Abstract
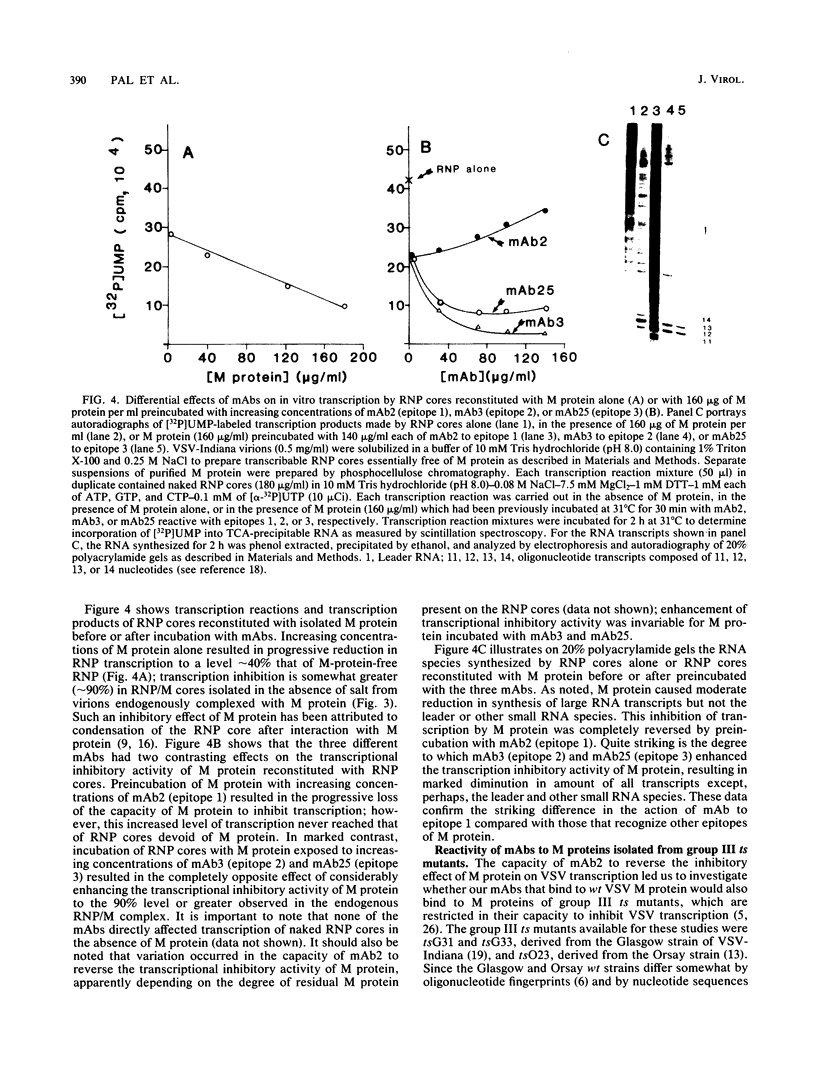
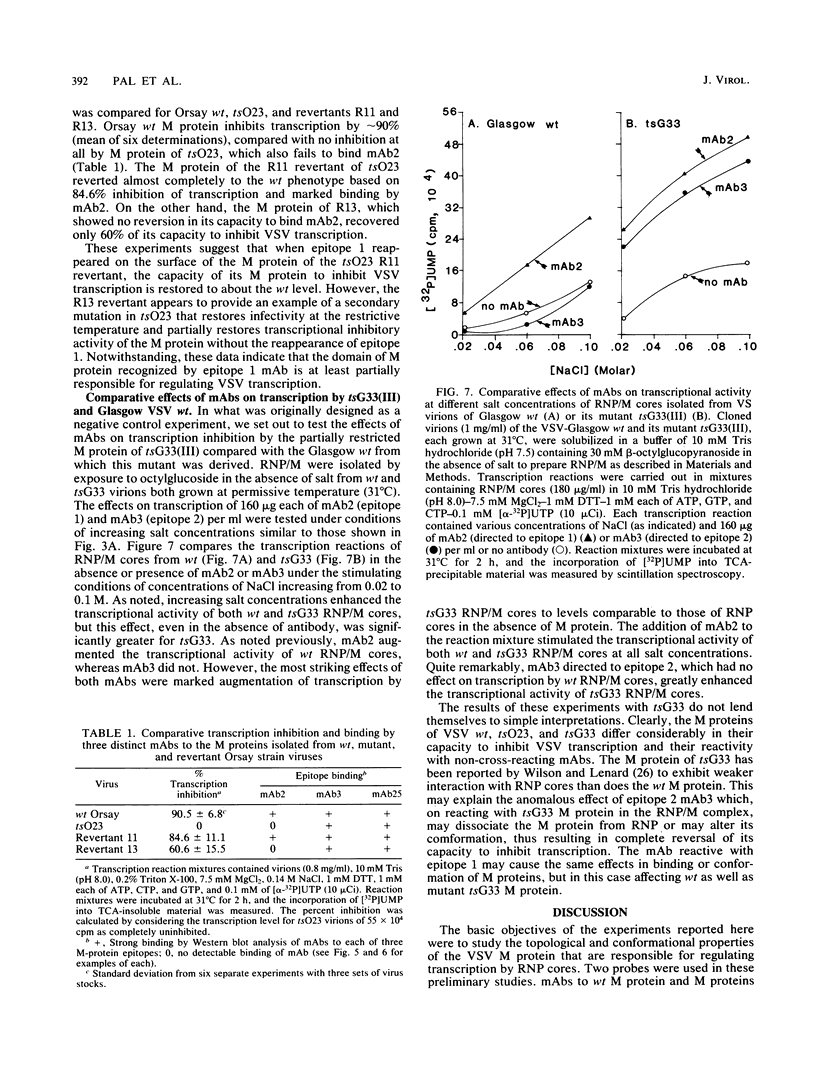
The ability of the matrix (M) protein of wild-type vesicular stomatitis virus (VSV) to regulate viral transcription was studied with monoclonal antibodies and temperature-sensitive (ts) mutants in complementation group III, the M proteins of which are restricted in transcription inhibition. The marked inhibition of transcription by VSV ribonucleoprotein (RNP) cores complexed with M protein (RNP/M) was reversed by antibody to epitope 1. Antibodies to epitopes 2 and 3 not only failed to reverse the transcription-inhibitory activity of isolated M protein but actually increased M-protein inhibition of transcription in a reconstituted system. Monoclonal antibodies to epitopes 2 and 3 strongly bound to M proteins from all wild-type and ts-mutant virions, but monoclonal antibody to epitope 1 completely failed to bind to the M protein of ts023(III) even though it reacted strongly with M proteins of mutants tsG31(III) and tsG33(III). The M protein of a tsO23 revertant (R11) completely recovered its capacity to inhibit transcription and to bind monoclonal antibody to epitope 1, whereas the M proteins of three other revertants remained restricted in their capacity to inhibit transcription and to bind monoclonal antibody to epitope 1. These studies indicate that exposure of epitope 1 on the surface of M protein is essential for inhibiting transcription by VSV RNP cores.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Banerjee A. K. Sequential transcription of the genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 May;73(5):1504–1508. doi: 10.1073/pnas.73.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenholz Y., Moore N. F., Wagner R. R. Enveloped viruses as model membrane systems: microviscosity of vesicular stomatitis virus and host cell membranes. Biochemistry. 1976 Aug 10;15(16):3563–3570. doi: 10.1021/bi00661a026. [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Reversal by certain polyanions of an endogenous inhibitor of the vesicular stomatitis virus-associated transcriptase. J Biol Chem. 1978 May 25;253(10):3361–3363. [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewley J. P., Bishop D. H., Kang C. Y., Coffin J., Schnitzlein W. M., Reichmann M. E., Shope R. E. Oligonucleotide fingerprints of RNA species obtained from rhabdoviruses belonging to the vesicular stomatitis virus subgroup. J Virol. 