Abstract
Nowadays, lumbar spondylosis is one of the most frequent causes of lower back pain. In order to improve our understanding of the lumbar spine anatomy and functionality over time, we compared the lumbar vertebrae of Neanderthals with those of anatomically modern humans. The fossil record reports on only two Neanderthal skeletons (i.e., Kebara 2 and Shanidar 3, both predating the appearance of modern humans) with full preservation of the entire lumbar spine. Examination of these early hominids showed that they display natural lumbar kyphosis, with only mild degenerative changes of the lumbar spine (ages at death: 30–35 years, Kebara 2; and 35–50 years, Shanidar 3). This finding is highly unexpected since Neanderthals are known to have had extraordinary physical activity due to demanding living conditions. The adult lumbar spines discussed here therefore show no correlation between high physical activity and degenerative spine disease as known from recent times. We speculate that both the kyphosis itself and the massive and heavily muscled skeleton of Neanderthals are causative for the minimal bone degeneration. We conclude that a kyphotic lumbar spine is the natural anatomy in these two Neanderthal individuals. Future research will reveal if this holds true for the entire Neanderthal species.
Keywords: Paleopathology, Neanderthal man, Lumbar spine, Kyphosis, Lordosis, Spondylosis
Introduction
The vertebral column is an important element in bipedalism in humans. Neanderthal man is the most well-known late archaic Homo species. They lived in areas ranging from western Europe to southwest Asia from about 150,000 to about 28,000 years ago (i.e., the late Pleistocene). Neanderthal fossils are quite common compared to those of earlier forms of Homo, because of the Neanderthal rites of intentional burial. However, due to the aeriferous components of the vertebrae bodies the lumbar spine is rarely preserved [5, 12]. Only in two specimens, termed Kebara 2 and Shanidar 3, the lumbar spine is fully preserved [2, 3, 9].
In order to increase our understanding of the lumbar spine in modern humans, we compared the fully preserved lumbar vertebrae of the two Neanderthal individuals with those of modern humans. We wanted to answer two main questions: do Neanderthals and anatomically modern humans display the same morphology of the lumbar spine, and do they share the same forms of degenerative changes ?
Case report
Kebara cave is a limestone cave locality of the wadi Kebara (northern Israel). By far the most significant discovery made at Kebara cave in 1982 was that of the most complete Neanderthal skeleton dating from 60,000 to 48,000 years ago (Kebara 2, nicknamed “Moshe”). This skeleton retains a large part of the torso (vertebral column, ribs and pelvis)—however, the head and lower limbs are missing [2, 3, 11, 14]. Our re-examination of this specimen revealed signs of lumbar anomalies. These include mild degenerative changes (Fig. 1) and differences in the lumbar spine morphology when compared to modern humans. Especially the height of the anterior and posterior vertebral bodies differ from the Homo sapiens morphology, which results in ventrally wedged vertebrae (Table 1; Fig. 2). The anterior and posterior vertebral body heights of the second Neanderthal skeleton showing a fully preserved lumbar spine, and called Shanidar 3, fit well into this picture (Table 1; see [9]).
Fig. 1.
All lumbar spine elements constituting a joint unit in the Neanderthal skeleton Kebara 2 were examined for evidence of degenerative changes, and only two regions of mild degenerative spine disease were found: a Marginal osteophytes without uneven joint surfaces (grade 1 in [17]) in the right L5–S1 facet joint (spondylarthrosis); b marginal osteophytes on the left lateral part of the vertebral bodies L3 and L4 (spondylosis)
Table 1.
Anterior and posterior heights of the lumbar vertebral bodies (ventral height/dorsal height in mm) in modern humans and two Neanderthal skeletons (Kebara 2 and Shanidar 3), revealing ventrally wedged L1–L4 vertebral bodies in both Neanderthals
| Vertebrae | Homo sapiensa | Homo neanderthalensis | |
|---|---|---|---|
| Kebara 2b | Shanidar 3c | ||
| Level | |||
| L1 | 25.0/25.8 | 24/26 | 23.4/29.2 |
| L2 | 27.9/25.2 | 23/26 | 24.2/28.4 |
| L3 | 27.4/26.0 | 22/27 | 26.7/28.8 |
| L4 | 26.7/26.4 | 25/26 | 29.0/29.3 |
| L5 | 28.7/23.1 | 27/22 | 30.0/25.7 |
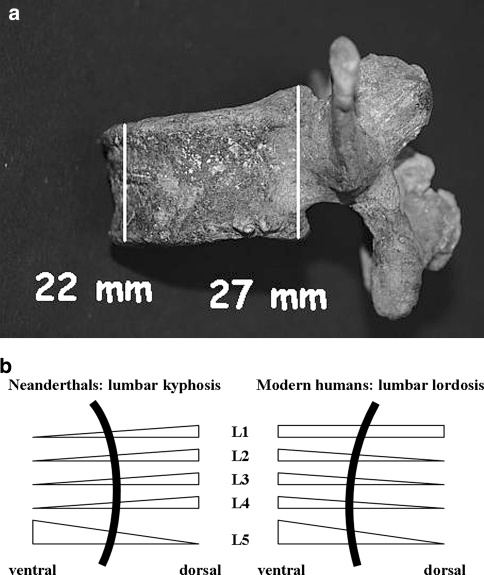
Fig. 2.
