Abstract
We present an extremely rare case of traumatic spinal cord herniation due to a brachial plexus avulsion injury and provide a review of the literature of spinal cord herniation. Spinal cord herniation is an uncommon condition that can occur spontaneously or as a result of surgery or trauma. This condition often presents with symptoms and signs as Brown-Séquard syndrome. Traumatic pseudomeningoceles after a brachial plexus avulsion injury have been reported. But transdural herniation of the spinal cord into this pseudomeningocele is an extremely rare and poorly documented condition. There is only two reports of this condition in a thoracic case. The authors report the case of a 22-year-old man presented with a 2-year history of quadriplegia. He was involved in a motorcycle accident, 3 years prior to his presentation. Four years after the initial right brachial plexus injury, he was not able to walk independently. Magnetic resonance imaging (MRI) and computerized tomography (CT) myelography revealed a lateral pseudomeningocele arising from the right C6–7 and C7–T1 intervetebral foramen and cervical spinal cord herniation into this pseudomeningocele. The patient underwent primary closure of pseudomeningocele to prevent spinal cord reherniation. He can walk with cane and use left arm unrestrictedly at the 2-year follow-up examination. Spinal cord herniation following traumatic nerve root avulsion is extremely rare but it should be considered in the differential diagnosis of patients presenting with delayed myelopathy or Brown-Séquard syndrome.
Keywords: Quadriplegia, Nerve root avulsion, Pseudomeningocele, Spinal cord herniation, Spinal cord tethering
Introduction
Wortzman et al. [21] reported the first case of spinal cord herniation in 1974. This is a rare but well-documented condition. Spinal cord herniation usually occurs spontaneously [1, 2], but sometimes following surgery [5, 8] or trauma [7, 12]. Idiopathic spinal cord herniation is usually seen ventrally and commonly in the thoracic spine. Furthermore the spinal cord frequently is shifted ventrally or ventrolaterally, so this condition often presents with symptoms and signs as Brown-Séquard syndrome [1, 2, 9, 14]. In contrast, postsurgical spinal cord herniation can be found anywhere in the spinal column [7, 12]. This traumatic type can occur after traumatic nerve root avulsion. There is only two previous case reports of spinal cord herniation occuring in thoracic region after traumatic nerve root avulsion [6, 22].
This report describes the case of a 22-year-old man with cervical spinal cord herniation who presented 3 years after sustaining a traumatic brachial plexus nerve root avulsion.
Case report
History
A 22-year-old man presented with a 2-year history of quadriplegia. He was involved in a motorcycle accident in 1998, 3 years prior to his presentation. At that time, he suffered marked right brachial plexus paralysis. The brachial plexus injury resulted in complete monoplegia. The elements of the brachial plexus from C-5 to T-1 were involved, with the nerve roots known to be avulsed. Eight months later, he gradually developed contralateral numbness of the left arm and left leg paralysis. His condition presented Brown-Séquard syndrome. Another 1 year later, he experienced some bladder dysfunction. Four years after the initial brachial plexus injury, he developed right leg paralysis and finally was not able to walk independently. He was treated in another hospital for motor neuron disease.
Examination
On examination, the patient could not walk, he was wheel-chair bound. He could not move his right arm at all and obvious wasting of his right arm was observed. The muscle weakness of his left arm was mild (Grade 4/5). He exhibited bilateral wasting of both thigh and calf muscles. There were severe hypoalgesia and hypoesthesia in left arm and left leg. The sensory examination of the joint position and vibration was diminished predominantly in left lower extremities. Hyperreflexia of the knee and ankle jerks were also present bilaterally. Babinski reflexes were positive bilatetally. Also he complained mild bowel and bladder dysfunction. A detailed physical examination showed no cranial nerve symptoms.
