Abstract
Solitary juvenile xanthogranuloma (JXG) in the spinal column is extremely rare and there has been no report of such a lesion involving C1 and C2 in English literature so far. Here, we report and characterize the first case of xanthogranuloma of the upper cervical spine. This case report draws attention to the fact that solitary xanthogranuloma should be considered among possible diagnoses of spinal tumor in children and young adults. An 18-year-old female patient presented to the hospital with intermittent pain in the right side of the neck. MRI studies revealed a huge soft tissue mass to the right side of the C1 and C2 vertebras, and osseous destruction can be found in the cervical spine CT scan. Complete surgical removal of the tumor and occipital–cervical instrumentation with autogenous bone graft were performed with no complications. The patient was free of pain immediately after the surgery with intact neurological functions. Follow-up MRI 6 and 12 months after the surgery showed no residue or recurrence of the tumor. Our report and the literature review indicate that isolated JXG does not show any predilections of localization inside the central nervous system. So a solitary xanthogranuloma should be considered among possible diagnoses of spinal tumor in susceptible patients. Localized JXG shows isointense signals in MRI and enhances homogeneously with gadolinium. Immunohistochemical studies can ensure the diagnosis. Whenever possible, total surgical removal alone seems to be curative.
Keywords: Juvenile xanthogranuloma, Cervical spine tumor, Total resection
Introduction
Xanthogranuloma is a proliferative disorder of non-Langerhans histiocytes [4]. In the vast majority of cases, xanthogranulomas occur as multiple, self-limited, cutaneous lesions of children in the first two decades of life, and thus have been defined as juvenile xanthogranuloma (JXG) [7, 12]. Extracutaneous manifestations of JXG are uncommon, and isolated JXG involving the spinal column is extremely rare [5]. Here, we report and characterize a case of solitary JXG in the upper cervical spine, which to the best of our knowledge is the first JXG case in the upper cervical spine, and make a systematic review about the relevant literatures.
Case report
History and examination
An 18-year-old female patient presented to our institution with complaints of intermittent pain in the right side of the neck lasting for about 1 week. The pain exacerbated at night and worsened with motion. Resting, physical therapy, and medication had little effect in controlling the pain.
Physical examination demonstrated a blood pressure of 120/70 mmHg. Inspection of the skin revealed no lesion. On palpation, a round-shaped mass could be found under the mastoid process in the right side of the neck. The mass was fixed and tenacious, with no obvious tenderness. Flexion–extension and rotation of the cervical spine were slightly limited. Superficial sensation in the right side of the neck was decreased. However, no dysesthesia appeared in other parts of the body. The results of the neurological and musculoskeletal examinations were otherwise unremarkable.
Radiological studies
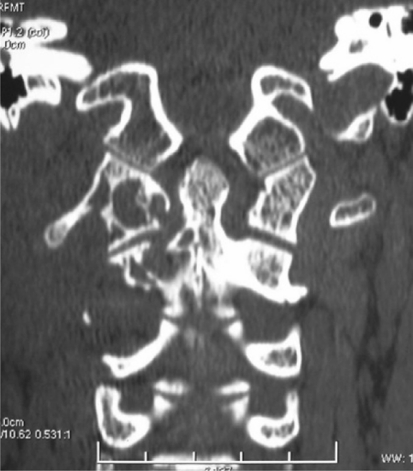
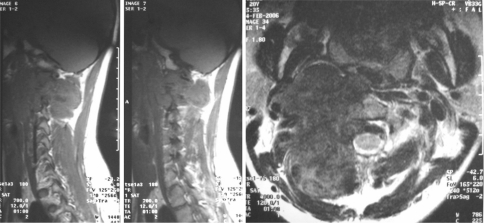
CT scan of the upper cervical spine revealed a large round mass about 4.0 × 5.0 × 3.5 cm in size with osseous destruction in the right lateral masses and appendix of the C1 and C2 vertebras (Fig. 1). And there were irregular calcifications in the mass. MRI studies revealed a soft tissue tumor with osseous destruction in the upper cervical spine, which extended well into the surrounding structures. The tumor had a clear boundary, and the right side of the vertebral artery was enwrapped in the tumor. The tumor showed isointense to hypointense mixed signals in T1WI and T2WI, but hyperintense signals after gadolinium enhancement, with focuses of necrosis in it. The spinal cord was severely compressed at C2 level, but no denatured signals came out (Fig. 2).