The bodies of modern humans' L2–L5 vertebrae are dorsally wedged, whereas L1–L4 vertebrae in the two tested Neanderthals are ventrally wedged. The vertebral body morphology in both Neanderthals reveals a ventral curvature of the spine, i.e., lumbar kyphosis. a Ventrally wedged vertebra L3 in the skeleton termed Kebara 2. b sketch of the sagittal curvature of the lumber spine in Neanderthals and modern humans
Discussion
Morphology of the spine
The question of the aetiology of the thoracic ventral curve (kyphosis) was addressed as early as 1879, when Aeby [1] stated that the bony configuration of the vertebral body, which is ventrally wedged, accounted for this curve. Hence, it appears that the kyphosis in the thoracic spine is present owing to phylogeny, while the cervical and lumbar curves are adaptive.
Normally, the vertebrae bodies L2 to L5 of modern humans are dorsally wedged, while the L1 vertebra is transitional (Table 1; Fig. 2) [4]. This contributes to the lumbar lordosis that develops secondarily to an upright position of the trunk.
The measurements in both Neanderthals reveal lumbar spines with an anterior (i.e., ventral) wedging of the vertebrae L1 to L4 (Table 1; Fig. 2). This contributes to lumbar kyphosis or a straight lumbar spine. Both Neanderthal lumbar spines thus evince one novel feature—these earlier hominids suffered from natural lumbar kyphosis.
The correct shape of a spine's curvature can be constructed after the alignment of the vertebral bodies and knowledge about the corresponding disc spaces. However, the morphological reconstruction of the disc space in fossils is difficult due to the intrinsic variation in this region and the lack of preservation. Measurements of the inter-vertebral height of the modern spine revealed a remarkable range of variation, suggesting that disk height is highly variable and therefore difficult to predict [18]. To our knowledge no data are available on Neanderthal disc space/inter-vertebral height. Because of these facts we abstained from reconstructing the inter-vertebral space in the presented cases. In summary, the lumbar spines observed here are undoubtedly ventrally bent (i.e., kyphotic), however, the exact degree of this ventral curvature may be subject to debate.
We reject the unlikely hypothesis that both Neanderthals show a wedging of the vertebrae L1–L4 due to the occurrence of multiple fractures. If true, this would be a surprising coincidence given the fact that this rare condition happened to the sole Neanderthals that show full preservation of the spine. Moreover, as known from recent times the majority of vertebral wedge deformities are found in the mid-thoracic (i.e., ca. Th6/Th7) and thoraco-lumbar regions (i.e., Th12–L2), because these parts bear the greatest dynamic load (biomechanical factor), and may therefore predispose to fracture [6]. Differently, fractures occurring in the region from L3 down to L5 are rare. In a group of men (mean age of 64.4 years) Ismail et al. [6] found vertebral wedge deformities with one vertebra affected (6.5%), two vertebrae (1.3%), and three or more vertebrae wedge-shaped (0.5%). Since fracture frequency increases with age, and since our examined Neanderthal men are markedly younger than the aforementioned group [3, 9, 11] we reject the hypothesis that the kyphosis originates from multiple fractures in the lumbar region. It is of note that the entire spine (i.e., from C1 down to L5) in Kebara 2 does not show any sign of pathology connected to severe degeneration, injury or combat.
Degenerative changes of the lumbar spine
There is evidence that occupational exposure in recent time has an effect on disc degeneration. However, these factors explain little of the variability in degeneration found in the adult population. Furthermore, the lack of a clear dose-response relation between time in various occupational loading conditions and degenerative findings adds to doubts about a strong causal link [7, 8, 10, 16]. Neanderthals had a high level of physical activity and mechanical demands, particularly the hunt of animals and the habitual transport of heavy objects over great distances.