Image
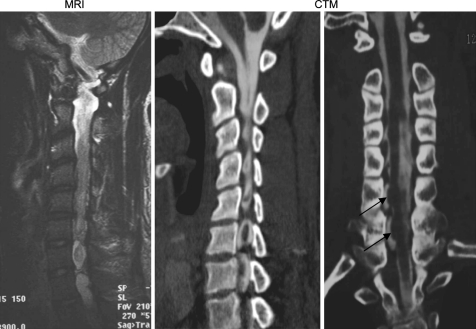
Simple radiographs demonstrated no abnormalites. The patient underwent magnetic resonance imaging (MRI) and computerized tomography (CT) myelography (Figs. 1, 2). CT myelography revealed a lateral pseudomeningocele arising from the right C6–7 and C7–T1 intervetebral foramen. There was spinal cord atrophy, and deviation. The condition was determined to be tethering of the spinal cord at the pseudomeningocele due to adhesion. Intramedullary hyperintensity was also present on T2 weighted sequences on MRI.
Fig. 1.
Sagittal and coronal images of the spinal cord herniation (MRI and CT myelography) The small arrow indicates the spinal cord herniation into the posttraumatic pseudomeningocele. The spinal cord was atrophic and shifted to herniated side
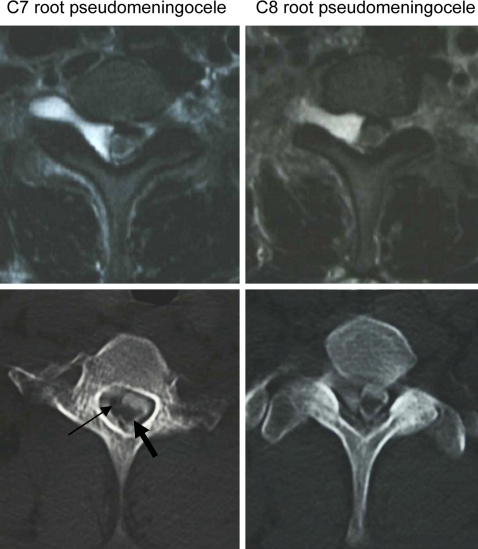
Fig. 2.
Axial image of the spinal cord herniation (MRI and CT myelography). The small arrow shows the spinal cord herniation. The large arrow shows that the original spinal cord was atrophic
Operation
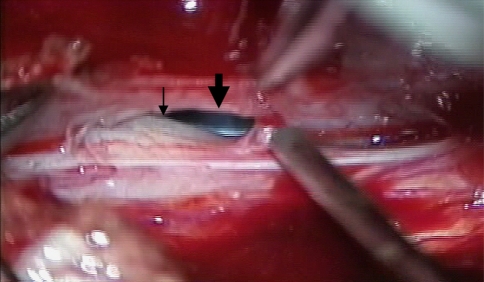
The patient underwent open-door laminoplasty from C5 to T2. The two pseudomeningoceles were identified at the right C6–7 and C7–T1 vertebra level. When the dura was opened, outpouching of the spinal cord was seen through both a 6 and 8 mm-diameter dural defect (Fig. 3). Clear communication of the CSF was identified between the spinal subarachnoid space and the pseudomeningocele. Primary closure of the pseudomeningocele was performed to prevent spinal cord reherniation, and a dural patch was used to close the posterior aspect of the dura.
Fig. 3.
Spinal cord herniation into a pseudomeningocele in a intraoperative view. The small arrow shows the spinal cord herniation and the large arrow shows pseudomeningocele due to nerve root avulsion
Postoperative course
There were no postoperative complications, and no recurrences have been observed to date. Three months postoperatively, the patient could walk with support but there was no subjective improvement of the left arm. He could walk with cane and use left arm unrestrictedly at the 2-year follow-up examination.
Discussion
Since MRI has come into widespread use, the incidence of spinal cord herniation has gradually been accepted [22]. As a result, the number of published reports of this condition has increased and over 30 are documented [1–3, 5–15, 19]. This disorder is characterized by the following observations; (1) the patient typically presents with symptoms of Brown-Séquard syndrome; (2) the neurological deficit is progressive unless treated; and (3) a surgical reduction of the spinal cord herniation is known to lead to an improvement of the motor deficit, even in cases in which the deficit was longstanding [2]. Spinal cord herniation can be classified as spontaneous, posttraumatic, and iatrogenic [6]. Idiopathic spinal cord herniation occurs exclusively in the thoracic spine, with a female/male ratio of approximately 2:1 and it usually affects middle-age patients [2, 9, 10, 13, 15].