1977 Jul;23(1):152–166. doi: 10.1128/jvi.23.1.152-166.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Little S. P., Hagen F. S., Huang A. S. The matrix (M) protein of vesicular stomatitis virus regulates transcription. Cell. 1978 Dec;15(4):1455–1462. doi: 10.1016/0092-8674(78)90069-7. [DOI] [PubMed] [Google Scholar]
- Combard A., Printz Ane C. Inhibition of vesicular stomatitis virus transcriptase complex by the virion envelope M protein. Biochem Biophys Res Commun. 1979 May 14;88(1):117–123. doi: 10.1016/0006-291x(79)91704-2. [DOI] [PubMed] [Google Scholar]
- De B. P., Thornton G. B., Luk D., Banerjee A. K. Purified matrix protein of vesicular stomatitis virus blocks viral transcription in vitro. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7137–7141. doi: 10.1073/pnas.79.23.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U. Reconstitution studies detect a single polymerase entry site on the vesicular stomatitis virus genome. Cell. 1982 Dec;31(3 Pt 2):635–642. doi: 10.1016/0092-8674(82)90319-1. [DOI] [PubMed] [Google Scholar]
- Emerson S. U. Vesicular stomatitis virus: structure and function of virion components. Curr Top Microbiol Immunol. 1976;73:1–34. doi: 10.1007/978-3-642-66306-2_1. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Flamand A. Etude génétique du virus de la stomatite vésiculaire: classement de mutants thermosensibles spontanés en groupes de complémentation. J Gen Virol. 1970 Sep;8(3):187–195. doi: 10.1099/0022-1317-8-3-187. [DOI] [PubMed] [Google Scholar]
- Grinnell B. W., Wagner R. R. Nucleotide sequence and secondary structure of VSV leader RNA and homologous DNA involved in inhibition of DNA-dependent transcription. Cell. 1984 Feb;36(2):533–543. doi: 10.1016/0092-8674(84)90246-0. [DOI] [PubMed] [Google Scholar]