Fig. 1.
Preoperative CT scan showed a large mass with osteolytic defect in the right lateral masses and appendix of the C1 and C2 vertebras
Fig. 2.
Preoperative MRI scan showed osseous destruction in the right lateral masses and appendix of the C1 and C2 vertebras, and a huge mass in the soft tissue to the right side of the C1 and C2 vertebras. a Axial view, b transverse view
Treatment
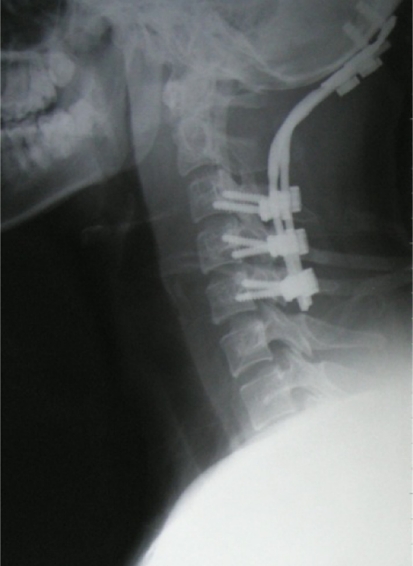
We performed an anterior cervical spine tumor resection and a posterior occipital–cervical fusion with instrumentation for this patient. We have adopted an incision extending from the right mastoid process to submaxilla. And through this approach, a round yellowish encapsulated tumor about 4.0 × 5.0 × 3.5 cm in size was exposed. The tumor was tenacious and with limited motion. It originated from the C2 nerve root and involved the right lateral mass of the C1 and the right part of the vertebral body of the C2. After ligation of the right vertebral artery, the tumor was blunt dissected and subtotally removed. Then, a posterior operation was performed. The tumor was totally resected after removal of the posterior elements of C1 and C2, and then an occipital–cervical instrumentation with autogenous bone graft was performed (Fig. 3).
Fig. 3.
Lateral view of the cervical spine after the operation of tumor resection and spine reconstruction
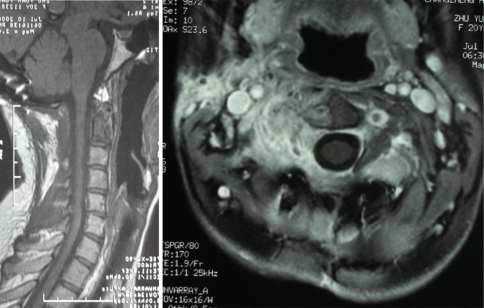
After surgery, the patient was relieved of pain, and could walk without difficulty. Follow-up visits have been scheduled at regular intervals to monitor the tumor recurrence and associated complications. Cervical spine MRI with gadolinium enhancement 6 and 12 months after the surgery demonstrated no residue or recurrence of the tumor (Fig. 4). Now the clinical and radiological follow-up has extended to 2 years with no relapse of the symptoms, and the patient has already returned to school.
Fig. 4.
Postoperative MRI study 12 months after the surgery showed no residue or recurrence of the tumor. a Axial view, b transverse view
Pathology
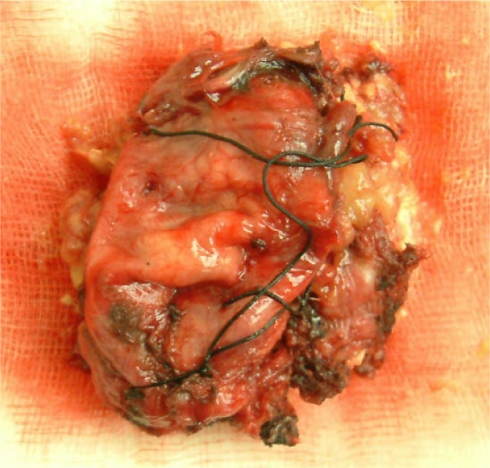
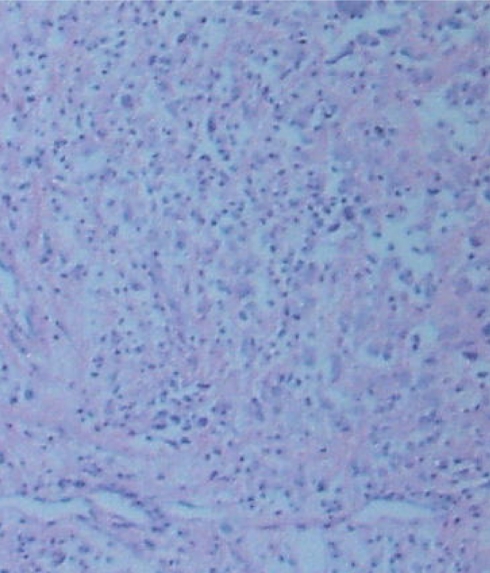
Gross examination showed a 4.0 × 5.0 × 3.5 cm, ovoid, circumscribed, and encapsulated specimen (Fig. 5). The cut surface was homogeneous yellow. Microscopic examination revealed a xanthic appearance, with typical Touton giant cells (Fig. 6). Immunohistochemical studies showed the tumor cells were strongly inmmunopositive for CD68, CD163, and vimentin, in addition with the presence of histiocytes and multinucleated Touton giant cells, the diagnosis of Xanthogranuloma was established. Other immunohistochemical findings include: My (−), Ly (−), aAT (+), and NSE (−).