But posttraumatic and iatrogenic spinal cord herniation remain a rare conditions with only a small number of reports in the literature [5, 7, 8, 12, 17, 18]. This type of herniation can affect younger patients and it occurs as a result traumatic injury or surgery [18, 20]. Posttraumatic and iatrogenic herniation can occur anywhere along the length of the spine, with cervical cases after a C1–2 wiring failure [8] and lumbar cases after a L-1 burst fracture [20]. In cases of traumatic spinal cord herniation, patients usually present after a postinjury interval of several years, and rarely within a few days of the initial injury [11, 18]. This is because it may take long time to develop the pseudomeningocele, and arachnoidal adhesions are likely developed over time. The adhesions lead to tethering of the spinal cord to the dural ring and thereby preventing the cord from returning into the spinal canal.
In 2003, DaSilva et al. first reported a 18-year male with a rare combination of spinal cord herniation and nerve root avulsion [6]. The patient suffered Brown-Séquard syndrome and surgical intervention improved his paralysis. Until now, there have been few reports of a pseudomeningocele developing after a plexus injury [4, 16, 19]. The incidence of pseudomeningocele after nerve root avulsion was high (21–57%), but until now there is only two reports of a spinal cord herniation developing into this pseudomeningocele [6, 22].
For the diagnosis of spinal cord herniation, MRI and CT myelography are essential. MRI can show acute, angular, ventral deviations, such as kinking of the spinal cord in the sagittal plane [15, 20]. Spinal cord atrophy, intramedullary hyperintensity, syrinx formation, and tethering have been also observed [13, 20]. Postmyelography CT scanning is helpful in demonstrating the normal CSF flow pattern dorsally and to exclude the possibility of a compressive posterior arachnoidal cyst [3]. In the present case, there were two dural hiatus at the origin of the C7 and C8 nerve root sleeves.
The treatment of traumatic spinal cord herniation is the same as that of idiopathic spinal cord herniation. The goals of the surgical treatment are to reduce the herniation, return the spinal cord to its normal position, and prevent recurrence. In severe and progressive cases, surgical repair is mandatory. The surgical lesion should be closed by suture or grafting if the spinal cord has extruded into the epidural space [11]. The outcome appears to be favorable in most surgically treated patients, however, persistent sensory symptoms are observed in some cases [9, 10, 14, 15].
This spinal cord herniation following nerve root avulsion is extremely rare. The neurologist or spinal surgeon should be aware of this condition because that is the key to establishing an early diagnosis. Preserving the functional status in patients presenting with myelopathy or Brown-Séquard syndrome in the early stages is therefore essential.
Conflict of interest statement
None of the authors has any potential conflict of interest.
