Abstract
Intolerance to the cold is common following peripheral nerve injury and surgery of the upper extremity. Despite its prevalence, the exact pathophysiology and natural history of this condition are not well understood. Subjective, self-report questionnaires have been created and validated as reliable measures of post-traumatic cold intolerance. The difficulty currently lies in assigning an objective measure to this predominantly subjective phenomenon. The present study evaluated the test–retest reliability of a proposed objective measure of cold intolerance, the Immersion in Cold-water Evaluation (ICE), and its correlation with subjective measures in healthy control subjects. Two age groups were also compared to investigate the effect of age on cold intolerance and temperature recovery. On two separate testing days, subjects completed three health-related questionnaires and submersed their dominant hands in cold water. The temperature of their second and fifth digits was monitored during recovery. Both the objective cold-provocation testing and the subjective self-report questionnaires were highly reliable albeit not significantly correlated. No significant temperature recovery trend was noted between the age groups. Post-traumatic cold intolerance is postulated to have both a vascular and neural etiology among other contributing causes. The protocol studied here was centered predominantly on the former etiology, examining peripheral blood flow and associated temperature recovery. This study established ICE as a reliable means to objectively measure cold response, supplementing information provided by previously validated self-report methods.
Keywords: Cold intolerance, Immersion in Cold-water Evaluation (ICE), Peripheral nerve injury
Introduction
Cold intolerance, or hypersensitivity, is common following peripheral nerve injury and surgery of the upper extremity. Its symptoms generally develop within the first few months following the initial damage and can include pain, ache, discomfort, dexterity loss, stiffness, dysaesthesia, paresthesias, and color change [2, 3, 16, 25]. These abnormal responses may endure for several hours following exposure to cold weather, environments, and substances and tend to diminish upon re-warming [7, 10, 19, 25]. Intolerance to the cold can become particularly disabling for those employed outdoors in colder climates as well as with regular use of refrigerators and air conditioning, implicating serious economic and livelihood implications for affected individuals [5, 15, 18]. Campbell and Kay [2] suggest that since these symptoms have been shown to occur both in isolation and in combination, the diagnosis of cold intolerance is not necessarily dependent on their simultaneous presentation. Cold-induced symptoms within individuals tend to manifest in a consistent manner. However, substantial variation exists between individuals on both the nature and severity of symptoms [14]. While the current definitions cover certain aspects of cold intolerance, they do not effectively define the condition itself.
The pathogenesis and natural history of cold intolerance remain ambiguous despite the condition’s increasing prevalence [10, 18]. Injury to the peripheral vasculature, defective vasoregulation, and nerve injury have been suggested as possible routes to cold intolerance [3]. Some studies deem cold intolerance to be a long-term and permanent condition, while others suggest it will improve naturally over time, as sensibility to the affected appendage returns [3, 5, 7, 19]. Improvement may be subjective since treatments often consist of behavioral modification, not direct remedy of the ailment: the problem may have only disappeared because the patient has learned to avoid the cold [5]. It has been argued that cold intolerance is not significantly correlated with age [25], smoking history [5], or mechanism of peripheral nerve injury [3]. However, other studies have found that younger subjects are less affected [27], smokers are more affected, and subjects with crushed nerve injuries are more likely to develop cold intolerance [10], further exemplifying the diversity and disagreement surrounding this issue. By creating valid and reliable measurement tools, both for subjective observations and objective physiological responses, the disagreement surrounding this highly prevalent issue may be settled.
A variety of self-answered questionnaires have been created to measure the subjective presence of cold intolerance. McCabe et al. [18] developed the Cold Sensitivity Severity Scale (CSSS), a five-item scale covering a range of cold exposures using simple questions and an adjectival scale. Irwin et al. [10] have since developed the Cold Intolerance Symptom Severity score (CISS), a six-item scale that explores additional dimensions of cold intolerance. The reliability and applicability of these scales have been formally evaluated, their validity continuously verified as other studies make use of these tools to measure problems experienced by patients [3, 5, 10, 24]. While evidence on these self-report scales has been positive, there remains a role for objective measures of the physiological parameters associated with cold intolerance. Previously used objective cold intolerance or “cold stress” tests include measurements of cutaneous temperature, blood flow, and blood pressure when exposed to cold [5]. The isolated cold stress test is one method that evaluates digital temperature and cutaneous perfusion during a room-temperature recovery period following cold exposure [13, 24]. However, when Ruch et al. completed this test with the CISS score, there was very little correlation between the two sets of results [25].
