Abstract
We report a rare case of Merkel cell carcinoma with extra-dural spinal metastasis causing paraplegia. There are only four reported cases in literature. A 57-year-old lady presented with a breast lump, multiple truncal skin swellings, low back pain and rapidly progressive paraplegia. MRI showed multiple epidural soft tissue masses causing neural compression. A biopsy from the truncal skin lesion was diagnosed as Merkel cell carcinoma (MCC). Posterior decompression and tumor debulking at all three sites of neural compression was performed. Histopathology of the epidural tumor was consistent with MCC and the diagnosis was confirmed by immuno-histochemistry staining for cytokeratin-20. She was started on chemotherapy and radiotherapy. One month after diagnosis she died due to extensive metastasis. The short term palliative response seen in our patient demonstrates the poor prognosis for patients with spinal metastasis.
Keywords: Merkel cell carcinoma, Spinal metastasis, Secondaries, Paraplegia
Case report
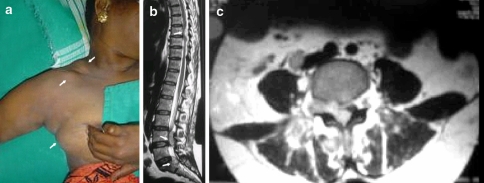
A 57-year-old diabetic lady presented with persistent low back pain and bilateral radiculopathy of 2 months duration followed by rapidly progressive weakness and numbness of the lower limbs. She also had loss of bowel and bladder control since 1 week. Multiple painless skin lumps over the chest and abdomen had appeared spontaneously a few months prior to presentation. There were no constitutional symptoms. General examination revealed multiple firm non-tender shiny skin lumps approximately 3–4 cm in diameter on the anterior abdominal wall, chest and on the right breast. There were also firm and non-tender mobile lymph-nodes of 2–3-cm size in the right posterior triangle of the neck (Fig. 1a). There was no tenderness or pain on movements of the spine. She had a flaccid paraplegia, a left extensor plantar response and sensory hypoesthesia below the ninth thoracic dermatome bilaterally. The radiographs and CT scan done prior to admission were normal (Fig. 1b, c). An MRI showed epidural soft tissue masses causing neural compression at the eighth thoracic, fourth lumbar and first sacral levels. There were also with multiple soft tissue lesions in the paraspinal and retroperitoneal space––features suggestive of metastasis. There was no history to suggest immuno-compromised status and human immunodeficiency virus infection was eliminated by the appropriate laboratory tests.
Fig. 1.
a Lymphnodes in the posterior triangle of the neck. Soft tissue swellings in the infra-clavicular region and the right breast. b T2 weighted MRI sagittal section showing the epidural soft tissue lesions at T8, L4 and S1 vertebral levels. c T2 weighted MRI axial section at L4 level demonstrating the epidural mass at the right neural foramina, and lesions in the retroperitoneal and paraspinal region
An FNAC of the lymph node, anterior abdominal wall mass and right breast was reported as a small cell tumor. An incisional biopsy from a skin lesion was reported as MCC. A laminectomy and tumor debulking at all three sites of compression was performed. Epidural tumor tissue sent for histopathology was reported as a high grade malignancy composed of epitheliod cells with scanty cytoplasm, markedly pleomorphic nulei having a “salt and pepper” chromatin (highly reminiscent of a neuro-endocrine origin). The tumor cells with numerous mitosis were predominantly disposed in a trabercular and cribriform pattern and also arranged around necrotic spaces. The features were strongly suggestive of a MCC. Immuno-histochemistry was positive staining for cytokeratin-20 confirming Merkel cell carcinoma.
Chemotherapy—Cisplatin 80 mg/m2, Doxorubicin 50 mg/m2 and Etoposide 300 mg/m2 were administered with concurrent radiotherapy. She began to show improvement in neurological status grade 1 to 2 power. One month after diagnosis there was a sudden deterioration in general condition and she died due to multiple metastasis.