References
- 1.Aizawa Idiopathic herniation of the thoracic spinal cord: report of three cases. Spine. 2001;26:E488–E491. doi: 10.1097/00007632-200110150-00030. [DOI] [PubMed] [Google Scholar]
- 2.Borges Idiopathic spinal cord herniation: a treatable cause of the Brown-Sequard syndrome-case report. Neurosurgery. 1995;36:1028–1033. doi: 10.1097/00006123-199505000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Brugieres Idiopathic spinal cord herniation: value of MR phase-contrast imaging. AJNR Am J Neuroradiol. 1999;20:935–939. [PMC free article] [PubMed] [Google Scholar]
- 4.Chow Predictive value of computed tomographic myelography in obstetrical brachial plexus palsy. Plast Reconstr Surg. 2000;106:971–977. doi: 10.1097/00006534-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Cobb Herniation of the spinal cord into an iatrogenic meningocele. Case report. J Neurosurg. 1973;39:533–536. doi: 10.3171/jns.1973.39.4.0533. [DOI] [PubMed] [Google Scholar]
- 6.DaSilva Upper thoracic spinal cord herniation after traumatic nerve root avulsion. J Neurosurg: Spine. 2003;99:306–309. doi: 10.3171/spi.2003.99.3.0306. [DOI] [PubMed] [Google Scholar]
- 7.Dunn Transdural herniation of the cervical spinal cord as a complication of a broken fracture-fixation wire. AJNR Am J Neuroradiol. 1987;8:724–726. [PMC free article] [PubMed] [Google Scholar]
- 8.Goodman Cervical pseudomeningocele after laminectomy as a cause of progressive myelopathy. Bull Los Angel Neurol Soc. 1974;39:121–127. [PubMed] [Google Scholar]
- 9.Isu Spinal cord herniation associated with an intradural spinal arachnoid cyst diagnosed by magnetic resonance imaging. Neurosurgery. 1991;29:137–139. doi: 10.1097/00006123-199107000-00027. [DOI] [PubMed] [Google Scholar]
- 10.Kumar Herniation of the spinal cord. Case report. J Neurosurg. 1995;82:131–136. doi: 10.3171/jns.1995.82.1.0131. [DOI] [PubMed] [Google Scholar]
- 11.Lee Spinal cord herniation after stabbing injury. Br J Neurosurg. 1997;11:84–86. doi: 10.1080/02688699746780. [DOI] [PubMed] [Google Scholar]
- 12.Marquardt Acute posttraumatic spinal cord herniation. Case report and review of the literature. J Neurosurg (Spine 2) 2001;94:316–318. doi: 10.3171/spi.2001.94.2.0316. [DOI] [PubMed] [Google Scholar]
- 13.Massicotte Idiopathic spinal cord herniation: report of eight cases and review of the literature. Spine. 2002;27:E233–E241. doi: 10.1097/00007632-200205010-00025. [DOI] [PubMed] [Google Scholar]
- 14.Masuzawa Spinal cord herniation into a congenital extradural arachnoid cyst causing Brown- Séquard syndrome. Case report. J Neurosurg. 1981;55:983–986. doi: 10.3171/jns.1981.55.6.0983. [DOI] [PubMed] [Google Scholar]
- 15.Miura Idiopathic spinal cord herniation. Neuroradiology. 1996;38:155–156. doi: 10.1007/BF00604805. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura Magnetic resonance myelography in brachial plexus injury. J Bone Joint Surg 79 B. 1997;5:764–769. doi: 10.1302/0301-620X.79B5.7679. [DOI] [PubMed] [Google Scholar]
- 17.Sachdev Posttraumatic pseudomeningomyelocele (enlarging fracture?) in a vertebral body. Case report. J Neurosurg. 1981;54:545–549. doi: 10.3171/jns.1981.54.4.0545. [DOI] [PubMed] [Google Scholar]
- 18.Vallee Ventral transdural herniation of the thoracic spinal cord: surgical treatment in four cases and review of literature. Acta Neurochir (Wien) 1999;141:907–913. doi: 10.1007/s007010050396. [DOI] [PubMed] [Google Scholar]
- 19.Walker Detection of nerve rootlet avulsion on CT myelography in patients with birth palsy and brachial plexus injury after trauma. J Roentgenol (AM) 1996;167:1283–1287. doi: 10.2214/ajr.167.5.8911196. [DOI] [PubMed] [Google Scholar]
- 20.Watters Transdural spinal cord herniation: imaging and clinical spectra. AJNR Am J Neuroradiol. 1998;19:1337–1344. [PMC free article] [PubMed] [Google Scholar]
- 21.Wortzman G. Spontaneous incarcerated herniation of the spinal cord into a vertebral body: a unique cause of paraplegia. Case report. J Neurosurg. 1974;41:631–635. doi: 10.3171/jns.1974.41.5.0631. [DOI] [PubMed] [Google Scholar]
- 22.Yokota Spinal cord herniation into associated pseudomeningocele after brachial plexus avulsion injury: case report. Neurosurgery. 2007;60:E205. doi: 10.1227/01.NEU.0000249195.76527.61. [DOI] [PubMed] [Google Scholar]