Review of the literature indicates little standardization between centers or studies utilizing cold stress testing. A simple protocol that is inexpensive, practical, and reliable has the potential to contribute to clinical practice and research. The present study sought to define and evaluate the test–retest reliability of a simple cold-provocation protocol in healthy, control subjects. It aimed to examine the correlation of this objective test with previously validated subjective measures as well as examining the effect of age on temperature recovery.
Materials and Methods
Subjects
Fifty-two healthy subjects were recruited from May 2006 to June 2007. All subjects were screened for the following exclusion criteria: (1) diagnosis of peripheral nerve injury, Raynaud’s or hand–arm vibration syndrome; (2) history of fingertip blanching, unstable cardiovascular or neurological conditions, or neck pain; (3) exposure to occupational vibration; and (4) previous digital nerve re-implantation. Subjects were instructed to refrain from consuming caffeine and alcohol and to refrain from smoking for at least 4 h before testing. The McMaster Research Ethics Board granted ethics approval for this project. All subjects gave informed written consent for their participation.
Protocol Development
A literature review was conducted to establish the range of temperatures, immersion times, and monitoring periods used in cold-provocation testing [4, 8, 16, 17]. These parameters were used to develop the potential ranges for the test protocol presented here. Pilot testing with healthy volunteers and patients was conducted to establish the lowest possible temperature that the majority of subjects could tolerate, while assessing variations in immersion times and monitoring periods. A temperature of 12°C (after tests at 10°C proved to be too cold) for 5 min was found to be tolerable by all volunteers, although symptoms of cold discomfort could be expected. Temperature recovery was initially monitored for 20 min following immersion. It was found that most recovery occurred within 5 min and that no significant improvement was noted past 10 min for all study participants. Ten minutes was then selected as a reasonable upper limit for the monitoring period even for patients with pathology. The Immersion in Cold-water Evaluation (ICE) was established based on this pilot testing.
Testing Protocol
All testing was completed in a room temperature maintained at 20 ± 2°C. Each subject was tested in the same location on both testing days using the same equipment throughout. Subjects were required to acclimatize to the testing room temperature upon arrival for 15 min. During this time, subjects completed the following self-report questionnaires: the Patient-Rated Wrist/Hand Evaluation (PRWHE), the Cold Symptom Severity Scale, and the SF-36 Health Survey [10, 22].
Each subject was directed to rest his dominant hand comfortably, palmar surface up, on a bench top approximately at the level of the subject’s heart. The subject was instructed to maintain a stable upper-body position and refrain from moving his hands, both while on the bench and during cold immersion. A DT48 King Medical Infrared Skin thermometer was used to record the temperature of the subject’s second and fifth digits at 1-min intervals for 2 min to identify a baseline, pre-immersion measure. The subject subsequently immersed his hand, up to the styloid process of the ulna, in an insulated container of 12°C water (Fig. 1). The temperature was monitored throughout the experiment using a floating Aquarius Digital Spa and Pool thermometer and a mercury-in-glass thermometer to verify the temperature readings. The water temperature was maintained within 1°C of the target value with the administration of additional ice or warm water, as required. The investigator stirred the contents of the container three times each minute to evenly disperse the water warmed by the subject’s hand.
Figure 1.
Cold provocation. Subjects immersed their hands in an insulated container of 12°C water. They were instructed to refrain from touching the sides or bottom of the container, as well as to resist the urge to move their hands.
Following 5 min of immersion in ice water, the subject removed his hand and placed it again palmar surface up on a towel on the bench. The investigator quickly pat-dried the subject’s hand and recorded the temperature of the second and fifth digits (Fig. 2). The temperature of these two digits was recorded at 1-min intervals for the next 10 min. The protocol was repeated using the subject’s non-dominant hand for comparison. Subjects returned within 1 week following the first testing date to complete the ICE protocol in its entirety.
