Abstract
Group II introns are found in organelles, bacteria, and archaea. Some harbor an open reading frame (ORF) with reverse transcriptase, maturase, and occasionally endonuclease activities. Group II introns require the assistance of either intron-encoded or free-standing maturases to excise from primary RNA transcripts in vivo. Some ORF-containing group II introns were shown to be mobile retroelements that invade new DNA sites by retrohoming or retrotransposition. Group II introns are also hypothesized to be the ancestors of the spliceosome-dependent nuclear introns and the small nuclear RNAs (snRNAs—U1, U2, U4, U5, and U6) that are part of the spliceosome. The ability of some fragmented group II introns to undergo splicing in trans supports the theory that the snRNAs evolved from portions of group II introns. Here, we developed a Tn5-based genetic screen to explore the trans-splicing potential of the Ll.LtrB group II intron from the Gram-positive bacterium Lactococcus lactis. Proficient trans-splicing variants of Ll.LtrB were selected using a highly sensitive trans-splicing/conjugation screen. We report that numerous fragmentation sites located throughout Ll.LtrB support splicing in trans, showing that this intron is remarkably more tolerant to fragmentation than expected from the fragmentation sites uncovered within natural trans-splicing group II introns. This work unveils the great versatility of group II intron fragments to assemble and accurately trans-splice their flanking exons in vivo.
Keywords: Lactococcus lactis, conjugation, spliceosome, snRNAs, Tn5
INTRODUCTION
Group II introns were originally discovered interrupting mitochondrial genes of lower eukaryotes, as well as mitochondrial and chloroplastic genes of higher plants. In contrast, no group II introns were found interrupting nuclear genes of eukaryotes. Bacterial group II introns were uncovered more recently and are present in one to a few copies per genome in both Gram-negative and Gram-positive bacteria, while in archaea, group II introns are infrequent (Michel and Ferat 1995; Lambowitz and Zimmerly 2004). Interestingly, some group II introns that contain an open reading frame (ORF) are also mobile retroelements. Following their excision, they invade new DNA sites using an RNA intermediate and target either identical or similar sequences by retrohoming or retrotransposition, respectively (Lambowitz and Zimmerly 2004). From an evolutionary point of view, group II introns are considered to be the ancestors of the spliceosome-dependent nuclear introns and the small nuclear RNAs (snRNAs—U1, U2, U4, U5, U6) that are part of the eukaryotic spliceosome machinery (Sharp 1985, 1991; Cech 1986; Jarrell et al. 1988; Jacquier 1990; Cavalier-Smith 1991; Michel and Ferat 1995).
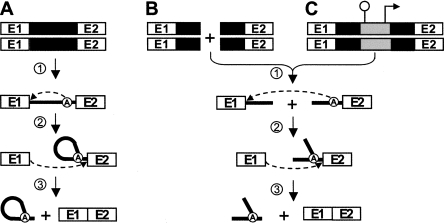
Group II introns are large RNA enzymes that need the assistance of maturases to remove themselves from pre-mRNAs in vivo. These ribozymes self-splice from RNA transcripts through two consecutive transesterification reactions (Fig. 1A; Michel and Ferat 1995; Lambowitz and Zimmerly 2004). Following transcription of the interrupted gene (Fig. 1A, step 1), the first nucleophilic attack at the exon 1–intron junction is initiated by the 2′-OH residue of a bulged adenosine (A) located near the 3′ end of the intron (Fig. 1A, step 2). The liberated 3′-OH of exon 1 then initiates the second transesterification reaction at the intron–exon 2 junction (Fig. 1A, step 3), which ligates the two flanking exons and releases the intron RNA in the form of a lariat. Eukaryotic nuclear introns, which require the spliceosome machinery to be excised, follow the same splicing pathway and are also released as lariats. This strongly suggests that group II and nuclear introns share a common ancestor (Sharp 1985; Cech 1986). The similarity of exon–intron boundaries between group II and nuclear introns also supports this hypothesis (Jacquier 1990). Moreover, substantial evidence indicates that the splicing reaction achieved by the spliceosome is fundamentally RNA-mediated, strengthening the argument that this large splicing machinery evolved from a primordial ribozyme (for review, see Valadkhan 2007). Finally, the discovery that some organellar group II introns fragmented into two or three pieces can splice in trans (Fig. 1B; for review, see Bonen 1993) further suggests that the snRNAs of the spliceosome were generated from group II intron fragments (Sharp 1991).