Fig. 5.
Gross sample of the cervical xanthogranuloma
Fig. 6.
Microscopical view of cervical xanthogranuloma
Discussion
The histiocytic disorders, first described by Adamson [1], encompass a spectrum of diseases in which the principal pathological cells are macrophages and dendritic cells [4]. JXG is one of the dendritic cell-related disorders, and has been classified as a non-LCH disease together with several other histiocytic entities including papular xanthoma, benign cephalic histiocytosis, sinus histiocytosis with massive lymphadenopathy (Rosai–Dorfman disease), and hemophagocytic histiocytosis [3]. The etiology of JXG is unknown. However, it is believed to result from a disordered macrophage response to a non-specific tissue injury, resulting in a granulomatous reaction [10].
Classic JXGs are benign, usually asymptomatic, and solitary or multiple red-to-yellowish papules and nodules composed of histiocytic cells that predominantly occur in infancy and childhood, which typically regress over several years [17]. The most frequently occurrence of the JXG lesion is on the skin of head and neck, but many extracutaneous sites have been reported. Extracutaneous involvement occurs in about 5–10% of all JXG cases. The eye, particularly the uveal tract, is the most frequent site of extracutaneous involvement. And other reported involving organs include the oropharynx, heart, lung, liver, spleen, adrenals, muscles, subcutaneous tissues, and the central nervous system, but the involvement of spine is extremely rare [2, 11, 15]. We could find only four such cases reported in English [8, 9, 15, 16] so far, and no report of a xanthogranuloma involving C1 and C2 (Table 1). The present case, to the best of our knowledge, is the first solitary JXG case involving the upper cervical spine, which originated from the C2 nerve root. In all xanthogranuloma cases involving the spine, the clinical features were mostly related with the anatomic localization as slow-growing tumors in general. MRI is the best method for obtaining details of the localization of the tumors and their relation to adjacent structures. The lesion may appear hypointense, isointense, or slightly hyperintense in T1WI and T2WI. And homogeneously enhancement with gadolinium can be observed in most cases.
Table 1.