Figure 2.
Temperature recording. Following 5 min of cold provocation, the temperature of the index and fifth digits was measured using a DT48 King Medical Infrared Skin thermometer. The temperature of these two digits was recorded at 1-min intervals for 10 min.
Data Analysis
All data were statistically analyzed using SPSS v15.0 software. All digit temperature recordings were reported as a percentage of pre-immersion temperature. Test–retest reliability of both the self-report questionnaires and objective measures was calculated using the intraclass correlation coefficient (ICC) with a 95% confidence interval. Correlations between subjective and objective results were analyzed with the Pearson correlation coefficient. Finally, any differences between groups were analyzed using linear regression and analysis of variance.
Results
Subjects
Fifteen male and 37 female subjects, aged 19–67 (mean age of 29.77 ± 1.84), were successfully screened for participation in this study. This sample was further divided into two age groups: >45 (11 subjects) and <45 (41 subjects), each with similar gender proportions (approximately 3:7, men to women). The subjects successfully completed all questionnaires and were able to remain immersed for the full duration of the ICE on two separate testing occasions.
Every subject complained of discomfort during the first 2 min of the ICE. Many described it as a “slight burning pain” and “throbbing tingle.” Most commented that they eventually felt a numbing sensation, free from pain after the third minute. Some, however, complained that the discomfort was never alleviated during immersion. A number of participants commented that their hands began to throb during the re-warming process. Two such subjects suffered from prolonged stiffness and minor loss of dexterity for some time following the experiment on both testing occasions. No long-term discomfort was reported.
Test–Retest Reliability
Both the subjective and objective measures had good test–retest reliability (ICC, 95% CI). The CSSS scale had higher reliability coefficients (0.90, 0.83–0.94) than the PRWHE (0.76, 0.59–0.86; Table 1). The lowest retest correlation coefficient occurred for cold provocation as measured on the index finger at time 0 (0.42, −0.01 to 0.67). This suggests that the most instability in scores occurs with the initial temperature drop associated with removing the hand from immersion, drying, and initiating measurement procedures. Test–retest reliability was generally good for temperature recovery, gradually becoming more significant as the re-warming period progressed (Table 2; Fig. 3).
Table 1.
Subjective test–retest reliability.
| ICC (95% CI) | |
|---|---|
| PRWHE | 0.74 (0.40–0.88) |
| CSSS | 0.89 (0.74–0.95) |
| SF36 | 0.69 (0.30–0.86) |
| Total | 0.87 (0.70–0.94) |
The test–retest reliability of the self-report questionnaires appeared highest with the test more specific for cold intolerance (CSSS) and least for the more general health report (SF-36).
PRWHE Patient-Rated Wrist Hand Evaluation; CSSS Cold Symptom Severity Scale; SF36 Short Form 36 Health Survey; ICC intraclass correlation coefficient; 95% CI 95% confidence interval
Table 2.
Objective test–retest reliability.
| Recovery time | ICC (95% CI) | |||
|---|---|---|---|---|
| Dominant index | Non-dominant index | Dominant 5th digit | Non-dominant 5th digit | |
| 0 min | 0.38 (−0.40 to 0.73) | 0.42 (−0.33 to 0.74) | 0.30 (−0.58 to 0.69) | 0.84 (0.63–0.93) |
| 2 min | 0.84 (0.63 to 0.93) | 0.88 (0.72 to 0.95) | 0.71 (0.34 to 0.87) | 0.76 (0.46–0.90) |
| 4 min | 0.82 (0.60 to 0.92) | 0.74 (0.42 to 0.89) | 0.78 (0.49 to 0.90) | 0.76 (0.44–0.89) |
| 6 min | 0.81 (0.57–0.92) | 0.77 (0.48–0.90) | 0.81 (0.58 to 0.92) | 0.83 (0.62–0.93) |
| 8 min | 0.80 (0.55–0.91) | 0.77 (0.47–0.90) | 0.81 (0.56 to 0.91) | 0.81 (0.58–0.92) |
| 10 min | 0.80 (0.54–0.91) | 0.79 (0.53–0.91) | 0.78 (0.50 to 0.90) | 0.74 (0.40–0.88) |
| Average | 0.86 (0.69–0.94) | 0.81 (0.57–0.92 | 0.84 (0.63 to 0.93) | 0.83 (0.61–0.92) |
The lowest test–retest reliability of the ICE protocol during the two testing occasions was observed directly following immersion, with increasing reliability as recovery time progressed. There was no statistically significant difference between subjects’ dominant and non-dominant hands.