FIGURE 1.
Group II intron splicing pathways. (A) Cis-splicing. Following transcription of the interrupted gene (step 1), the 2′-OH residue of the branch point nucleotide (A) performs a nucleophilic attack at the exon 1–intron junction (step 2). Then, the liberated 3′-OH at the end of exon 1 performs the second nucleophilic attack at the intron–exon 2 junction (step 3), ligating the two exons and releasing the intron in the form of a lariat. (B) Trans-splicing. Some fragmented group II introns that are expressed from separate loci (step 1) can splice in trans to ligate their flanking exons and release a Y-branched intron molecule (steps 2 and 3). (C) Trans-splicing of Tn5-interrupted group II introns. Insertion of a Tn5 transposon, harboring a transcriptional terminator and a constitutive promoter, within the intron results in the expression of the gene as two separate transcripts (step 1). These two transcripts may undergo trans-splicing and ligate their flanking exons (steps 2 and 3). Group II intron, black box (DNA) and black line (RNA); branch point, circled A; exon 1 and 2, E1 and E2; Tn5 transposon, gray box; pepN transcriptional terminator, schematic stem–loop; P23 constitutive promoter, right-angle arrow.
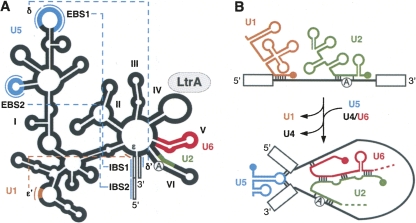
Despite a lack of similitude at the primary sequence level, the secondary structure of group II introns is well conserved (Fedorova and Zingler 2007). These RNA elements fold into six domains (DI–DVI) that radiate from a central wheel (Fig. 2A). Domain I (DI), the largest functional domain, serves as a scaffold to dock the remaining domains (DII–DVI). The lower stem of DII is believed to recruit DIII to the catalytic core, with DIII acting as a catalytic effector (Fedorova and Zingler 2007). DIV is dispensable for splicing (Jarrell et al. 1988) and highly variable in size since it may contain an ORF. Accordingly, upon folding of the intron, this domain is thought to protrude from the catalytic core. DV is the catalytic domain and exhibits the most sequence conservation among group II introns; its interaction with DI forms the minimal catalytic core (Fedorova and Zingler 2007). DVI carries the bulged adenosine (A) residue, also termed the branch point, responsible for the first nucleophilic attack of the splicing pathway (Fig. 1; Fedorova and Zingler 2007). In addition to local interactions, a series of long-range tertiary contacts between the various domains contribute to the three-dimensional folding and compaction of group II introns, and must take place to enable splicing (Fedorova and Zingler 2007).
FIGURE 2.
Functional and/or structural equivalences between portions of group II introns and the snRNAs of the spliceosome. (A) Schematic of group II intron conserved secondary structure. The six domains (I–VI) and pertinent long-range tertiary interactions are represented (dotted lines). (B) Interactions between snRNAs and spliceosomal introns. The functional and/or structural correspondences between snRNAs and portions of group II introns are color-coded. Group II intron, thick black line; spliceosomal intron, thin black line; branch point, circled A; exons, open boxes; exon binding sites 1 and 2; EBS1 and 2; intron binding sites 1 and 2, IBS1 and 2.