Discussion
Toker published the first complete description of Merkel cell carcinoma (MCC) in 1972, originally describing it as “trabecular carcinoma” [22]. Merkel cell carcinoma is a rare neuroendocrine malignancy (APUDoma) of the skin, generally occurring in elderly patients, [7] the mean age at presentation being about 75 years [17]. Merkel cell carcinoma is principally a disease of the Caucasian race [12] with an incidence of 0.44 per 100,000 [8] and is abnormally high (8%) among immunosuppressed patients [2, 6]. An analysis of the Surveillance, Epidemiology and End Results (SEER) database conducted by Hodgson (2005) reported that the incidence of MCC has increased threefold between 1986 and 2001 [8].
The tumor is usually located in sun-exposed areas of the skin [11] the most common site being the head and neck region [20] followed by the limbs and trunk. Nearly 3% of MCC may present as a metastatic disease of unknown primary, the most frequent sites of metastasis being distant lymph-nodes, distant skin, lung, central nervous system and bone [10]. Merkel cell carcinoma grows rapidly and is characterized by significant incidence of local recurrence (30–45%), early involvement of the loco-regional lymph nodes (40–70%) and distant metastases (30–50%) [11] (Fig. 1).
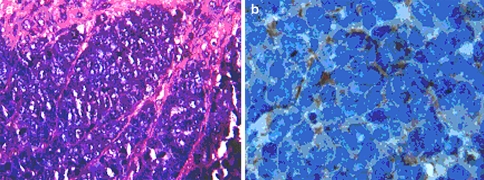
The histology of MCC is typical of small round blue cell tumors an entity that includes a wide variety of highly malignant tumors: the Ewings family of tumors, rhabdomyosarcoma, neuroblastoma, lymphoma, desmoplastic small cell tumor and small cell lung carcinoma (SCLC) [18, 21]. A characteristic histological finding in MCC is the presence of tumor tissue within the dermis with repeated extensions to the underlying subcutaneous tissue without involvement of the epidermis, papillary dermis and adnexal structures. The typical haematoxylin-eosin stained cytomorphological features of MCC are described in the legend of Fig. 2a. Using conventional light microscopy, MCC may be misdiagnosed as any other poorly differentiated small cell neoplasm, [11] therefore electron microscopy and immuno-histochemical staining (neuron-specific enolase and cytokeratin-20, chromogranin A) play an important role in the diagnosis [25]. In our case the diagnosis was by typical histology which was confirmed by imuno-histochemistry staining positive for cytokeratin-20.
Fig. 2.
a Light microscopy, Haematoxylin-eosin, x400. Demonstrating the typical histology of MCC. Small blue cells with sparse cytoplasm, uniform monotonous medium-sized nuclei and abundant mitoses. Chromatin is displayed in typical salt and pepper pattern. b Immuno-histochemistry staining, x1000, positive for cytokeratin-20 paranuclear dot
The most common staging system is that described by Yiengpruksawan et al. [26], stage I disease for isolated local lesion, stage II disease is characterized by metastatic spread to regional lymph nodes and stage III has evidence of distant metastatic disease at the time of initial presentation. In the analysis of 1034 cases of MCC, Agelli and Clegg [1] found the 5 year relative survival for patients with localized, regional and distant MCC was approximately 75%, 59% and 25% respectively.
Treatment of MCC depends on the stage of the disease, and the patient’s age and overall condition. MCC should be treated aggressively with wide excision of the primary lesion with a 2–3 cm margin, and prophylactic lymphadenectomy followed by irradiation to the primary site [4, 17]. Merkel cell carcinoma is a highly radiosensitive tumor [9] and radiation is most commonly used as an adjuvant therapy after surgery. Radiation may be used as the only treatment especially in patients who are too weak to undergo surgical treatment [14, 16]. Chemotherapy is reserved for systemic disease, though the success of this treatment is limited and no chemotherapy protocol has been shown to improve survival [15, 17]. Doxorubicin/cyclophosphamide based regimen is most commonly used; the other agents include cisplatin, vincristine, etoposide, methotrexate, bleomycin. The type of initial therapy apparently has no effect on the survival rates for distant metastasis [5].