ICC Intraclass correlation coefficient; 95% CI 95% confidence interval
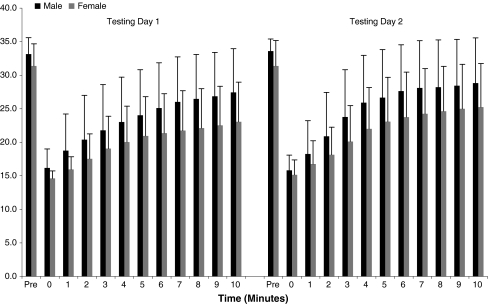
Figure 3.
Temperature recovery. Test–retest reliability was good for temperature recovery, graphically represented by the similar pattern in bar height—a measurement of the index finger temperature of male and female subjects on both testing days. Recovery was slightly higher in men than women, although not statistically significant. Considerable variation existed between subjects, as evident by the large standard deviation values.
Correlation Between Measures
There were no significant correlations between subjective and objective measures (Pearson correlation coefficient, p; −0.30 to 0.26, p > 0.05), regardless of which questionnaire was used. However, there was a significant correlation between subjective measures CSSS and PRWHE (0.35–0.49, p < 0.05). There was a significant positive correlation between age and temperature recovery (0.45–0.47, p < 0.05), indicating that recovery rate varied with age. These results must be viewed with caution given the low representation in the >45 age group (Fig. 4).
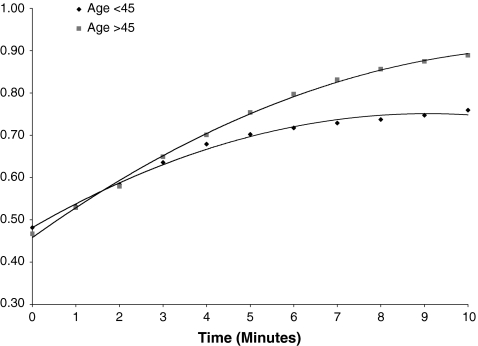
Figure 4.
Recovery between age groups. Subjects in the >45 age group appeared to recover more quickly and completely than subjects in the <45 age group. However, this statistically significant observation may not be reliable due to lack of representation in the older age group.
Temperature Recovery Trends
There was substantial variability between subjects in terms of the pattern of temperature recovery and the final value that each subject attained. Some subjects’ digit temperatures gradually increased, approximately 1° at a time, while others increased non-linearly, the temperature taking large, erratic jumps. The majority of subjects attained 70% or less recovery on average, with only a minority reaching 80% recovery. A small minority recovered beyond baseline temperature (e.g., >100%) within specific trials. There was no identified age, gender, or hand-dominance association to explain these occurrences. There was also no significant difference between subjects’ dominant and non-dominant hands.
A significant difference in recovery between age groups was observed, as described above. Subjects in the >45 age group appeared to recover more quickly and completely than subjects in the <45 age group (Fig. 4). Again, this analysis is underpowered because of the low representation in the older age group.
Discussion
Protocol Rationale
Cold immersion testing has been deemed a relevant method for evaluating the thermoregulatory potential of the upper extremities [13]. Varying techniques have been developed both to implement this stress and to measure its consequences. The ICE protocol tested in this study involved cold water provocation and subsequent monitoring of digit surface temperature. Nylander et al. [20] observed the seemingly intuitive finding that cold pain is experienced when temperature in the fingertips is low. The application of a mild temperature stress will stimulate vasoconstriction and decrease blood flow to the extremities [23]. The rationale behind this test for post-traumatic cold intolerance thus lies in the link between thermoregulation, peripheral blood flow, and the autonomic nervous system.