All lumbar spine elements in the Neanderthal skeleton Kebara 2 were examined for evidence of degenerative changes, and only two regions of mild degenerative disease were found (Fig. 1). The vertebral bodies of another Neanderthal, called Shanidar 3, reveal marginal osteophytes at levels L1–L2 and L2–L3, indicating only mild degenerative changes too (for comparison see photographs and radiograph published in [9]). This finding is highly unexpected since Neanderthals are known to have had extraordinary physical activity. Considering their demanding living conditions one may expect a high prevalence of degenerative spine disease. However, we stress that recent studies largely failed to show a clear correlation between physical activity and degenerative spine disease [7, 8, 10, 16]. The surprisingly unaltered condition of the adult Neanderthal spines must be addressed in future research.
Besides the points mentioned earlier, it would be difficult to directly compare the degree of degeneration in the fossils with today's generalized human population whose life expectancy has been dramatically extended by modern medicine and improved life circumstances. In contrast, there is a steady increase in Neanderthal mortality patterns from neonates to adolescents and then a marked decrease in old adults (40+ years). In fact, 80% of these adults died before they reached age 40 [13]. The age of the two discussed adult Neanderthal specimens was estimated to 30–35 and 35–50 years at the time of death for Kebara 2 and Shanidar 3 respectively [3, 9, 11]. Thus, considering their ancient environment both Neanderthals reached an individual age that is remarkably old. Today we find mild and moderate osteoarthritis (39.4 and 13.8%, respectively) in the male lumbar spine at the age of 45–49 years. Moreover, severe osteoarthritis is uncommon in individuals younger than 45 years [15]. We therefore conclude that the degree of degenerative changes in both Neanderthal specimens correlate well with the pathology seen in modern humans of equivalent age.
Today, lumbar spondylosis and spondylarthrosis are frequent causes of lower back pain. Considering that pain has no visual effect on bone, the degree of putative pain is difficult to determine when there is lack of osseous pathology in the Neanderthal skeleton. Since both Neanderthal lumbar spines, unexpectedly, reveal only minimal degenerative changes this species presumably did not suffer from lower back pain.
The normal kyphosis of the thoracic spine, the stability by the rib cage, and the angulation of the facets prevent the vertebrae from increased degeneration in this region. One may speculate that, likewise the situation of thoracic kyphosis in modern humans, lumbar kyphosis in the Neanderthal species prevented an early onset of degenerative changes. However, the factors that lead to degenerative disease in the lumbar spine of modern humans are not completely known and may not be completely attributed to the lordosis.
In addition, one may consider the greater stability of the entire Neanderthal skeleton. This species was generally larger boned and more heavily muscled than most humans nowadays. This could serve as a further explanation for the unexpected low level of degenerative changes of the kyphotic lumbar spine. Unfortunately, this hypothesis is not readily testable; only discovery and thorough examination of more Neanderthal lumbar spines and further clinical research can resolve this question.
Evolutionary interpretation
Despite only two Neanderthal samples available for such a kind of research, an evolutionary interpretation can be made: the bidirectional evolution of the late Homo, by splitting into H. sapiens and Homo neanderthalensis, led to multiple morphological and functional subvariants in both branches of the evolutionary tree from which only the “sapiens model” was finally more successful. We stress that a comparison of the evolutionary fitness between Neanderthals and H. sapiens is too complex and that the success of the latter cannot be used to judge the relative inferiority or superiority of anatomical Neanderthal features (including the lumbar curvature). The kyphosis of the Neanderthals' lumbar spine is an interesting feature in the evolution of primate bipedalism. We speculate that a pathology in H. sapiens may have been a regular anatomy in H. neanderthalensis.
Acknowledgments
The authors are grateful to Yoel Rak at the Department of Anatomy and Anthropology, Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel, for the invitation to examine the Neanderthal skeleton termed Kebara 2.
Conflict of interest statement None of the authors has any potential conflict of interest.