Osseous involvement has been described in the facial bones, cranium and spine [13]. Merkel cell carcinoma presenting with neurological complications is extremely rare [19]. There are only four cases [3, 13, 23, 24] with spinal metastasis reported in literature, however none of them had significant neurological deficits. In two cases the metastasis was to the epidural space and in the other two cases to the osseous spine and epidural space. Two of the four patients died early due to extensive metastasis in spite of aggressive treatment.
Our patient presented with metastatic disease and neurological deficits due to multi-level spinal cord compression, which to the best of our knowledge has not been reported. In view of the clinical findings our differential diagnosis was lymphoma with extradural mass lesions or breast carcinoma with multiple metastases. Biopsy of the lesions demonstrated MCC. We believe metastasis from MCC should be considered in the differential diagnosis of an epidural soft tissue mass in patients with apparently benign skin swellings. The short term palliative response seen in our patient demonstrates the poor prognosis for patients with spinal metastasis.
Conflict of interest statement
None of the authors has any potential conflict of interest.
References
- 1.Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832–841. doi: 10.1016/S0190-9622(03)02108-X. [DOI] [PubMed] [Google Scholar]
- 2.Buell JF, Trofe J, Hanaway MJ, et al. Immunosuppression and Merkel cell carcinoma. Transplant Proc. 2002;34:1780–1781. doi: 10.1016/S0041-1345(02)03065-8. [DOI] [PubMed] [Google Scholar]
- 3.Chao TC, Park JM, Rhee H, et al. Merkel cell tumor of the back detected during pregnancy. Plast reconstr Surg. 1990;86(2):347–351. doi: 10.1097/00006534-199008000-00028. [DOI] [PubMed] [Google Scholar]
- 4.Datta CK, Mendoza CB., Jr Merkel cell carcinoma: an aggressive neoplasm. W V Med J. 1999;95(3):127–129. [PubMed] [Google Scholar]
- 5.Gillenwater AM, Hessel AC, Morrison WH, et al. Merkel cell carcinoma of the head and neck: effect of surgical excision and radiation on recurrence and survival. Arch Otolaryngol Head Neck Surg. 2001;127:149–154. doi: 10.1001/archotol.127.2.149. [DOI] [PubMed] [Google Scholar]
- 6.Gooptu C, Woollons A, Ross J, et al. Merkel cell carcinoma arising after therapeutic immusuppression. Br J Dermatol. 1997;137:637–641. doi: 10.1111/j.1365-2133.1997.tb03802.x. [DOI] [PubMed] [Google Scholar]
- 7.Hanke CW, Conner AC, Temofeew RK, et al. Merkel cell carcinoma. Arch Dermatol. 1989;125:1096–1100. doi: 10.1001/archderm.125.8.1096. [DOI] [PubMed] [Google Scholar]
- 8.Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89:1–4. doi: 10.1002/jso.20167. [DOI] [PubMed] [Google Scholar]
- 9.Leonard JH, Ramsay JR, Kearsley JH, et al. Radiation sensitivity of Merkel cell carcinoma cell lines. Int J Radiat Oncol Biol Phys. 1995;32:1401–1407. doi: 10.1016/0360-3016(94)00610-W. [DOI] [PubMed] [Google Scholar]
- 10.Medina-Franco H, Urist MM, Fiveash J, et al. Multimodality treatment of Merkel cell carcinoma: case series and literature review of 1024 cases. Ann Surg Oncol. 2001;8(3):204–208. doi: 10.1007/s10434-001-0204-4. [DOI] [PubMed] [Google Scholar]
- 11.Metzinger SE, Wolfer RS, Disa JJ, et al. Recurrent Merkel cell carcinoma of the upper extremity. South Med J. 2000;93(3):340–345. [PubMed] [Google Scholar]