Thermoregulatory mechanisms in the body rely heavily on the regulation of cutaneous blood flow. Exposure to the cold results in cutaneous vessel vasoconstriction, shunting blood from the superficial to the deep venous system, transferring heat from the arteries to the veins, and effectively reducing skin temperature to maintain core levels [24]. Many blood vessels receive direct innervation from the sympathetic nervous system, enabling the maintenance of arterial and venous pressure [1]. Major peripheral nerves may act as important routes for the passage of adrenergic nerve fibers responsible for instigating vasoconstriction [24]. Peripheral neuropathies would consequently have a detrimental effect on vasomotor function.
Test–Retest Reliability, Correlation and Recovery Trends
High test–retest reliability was observed for both the subjective and objective testing methods. Despite high variability between subjects, there was significantly less variability within each subject, enabling such high reliability. This is consistent with findings of clinical practice where patients seem to have similar symptoms over time, but different patients manifest the problem differently. These findings of high reliability for self-reported cold intolerance are also consistent with that reported by previous authors [3, 5, 10]. The additional contribution of this particular study is in establishing the reliability of the ICE protocol.
Investigators completed all trials using the same equipment, environment, and technique, improving the reliability by standardizing and reducing the variability of the ICE protocol. The room temperature remained at 20 ± 2°C throughout testing, although no attempt was made to actively control it given that most clinics would be unable to do so. Another potential source of variability that was not controlled for was the time of testing with respect to the time of day and the time of year. Body temperature may vary with a circadian rhythm and activities like eating or exercise throughout different points of a 24-h period. Testing was also completed during a range of time in which weather patterns would have been variable. In this regard, neither one’s core body temperature nor the external temperature were controlled and could have affected the objective test–retest reliability and the correlation between the subjective and objective testing, respectively. However, the finding of high test–retest reliability suggests that the applicability of the ICE is reliable under normal conditions providing that a 15-min acclimatization period is adhered to. Craigen et al. [5] found that the ambient environmental temperature does not seem to affect the subjective ranking of cold-intolerance severity, suggesting that the lack of correlation may be unrelated to external temperature differences.
Although the ICE protocol and self-report were both reliable, they were not significantly interrelated. It is possible that some of the uncontrolled factors mentioned above contributed to this lack of relationship but unlikely that they can totally account for the observed lack of correlation. It is more likely that these are separate phenomena, with the ICE measuring vascular responses manifested in the periphery and self-report measuring the subjective experience of cold symptoms (which may include pain and other subjective sensations). A variety of peripheral and central neurovascular, psychosocial, cultural, environmental, and medical factors might contribute perceptions of discomfort or unpleasantness.
Despite an initial interest in examining the effect of age on temperature recovery, an insufficient number of older volunteers participated in the study, reducing the competency of results in this regard. A trend was observed between age groups with the subjects over age 45 recovering more quickly and completely than those less than age 45. This trend in the older subject group is inconsistent with reports of age-related decrease in vascular functioning. Feger and Braune [8] report a significant decrease in skin blood flow with respect to age in their study using laser-Doppler flowmetry. Santiago et al. suggest that thermoregulatory stress tolerance is reduced in aging subjects while thermal sensory thresholds are increased [29]. However, the size of the two age groups in the present study was highly inequitable, perhaps resulting in insufficient power to realistically make this claim.
Theories of Pathophysiology
Cold intolerance may persist following injury or surgery to the upper extremity, despite the sufficient return of general motor and sensory functioning [10]. Suggestions for its pathophysiology include both a neural and vascular etiology, along with the possible contribution of humoral factors and central control mechanisms.