- Newcomb W. W., Tobin G. J., McGowan J. J., Brown J. C. In vitro reassembly of vesicular stomatitis virus skeletons. J Virol. 1982 Mar;41(3):1055–1062. doi: 10.1128/jvi.41.3.1055-1062.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Grinnell B. W., Snyder R. M., Wiener J. R., Volk W. A., Wagner R. R. Monoclonal antibodies to the M protein of vesicular stomatitis virus (Indiana serotype) and to a cDNA M gene expression product. J Virol. 1985 Aug;55(2):298–306. doi: 10.1128/jvi.55.2.298-306.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney D. F., Emerson S. U. In vitro synthesis of triphosphate-initiated N-gene mRNA oligonucleotides is regulated by the matrix protein of vesicular stomatitis virus. J Virol. 1982 Jun;42(3):897–904. doi: 10.1128/jvi.42.3.897-904.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloemer R. H., Wagner R. R. Association of vesicular stomatitis virus glycoprotein with virion membrane: characterization of the lipophilic tail fragment. J Virol. 1975 Aug;16(2):237–240. doi: 10.1128/jvi.16.2.237-240.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk W. A., Synder R. M., Benjamin D. C., Wagner R. R. Monoclonal antibodies to the glycoprotein of vesicular stomatitis virus: comparative neutralizing activity. J Virol. 1982 Apr;42(1):220–227. doi: 10.1128/jvi.42.1.220-227.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener J. R., Pal R., Barenholz Y., Wagner R. R. Influence of the peripheral matrix protein of vesicular stomatitis virus on the membrane dynamics of mixed phospholipid vesicles: fluorescence studies. Biochemistry. 1983 Apr 26;22(9):2162–2170. doi: 10.1021/bi00278a017. [DOI] [PubMed] [Google Scholar]
- Wilson T., Lenard J. Interaction of wild-type and mutant M protein vesicular stomatitis virus with nucleocapsids in vitro. Biochemistry. 1981 Mar 3;20(5):1349–1354. doi: 10.1021/bi00508a048. [DOI] [PubMed] [Google Scholar]
- Zakowski J. J., Petri W. A., Jr, Wagner R. R. Role of matrix protein in assembling the membrane of vesicular stomatitis virus: reconstitution of matrix protein with negatively charged phospholipid vesicles. Biochemistry. 1981 Jun 23;20(13):3902–3907. doi: 10.1021/bi00516a037. [DOI] [PubMed] [Google Scholar]
- Zakowski J. J., Wagner R. R. Localization of membrane-associated proteins in vesicular stomatitis virus by use of hydrophobic membrane probes and cross-linking reagents. J Virol. 1980 Oct;36(1):93–102. doi: 10.1128/jvi.36.1.93-102.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]