- 12.Miller RW, Rabkin CS. Merkel cell carcinoma and melanoma: etiological similarities and differences. Cancer Epidemiol Biomarkers Prev. 1999;8:153–158. [PubMed] [Google Scholar]
- 13.Moayed S, Maldijianb C, Adam R, et al. Magnetic resonance imaging apparence of metastatic Merkel cell carcinoma to the sacrum and epidural space. Magn Reson Imaging. 2000;18:1039–1042. doi: 10.1016/S0730-725X(00)00176-4. [DOI] [PubMed] [Google Scholar]
- 14.Mortier L, Mirabel X, Fournier C, et al. Radiotherapy alone for primary Merkel cell carcinoma. Arch Dermatol. 2003;139:1587–1590. doi: 10.1001/archderm.139.12.1587. [DOI] [PubMed] [Google Scholar]
- 15.Nathu RM, Mendenhall WM, Parsons JT. Merkel cell carcinoma of the skin. Radiat Oncol Investig. 1998;6(5):233–239. doi: 10.1002/(SICI)1520-6823(1998)6:5<233::AID-ROI5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 16.Pacella J, Ashby M, Ainslie J, et al. The role of radiotherapy in the management of primary cutaneous neuroendocrine tumors (Merkel cell or trabecular carcinoma): experience at the Peter MacCallum Cancer Institute. Int J Radiat Oncol Biol Phys. 1988;14:1077–1084. doi: 10.1016/0360-3016(88)90382-3. [DOI] [PubMed] [Google Scholar]
- 17.Pergolizzi J, Jr, Sardi A, Pelczar M, et al. Merkel cell carcinoma: an aggressive malignancy. Am Surg. 1997;63(5):450–454. [PubMed] [Google Scholar]
- 18.Pisick E, Skarin AT, Salgia R. Recent advances in the molecular biology, diagnosis and novel therapies for various small blue cell tumors. Anticancer Res. 2003;23:3379–3396. [PubMed] [Google Scholar]
- 19.Snodograss SM, Landy H, Markoe AM, et al. Neurological complications of Merkel cell carcinoma. J Neuro Oncol. 1994;22:231–234. doi: 10.1007/BF01052924. [DOI] [PubMed] [Google Scholar]
- 20.Tai PT, Yu E, Winquist E, et al. Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: case series and review of 204 cases. J Clin Oncol. 2000;18:2493–2499. doi: 10.1200/JCO.2000.18.12.2493. [DOI] [PubMed] [Google Scholar]
- 21.Tarkkanen M, Knuutila S. The diagnostic use of cytogenetic and molecular genetic techniques in the assessment of small round cell tumours. Curr Diagn Pathol. 2002;8:338–348. doi: 10.1016/S0968-6053(02)90135-3. [DOI] [Google Scholar]
- 22.Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105:107–110. doi: 10.1001/archderm.105.1.107. [DOI] [PubMed] [Google Scholar]
- 23.Turgut M, Gokpinar D, Barutca S, et al. Lumbosacral metastatic extradural Merkel cell carcinoma causing nerve root compression–case report. Neurol Med Chir (Tokyo) 2002;42(2):78–80. doi: 10.2176/nmc.42.78. [DOI] [PubMed] [Google Scholar]
- 24.Turgut M, BakaM, Yurtseven M. Metastatic Merkel cell carcinoma to the sacrum and epidural space: case report. Magn Reson Imaging. 2004;22(9):1340. doi: 10.1016/j.mri.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Warner TFCS, Uno H, Hafez GR, et al. Merkel cells and Merkel cell tumors: ultrastructure, immunochemistry and review of the literature. Cancer. 1983;52:238–245. doi: 10.1002/1097-0142(19830715)52:2<238::AID-CNCR2820520209>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 26.Yiengpruksawan A, Coit DG, Thaler HT, et al. Merkel cell carcinoma. prognosis and management. Arch Surg. 1991;126(12):1514–1519. doi: 10.1001/archsurg.1991.01410360088014. [DOI] [PubMed] [Google Scholar]