Thermal and pain sensations are both mediated through small-diameter, slowly conducting nerve fibers: unmyelinated C and lightly myelinated A-δ fibers [1]. These two types of afferent fibers in the distal axons of primary sensory neurons respond to nociceptive stimuli, including temperatures below 15°C, if the intensity of the stimulus is high enough [29]. In cold-intolerant subjects, it may be that this cold stimulus threshold is lowered, requiring less intensity to elicit a pain response. These fibers are also thought to contribute to autonomic control [26]. Small fibers are therefore implicated in sensory and autonomic dysfunctions such as pain manifestation and enhancement.
Neuropathic pain can occur with damage to the peripheral or central nervous system. Peripheral sensitization involves the release of cellular mediators following peripheral nerve injury that act to sensitize nociceptors by altering the number and location of ion channels in both the nerve fiber and dorsal root ganglia. This enables a greater influx of action potential-generating ions, subsequently lowering the depolarization threshold so that the response of nociceptors to stimuli such as cold is heightened [21].
Another mechanism involves cross-talk among neighboring injured and uninjured fibers. The persistent changes occurring due to nerve injury can enable chemically mediated electrical connections from the affected to the unaffected fibers. This causes stimuli that would not typically be painful to initiate activity in the usually inactivated nociceptors [21]. De Medinaceli et al. suggest that the incomplete recovery of regenerated fiber diameter may contribute to cold intolerance. Conduction velocity is slowed in small-diameter fibers and with exposure to cold. The reduction in fiber diameter from trauma can permanently distort the neural signal so that it is perceived as nociceptive. This condition of peripheral desynchronization is further aggravated by the cold [6].
Klein-Weigel and colleagues noted a significant reduction in skin vessel density in cold-intolerant fingertips. This reduction in vessel density may reflect a decline in thermal-modulation capabilities in these areas. These results can be extrapolated to previous findings with defects in the restoration of vascular nerve plexuses to suggest that this may lead to cold-intolerance symptoms [12]. While these results do not necessarily confirm the hypothesis that macrovascular and microcirculatory failures are detrimental to cold tolerance in digit replants, they do imply that a reduction in skin vessel density may correspond to hindrance in thermal modulation. If this is the case, the cold-provocation protocol tested in the present study should reveal disturbed temperature recovery in nerve-injury subjects.
Measuring Cold Intolerance
While the natural history and pathophysiology of cold intolerance remain disputable, it is apparent to most clinicians that these symptoms can cause profound disablement and loss of productivity. There is little high-quality evidence to establish effectiveness of conservative, medical, or surgical interventions to treat these problems.
Self-report scales have proven to be excellent in determining the subjective presence of cold intolerance. The scales that are most reliable and sensitive to the vast array of symptoms are those that include questions applicable to daily living and varied severities of cold exposures. The CSSS is most relevant for testing the perception of cold sensitivity with regards to specific cold-intolerant scenarios [18]. The PRWHE rates wrist-related pain and disability in functional activities [9]. Together, these two scales may explain both the perception of and functional disability associated with post-traumatic cold intolerance.
Although previous authors have established that self-report questionnaires can be reliable, these alone provide insufficient measurement of the clinical phenomena for high-quality research. More objective tests of cold intolerance must be reproducible, sensitive, specific, and easily performed to be clinically applicable. The ICE protocol assumes that peripheral vascular response provides data that are at least moderately related to the underlying (vascular) etiology of cold intolerance and that readily available instrumentation adequately measures these changes. It should be recognized that in using readily available instrumentation, sensitivity may be less tenable than with more sophisticated technology [28].
A recent study by Feger and Braune [8] attempted to identify a standardized assessment for sympathetically mediated skin perfusion using laser-Doppler flowmetry and a modified cold pressor test. A ±2°C cooling pad was placed on the palmar surface of healthy subjects’ forearms for 20 s to induce vasoconstriction, excluding the confounding vasoconstriction effect that can result from inevitable hand movement in water-immersion protocols [8]. Laser-Doppler flowmetry provides a continuous, noninvasive measurement of microvascular perfusions with respect to relative changes in blood velocity and volume [23]. Both this provocation and measurement may be more accurate and reliable than simple water immersion and thermography. Test selection will depend on information needs, equipment availability, and practical considerations.