Solitary juvenile xanthogranuloma cases involving the spine reported in the literature (2007)
| Shimosawa et al. [16] | Kitchen et al. [10] | Kim et al. [9] | Rampini et al. [15] | Present case | |
|---|---|---|---|---|---|
| Age | 13 months | 15 years | 16 months | 34 months | 18 years |
| Sex | Female | Female | Female | Female | Female |
| Symptoms and signs | Difficult stance, mild spastic paraparesis | Lower back and leg pain, Lase`gue positive | Difficult stance and gait, spastic paraparesis | Intense cervical and right brachial pain, tetraparesis muscular hypotrophy | Intermittent pain in the right side of the neck |
| Tumor localization | Intradural extramedullary T6–T9 | S1 nerve root sheath | Intradural extramedullary T1–T2 | Intradural extramedullary C5–C7 | C2 nerve root |
| CT | – | No contrast enhancement | – | – | Irregular calcifications in the mass after enhancement |
| MRI | Hypointense in T1; hypointense in T2; no contrast enhancement | Isointense in T1; hypointense in T2; isointense in DP; no contrast administered | Isointense in T1; hyperintense in T2; homogeneous enhancement | T1 isointense; T2 isointense; homogeneous enhancement | Isointense to hypointense in T1 and T2, hyperintense enhancement |
| Surgical approach | Five-level laminoplastic laminectomy | One-level laminectomy, sacral-hiatus resection | Four-level laminectomy | Four-level open-door laminoplasty | C1–C2 laminectomy |
| Tumor relation to adjacent structures | Adherent to inner layer of dura mater; arachnoid intact | Adherent to S1 nerve root sheath | Adherent to inner layer of dura mater; arachnoid intact | Adherent to inner layer of dura mater; arachnoid intact | Originated from C2 nerve root and involved the right lateral mass of the C1 and C2 |
| Surgical resection | Total | Total | Total | Total | Total |
JXG is difficult to distinguish intraoperatively with other tumors of neural origin (e.g., schwannoma, neurofibroma, nerve sheath myxoma, malignant nerve sheath tumor). Therefore, the pathological and immunohistochemical studies remain the golden standard for achieving a diagnosis of JXG. The tumor is typically a round lesion, with a yellowish to grayish appearance on the cut surface. Microscopically, a diffuse and/or nodular pattern of growth and a pushing border are apparent at low magnification. The typical cellular composition of the lesions consist one or more of the three basic cellular types: mononuclear cells, multinucleated cells with or without Touton features, and spindle cells. When present, the characteristic Touton giant cells can be found in a background of mononuclear cells. If the tumor involves the bony structure, large osteolytic lesions with well-demarcated margins can be found along with osteoclast-like giant cells that are intermixed with Touton giant cells and mononuclear cells in the bony lesions [12]. Immunohistochemistry has an important role in the diagnosis of JXG. It is our experience that regardless of the cellular composition of a JXG, the mononuclear cells, giant cells, and spindle cells are consistently immunoreactive for vimentin, CD68, CD163, fascin, CD14 and factor XIIIa and non-reactive for CD1a. And in most cases, S-100 protein is also non-reactive, but scattered cells may show weak cytoplasmic reactivity, unlike the more diffuse and intense reaction of Langerhans cells [10, 12–14].
The pathological differentiation of a lesion as LCH or non-LCH is of considerable clinical importance. Unlike the majority of non-Langerhans histiocytoses, LCH is associated with considerable morbidity and mortality, often requiring aggressive therapy [17]. Langerhans cells are defined by the presence of Birbeck granules, which are observable in electron micrographs as pentilaminar cytoplasmic inclusions that often have a tennis racket appearance [4]. Although not diagnostic, Langerhans cells may also be identified by the presence of S-100 and CD1a staining by immunohistochemistry [4, 6]. In the present case, none of the characteristics of Langerhans cells was observed.
Currently, there is no standard treatment for JXG. The severity and location of the lesion dictates the course of treatment. Spontaneous regression of the skin lesions is the natural course, but in cases involving the spine, there’s no regression documented so far. For such an invasive benign tumor in the upper cervical spine, total removal of the tumor seems to be curative [15]. The recurrence of the tumor is unlikely to happen after the total resection, and neurological functions can be well preserved if no damage to the neural structures had been done during the tumor resection. If total resection cannot be achieved, the patients should be followed and given close medical observation after a subtotal resection. And if the symptom recurred, a second operation may be appropriate.
Conflict of interest statement
During the preparation of this article, no author has received any financial support or any other commercial associations that might pose a conflict of interest in connection with the submitted article. This article is totally the authors’ own work.
Footnotes
Each author’s involvement in the production of the manuscript: Cao Dong, in charge of the whole preparation and submission process of this article. Ma Junming, in charge of the gathering of the clinical data. Yang Xinghai, in charge of the editorial work of the article. Xiao Jianru, chief of all the clinical and editorial work, the one who contributes most in the publication of this article.