References
- 1.Aeby C. Die Altersverschiedenheiten der menschlichen Wirbelsäule. Arch Anat Physiol. 1879;10:77. [Google Scholar]
- 2.Arensburg B, Bar Yosef O, Chech M, Goldberg P, Laville H, Meignen L, Rak Y, Tchernov E, Tillier AM, Vandermeersch B Une sépulture néanderthalien dans la grotte de Kebara (Israel) (1985) Compte Rendus des Séances de l´Académie des Science (Paris), Série II 300:227–230
- 3.Arensburg B (1991) The vertebral column, thoracic cage and hyoid bone. In: Bar Yosef O. and Vandermeersch B. Le Squelette Mousterian de Kebara 2:113–146. Editions du C.N.R.S. (Paris)
- 4.Berry JL, Moran JM, Berg WS, Steffee AD. A morphometric study of human lumbar and selected thoracic vertebrae. Spine. 1987;12(4):362–367. doi: 10.1097/00007632-198705000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Crubézy E, Trinkaus E. Shanidar 1: a case of hyperostotic disease (DISH) in the middle Paleolithic. Am J Phys Anthropol. 1992;89:411–420. doi: 10.1002/ajpa.1330890402. [DOI] [PubMed] [Google Scholar]
- 6.Ismail AA, Cooper C, Felsenberg D, Varlow J, Kanis JA, Silman AJ, O'Neill TW, study group Number and type of vertebral deformities: epidemiological characteristics and relation to back pain and heigh loss. Osteoporos Int. 1999;9:206–213. doi: 10.1007/s001980050138. [DOI] [PubMed] [Google Scholar]
- 7.Jackson RP, McManus AC. Radiographic analysis of sagittal plane alignment and balance in standing volunteers and patients with low back pain matched for age, sex, and size. Spine. 1994;19:1611–1618. doi: 10.1097/00007632-199407001-00010. [DOI] [PubMed] [Google Scholar]
- 8.Lane NE, Bloch DA, Jones HH, Marshall WH, Wood PD, Fries JF. Long-distance running, bone density, and osteoarthritis. JAMA. 1986;255:1147–1151. doi: 10.1001/jama.255.9.1147. [DOI] [PubMed] [Google Scholar]
- 9.Ogilvie MD, Hilton CE, Ogilvie CD. Lumbar anomalies in the Shanidar 3 Neandertal. J Hum Evol. 1998;35:597–610. doi: 10.1006/jhev.1998.0249. [DOI] [PubMed] [Google Scholar]
- 10.Panush RS, Schmidt C, Caldwell JR, Edwards NL, Longley S, Yonker R, Webster E, Nauman J, Storck J, Pettersson H. Is running associated with degenerative joint disease? JAMA. 1986;255:1152–1154. doi: 10.1001/jama.255.9.1152. [DOI] [PubMed] [Google Scholar]
- 11.Rak Y, Arensburg B. Kabara 2 neanderthals pelvis: first look at a complete inlet. Am J Phys Anthropol. 1987;73(3):227–231. doi: 10.1002/ajpa.1330730209. [DOI] [PubMed] [Google Scholar]
- 12.Trinkaus E. Pathology and the posture of the La Chapelle-aux-Saints Neanderthal. Am J Phys Anthropol. 1985;67:19–41. doi: 10.1002/ajpa.1330670105. [DOI] [PubMed] [Google Scholar]
- 13.Trinkaus E. Neanderthal mortality patterns. J Archaeol Sci. 1995;22:121–142. doi: 10.1016/S0305-4403(95)80170-7. [DOI] [Google Scholar]
- 14.Valladas H, Joron JL, Valladas G, Arensburg B, Bar-Yosef O, Belfe Cohen A, Goldberg P, Laville H, Meignen L, Rak Y, Tchernov E, Tillier M, Vandermeersch B. Thermoluminescence dates for the Neanderthal burial site at Kebara in Israel. Nature. 1987;330:159–160. doi: 10.1038/330159a0. [DOI] [Google Scholar]
- 15.Saase JLCM, Romunde LKJ, Cats A, Vandenbroucke JP, Valkenbrug HA. Epidemiology of osteoarthritis: zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann Rheum Dis. 1989;48:271–280. doi: 10.1136/ard.48.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Videman T, Battié MC. The influence of occupation on lumbar degeneration. Spine. 1999;24:1164–1168. doi: 10.1097/00007632-199906010-00020. [DOI] [PubMed] [Google Scholar]
- 17.Weber J, Czarnetzki A, Spring A. Paleopathological feature of the cervical spine in the early middle ages: natural history of degenerative diseases. Neurosurgery. 2003;53:1418–1424. doi: 10.1227/01.NEU.0000094951.54541.18. [DOI] [PubMed] [Google Scholar]
- 18.Zhou SH, McCarthy ID, McGregor AH, Coombs RRH, Hughes SP. Geometrical dimensions of the lower lumbar vertebrae—analysis of data from digitised CT images. Eur Spine J. 2000;9:242–248. doi: 10.1007/s005860000140. [DOI] [PMC free article] [PubMed] [Google Scholar]