Since the ICE protocol measures digital recovery, it may not capture aspects of intolerance such as a neural component of etiology. De Medinaceli and colleagues sought to create a model of the peripheral nerve message to better understand the role of these nerves in the transmission of pain, especially cold-induced pain following neuropathy. The investigators were able to gather critical information concerning peripheral desynchronization and distorted peripheral messages indicative of cold-induced pain [6]. These findings suggest a need for further discovery into the functioning and measurement of peripheral nerve conductance. This and related work are needed for better measurement and design of interventions.
Limitations and Future Study
The present study was limited by the nature of the convenience population that volunteered. Most subjects were in their early 20s with only 11 comprising the >45 age group. It would be ideal to include a broader range of ages, especially older subjects, to truly examine the effect of aging on cold tolerance. Including a larger overall sample size with a more equal gender representation may also prove beneficial in determining the significance of some of the observed trends.
In trying to achieve a protocol suitable for a wide variety of applications, it is important to recognize that testing across multiple pathologies is required to fully establish the validity and applicability of the test. As noted, discomfort during testing is not uncommon, and others may find protocols that provide equal information with less discomfort. Ishitake et al. found that there were fewer negative side effects when hands were immersed up to the metacarpophalangeal joints as opposed to deeper submersion to the wrist. The results were comparable but with higher subject preference for the new depth due to reduced finger pain [11].
This study can be considered both developmental and promising. The availability of a standardized protocol with established reliability will provide clinicians and clinical researchers with a viable management option. Future studies involving patient populations and longitudinal data collection will be essential to further establish responsiveness. This study determined that ICE is a reliable means to provide objective and distinct information on cold response that can augment information provided by self-report.
Acknowledgements
Sincerest thanks go to M. Lomotan for her assistance with the logistical aspects of this study as well as in its preliminary data analysis. The following students must also be acknowledged for their contributions to the pilot study: L. Goring, J. Kasaboski, N. Rowling, S. Smith, L. Yates, N. Bridgelal, S. Rich, and J. Richardson.
References
- 1.Bear MF, Connors BW, Paradiso MA. The somatic sensory system. In Neuroscience: Exploring the Brain, 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 387–421.
- 2.Campbell DA, Kay SP. What is cold intolerance. J Hand Surg. 1998;23B(1):3–5. [DOI] [PubMed]
- 3.Collins ED, Novak CB, Mackinnon SE, et al. Long-term follow-up evaluation of cold sensitivity following nerve injury. J Hand Surg 1996;21A:1078–85. [DOI] [PubMed]
- 4.Coughlin PA, Chetter IC, Kent PJ, et al. The analysis of sensitivity, specificity, positive predictive value and negative predictive value of cold provocation thermography in the objective diagnosis of the hand–arm vibration syndrome. Occup Med 2000;51(2):75–80. [DOI] [PubMed]
- 5.Craigen M, Kleinert JM, Miller Crain G, et al. Patient and injury characteristics in the development of cold sensitvity of the hand: A prospective cohort study. J Hand Surg 1999;24A:8–15. [DOI] [PubMed]
- 6.de Medinaceli L, Hurpeau J-C, Merle M, et al. Cold and post-traumatic pain: modeling of the peripheral nerve message. BioSystems 1997;43:145–67. [DOI] [PubMed]