References
- 1.Adamson HG. Congenital xanthoma multiplex. Br J Dermatol. 1905;17:222. [Google Scholar]
- 2.Bostrom J, Janssen G, Messing-Junger M, Felsberg JU, Neuen-Jacob E, Engelbrecht V, Lenard HG, Bock WJ, Reifenberger G. Multiple intracranial juvenile xanthogranulomas: case report. J Neurosurg. 2000;93:335–341. doi: 10.3171/jns.2000.93.2.0335. [DOI] [PubMed] [Google Scholar]
- 3.Burgdorf WHC, Zelger B. The nonLangerhan’s cell histiocytosis in childhood. Cutis. 1996;58:201–207. [PubMed] [Google Scholar]
- 4.Dehner LP. Juvenile xanthogranulomas in the first two decades of life. Am J Surg Pathol. 2003;27(5):579–593. doi: 10.1097/00000478-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Favara BE, Feller AC, Pauli M, Jaffe ES, Weiss LM, Arico M, Bucsky P, Egeler RM, Elinder G, Gadner H, Gresik M, Henter JI, Imashuku S, Janka-Schaub G, Jaffe R, Ladisch S, Nezelof C, Pritchard J. Contemporary classification of histiocytic disorders: the WHO committee on histiocytic/reticulum cell proliferations—reclassification working group of the histiocyte society. Med Pediatr Oncol. 1997;29:157–166. doi: 10.1002/(SICI)1096-911X(199709)29:3<157::AID-MPO1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 6.Freyer DR, Kennedy R, Bostrom BC, Kohut G, Dehner LP. Juvenile xanthogranuloma: forms of systemic disease and their clinical implications. J Pediatr. 1996;129:227–237. doi: 10.1016/S0022-3476(96)70247-0. [DOI] [PubMed] [Google Scholar]
- 7.George DH, Scheithauer BW, Hilton DL, Fakhouri AJ, Kraus EW. Juvenile xanthogranuloma of peripheral nerve: a report of two cases. Am J Surg Pathol. 2001;25:521–526. doi: 10.1097/00000478-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez-Martin A, Baselga E, Drolet BA, Esterly NB. Juvenile xanthogranuloma. J Am Acad Dermatol. 1997;36:355–369. doi: 10.1016/S0190-9622(97)80207-1. [DOI] [PubMed] [Google Scholar]
- 9.Kim D, Kim T, Choi J. Intradural extramedullary xanthoma of the spine. A rare lesion arising from the dura mater of the spine: case report. Neurosurgery. 1996;39:182–185. doi: 10.1097/00006123-199607000-00042. [DOI] [PubMed] [Google Scholar]
- 10.Kitchen ND, Davies MS, Taylor W. Juvenile xanthogranuloma of nerve root origin. Br J Neurosurg. 1995;9:233–237. doi: 10.1080/02688699550041629. [DOI] [PubMed] [Google Scholar]
- 11.Kraus MD, Haley JC, Ruiz R, Essary L, Moran CA, Fletcher CD. “Juvenile” xanthogranuloma: an immunophenotypic study with a reappraisal of histogenesis. Am J Dermatopathol. 2001;23:104–111. doi: 10.1097/00000372-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Lesniak MS, Viglione MP, Weingart J. Multicentric parenchymal xanthogranuloma in a child: case report and review of the literature. Neurosurgery. 2002;51:1493–1498. doi: 10.1097/00006123-200212000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Nascimento AG. A clinicopathologic and immunohistochemical comparative study of cutaneous and intramuscular forms of juvenile xanthogranuloma. Am J Surg Pathol. 1997;21:645–652. doi: 10.1097/00000478-199706000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Newman CC, Raimer SS, Sanchez RL. Nonlipidized juvenile xanthogranuloma: a histologic and immunohistochemical study. Pediatr Dermatol. 1997;14:98–102. doi: 10.1111/j.1525-1470.1997.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 15.Rampini PM, Alimehmeti RH, Egidi MG, Zavanone ML, Bauer D, Fossali E, Villani RM. Isolated cervical juvenile xanthogranuloma in childhood. Spine. 2001;26:1392–1395. doi: 10.1097/00007632-200106150-00026. [DOI] [PubMed] [Google Scholar]
- 16.Shimosawa S, Tohyama K, Shibayama M. Spinal xanthogranuloma in a child: case report. Surg Neurol. 1993;39:138–142. doi: 10.1016/0090-3019(93)90092-F. [DOI] [PubMed] [Google Scholar]
- 17.Zelger BWH, Sidroff A, Orchard G, Cerio R. Non-Langerhans cell histiocytoses: a new unifying concept. Am J Dermatopathol. 1996;18:490–504. doi: 10.1097/00000372-199610000-00008. [DOI] [PubMed] [Google Scholar]