 294–304; discussion 304–297 [DOI] [PubMed] [Google Scholar]
- Schommer N. C., Kudielka B. M., Hellhammer D. H., Kirschbaum C. (1999). No evidence for a close relationship between personality traits and circadian cortisol rhythm or a single cortisol stress response. Psychol. Rep. 84, 840–842 [DOI] [PubMed] [Google Scholar]
- Sheline Y. I., Mintun M. A., Moerlein S. M., Snyder A. Z. (2002). Greater loss of 5-HT(2A) receptors in midlife than in late life. Am. J. Psychiatry 159, 430–435 [DOI] [PubMed] [Google Scholar]
- Sigmon S. T., Pells J. J., Schartel J. G., Hermann B. A., Edenfield T. M., LaMattina S. M., Boulard N. E., Whitcomb-Smith S. R. (2007). Stress reactivity and coping in seasonal and nonseasonal depression. Behav. Res. Ther. 45, 965–975 [DOI] [PubMed] [Google Scholar]
- Simeon D., Knutelska M., Yehuda R., Putnam F., Schmeidler J., Smith L. M. (2007). Hypothalamic-pituitary-adrenal axis function in dissociative disorders, post-traumatic stress disorder, and healthy volunteers. Biol. Psychiatry 61, 966–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tochigi M., Umekage T., Kato C., Marui T., Otowa T., Hibino H., Otani T., Kohda K., Kato N., Sasaki T. (2005). Serotonin 2A receptor gene polymorphism and personality traits: no evidence for significant association. Psychiatr. Genet. 15, 67–69 [DOI] [PubMed] [Google Scholar]
- van Heeringen C., Audenaert K., Van Laere K., Dumont F., Slegers G., Mertens J., Dierckx R. A. (2003). Prefrontal 5-HT2a receptor binding index, hopelessness and personality characteristics in attempted suicide. J. Affect. Disord. 74, 149–158 [DOI] [PubMed] [Google Scholar]
- Versijpt J. V. L. K., Dumont F, Decoo D, Vandecapelle M, Santens P, Goethals I, Audenaert K, Slegers G, Dierckx R. A., Korf J. (2003). Imaging of the 5-HT2A system: age-, gender-, and Alzheimer's disease-related findings. Neurobiol. Aging 24, 553–561 [DOI] [PubMed] [Google Scholar]
- Vitaliano P. P., Russo J., Carr J. E., Maiuro R. D., Becker J. (1985). The ways of coping checklist: revision and psychometric properties. Multivariate Behav. Res. 20, 3–26 [DOI] [PubMed] [Google Scholar]
- Vollman M. W., Lamontagne L. L., Hepworth J. T. (2007). Coping and depressive symptoms in adults living with heart failure. J. Cardiovasc. Nurs. 22, 125–130 [DOI] [PubMed] [Google Scholar]
- Walderhaug E., Lunde H., Nordvik J. E., Landrø N. I., Refsum H., Magnusson A. (2002). Lowering of serotonin by rapid tryptophan depletion increases impulsiveness in normal individuals. Psychopharmacology 164, 385–391 [DOI] [PubMed] [Google Scholar]
- Westrin A., Nimeus A. (2003). The dexamethasone suppression test and CSF-5-HIAA in relation to suicidality and depression in suicide attempters. Eur. Psychiatry 18, 166–171 [DOI] [PubMed] [Google Scholar]
- Wilson R. S., Bennett D. A., Mendes de Leon C. F., Bienias J. L., Morris M. C., Evans D. A. (2005). Distress proneness and cognitive decline in a population of older persons. Psychoneuroendocrinology 30, 11–17 [DOI] [PubMed] [Google Scholar]
- Wingen M., Kuypers K. P., Ramaekers J. G. (2006). Selective verbal and spatial memory impairment after 5-HT1A and 5-HT2A receptor blockade in healthy volunteers pre-treated with an SSRI. J. Psychopharmacol. [DOI] [PubMed] [Google Scholar]
- Wingen M., Kuypers K. P., Ramaekers J. G. (2007). The role of 5-HT1a and 5-HT2a receptors in attention and motor control: a mechanistic study in healthy volunteers. Psychopharmacology 190, 391–400 [DOI] [PubMed] [Google Scholar]
- Wust S., Wolf J., Hellhammer D. H., Federenko I., Schommer N., Kirschbaum C. (2000). The cortisol awakening response - normal values and confounds. Noise Health 2, 79–88 [PubMed] [Google Scholar]
- Yesavage J. A., Brink T. L., Rose T. L., Lum O., Huang V., Adey M., Leirer V. O. (1982). Development and validation of a geriatric depression screening scale: a preliminary report. J. Psychiatr. Res. 17, 37–49 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Damianoska K. J., Carrasco G. A., Dudas B., D'Souza D. N., Tetzlaff J., Garcia F., Hanley N. R., Scripathirathan K., Petersen B. R., et al. (2002). Evidence that 5-HTR2A receptors in the hypothalamic paraventricular nucleus mediate neuroendocrine responses to (−)DOI. J. Neurosci. 22, 9635–9642 [DOI] [PMC free article] [PubMed] [Google Scholar]