- 7.Engkvist O, Wahren LK, Wallin G, et al. Effects of regional intravenous guanethidine block in posttraumatic cold intolerance in hand amputees. J Hand Surg 1985;10B:145–50. [DOI] [PubMed]
- 8.Feger J, Braune S. Measurement of skin vasoconstrictor response in healthy subjects. Auton Neurosci 2005;120:88–96. [DOI] [PubMed]
- 9.Harris JE, MacDermid JC, Roth J. The International Classification of Functioning as an explanatory model of health after distal radius fracture: A cohort study. Health Qual Life Outcome 2005;3:7381. [DOI] [PMC free article] [PubMed]
- 10.Irwin MS, Gilbert SEA, Terenghi G, et al. Cold intolerance following peripheral nerve injury. J Hand Surg 1997;22B(3):308–16. [DOI] [PubMed]
- 11.Ishitake T, Kihara T, Matoba T. A revised cold water immersion test for assessing peripheral circulatory function. Kurume Med J 1996;43(1):11–5. [DOI] [PubMed]
- 12.Klein-Weigel P, Pavelka M, Dabernig J, et al. Macro- and microcirculatory assessment of cold sensitivity after traumatic finger amputation and microsurgical replantation. Arch Orthop Trauma Surg 2007;127(5):355–60. [DOI] [PubMed]
- 13.Koman LA, Nunley JA, Goldner JL, et al. Isolated cold stress testing in the assessment of symptoms in the upper extremity: Preliminary communication. J Hand Surg 1984;9A:305–13. [DOI] [PubMed]
- 14.Lithell M, Backman C, Nystrom A. Pattern recognition in post-traumatic cold intolerance. J Hand Surg 1997;22B(6):783–7. [DOI] [PubMed]
- 15.Lithell M, Backman C, Nystrom A. Cold intolerance is not more common or disabling after digital replantation than after other treatment of compound digital injuries. Ann Plast Surg 1998;40:256–9. [DOI] [PubMed]
- 16.MacDermid JC. Measurement of health outcomes following tendon and nerve repair. J Hand Ther 2005;18:297–312. [DOI] [PubMed]
- 17.Mason HJ, Poole K, Saxton J. A critique of a UK standardized test of finger rewarming after cold provocation in the diagnosis and staging of hand–arm vibration syndrome. Occup Med 2003;53:325–30. [DOI] [PubMed]
- 18.McCabe SJ, Mizgala C, Glickman C. The measurement of cold sensitivity of the hand. J Hand Surg 1991;16A:1037–40. [DOI] [PubMed]
- 19.Nancarrow JD, Rai SA, Sterne GD, et al. The natural history of cold intolerance of the hand. Injury 1996;27(9):607–11. [DOI] [PubMed]
- 20.Nylander G, Nylander E, Lassvik C. Cold sensitivity after replantation in relation to arterial circulation and vasoregulation. J Hand Surg 1987;12B(1):78–81. [DOI] [PubMed]
- 21.Pasero C. Pathophysiology of neuropathic pain. Pain Manag Nurs 2004;5(4):3–8. [DOI] [PubMed]
- 22.Patel AA, Donegan D, Albert T. The 36-item short form. J Am Acad Orthop Surg 2007;15(2):126–34. [DOI] [PubMed]
- 23.Pollock FE, Koman LA, Smith BP, et al. Measurement of hand microvascular blood flow with isolated cold stress testing and laser Doppler fluxmetry. J Hand Surg 1993;18(A):143–50. [DOI] [PubMed]
- 24.Ruch DS, Vallee J, Li Z, et al. The acute effect of peripheral nerve transection on digital thermoregulatory function. J Hand Surg 2003;28A:481–8. [DOI] [PubMed]
- 25.Ruijs ACJ, Jaquet J-B, Daanen HAM, et al. Cold intolerance of the hand measured by the CISS questionnaire in a normative study population. J Hand Surg 2006;31B(5):533–6. [DOI] [PubMed]
- 26.Santiago S, Ferrer T, Espinosa ML. Neurophysiological studies of thin myelinated (A delta) and unmyelinated (C) fibers: application to peripheral neuropathies. Neurophysiol Clin 2000;30:27–42. [DOI] [PubMed]
- 27.Schlenker JD, Kleinert HE, Tsai T-M. Methods and results of replantation following tramatic amputation of the thumb in sixty-four patients. J Hand Surg 1980;5:63–70. [DOI] [PubMed]
- 28.Shy ME, Frohman EM, So YT, et al. Quantitative sensory testing: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2003;60(6):898–904. [DOI] [PubMed]
- 29.Toibana N, Sakakibara H, Hirata M, et al. Thermal perception threshold testing for the evaluation of small sensory nerve fiber injury in patients with hand–arm vibration syndrome. Ind Health 2000;38(4):366–71. [DOI] [PubMed]