Functional and/or structural similarities exist between portions of group II introns and snRNAs (Fig. 2). For instance, DI is responsible for selection of the splice sites during group II intron splicing. The 5′ end of the intron is recognized through Watson–Crick base pairs (Fig. 2A, ε–ε′; Jacquier 1990; Fedorova and Zingler 2007). This is similar to the recognition of the 5′ end of spliceosomal introns by U1 (Fig. 2B; Jacquier 1990; Nilsen 1994; Patel and Steitz 2003). The 5′ splice site is also identified through a series of base pair interactions between intron binding sites (IBS) 1 and 2, which are at the 3′ end of exon 1, and exon binding sites (EBS) 1 and 2 in DI (Fig. 2A). The 3′ splice site is recognized by either the δ–δ′ (Fig. 2A) or EBS3–IBS3 base pair interactions between DI and exon 2 (Michel and Ferat 1995; Fedorova and Zingler 2007). The DI subdomain that harbors the EBS and δ sequences is thus functionally analogous to U5, which also recognizes the extremities of both exons at the intron–exon junctions in spliceosome-mediated splicing (Fig. 2B; Newman and Norman 1992; Nilsen 1994; Patel and Steitz 2003). In accordance, it was shown that a slightly modified U5 can complement the ID3 domain in group II intron catalysis (Hetzer et al. 1997). On the other hand, NMR studies showed that the stem–loop region of the catalytic snRNA U6, responsible for coordinating the metal ion in the spliceosome catalytic core, is structurally similar to DV, which plays the same role in group II intron splicing (Seetharaman et al. 2006). A slightly modified DV was shown to functionally complement U6 in the U12-dependant spliceosome, which further strengthens the putative evolutionary link between these two RNA elements (Shukla and Padgett 2002). Finally, binding of the U2 snRNA to the distal end of nuclear introns causes the branch point nucleotide to bulge out in a structure similar to DVI of group II introns (Fig. 2; Jacquier 1990). These functional and/or structural similarities between portions of group II intron and the snRNAs further support the evolutionary hypothesis that snRNAs were derived from group II intron fragments.
The Ll.LtrB intron from the Gram-positive bacterium Lactococcus lactis is one of the most characterized bacterial group II introns (Lambowitz and Zimmerly 2004). This 2.5-kb intron carries an ORF coding for LtrA, a 599 amino acid protein with reverse transcriptase, maturase, and endonuclease functions (Matsuura et al. 1997). LtrA is an essential splicing co-factor for Ll.LtrB. The maturase activity of LtrA promotes intron splicing by stabilizing the catalytically active tertiary conformation of the ribozyme (Matsuura et al. 1997). Ll.LtrB was found on two highly similar conjugative elements of L. lactis: a conjugative plasmid, pRS01 (Mills et al. 1996), and a chromosomal sex factor (Shearman et al. 1996). In both elements, Ll.LtrB interrupts the ltrB gene coding for relaxase (LtrB) at the same position. Relaxase initiates conjugation by nicking the conjugative element at the origin of transfer (oriT) and delivers the DNA to recipient cells (Byrd and Matson 1997). This enzyme is thus essential for conjugation, and splicing of Ll.LtrB from its pre-mRNA transcript is necessary for LtrB production and subsequent DNA transfer. Therefore, Ll.LtrB splicing controls conjugation efficiency of its host elements (Mills et al. 1996; Shearman et al. 1996). We previously developed a sensitive splicing/conjugation assay in L. lactis to show that Ll.LtrB trans-splices when fragmented at natural group II intron fragmentation sites. We also demonstrated that, similarly to cis-splicing, LtrA is essential for Ll.LtrB trans-splicing in vivo (Belhocine et al. 2007).
Here, we engineered a Tn5-based genetic screen to explore what fragmentation sites throughout Ll.LtrB allow trans-splicing in vivo. Trans-splicing Ll.LtrB variants were selected using a trans-splicing/conjugation assay. Our data reveal that Ll.LtrB can trans-splice efficiently when fragmented at various locations throughout its structure. Therefore, this study demonstrates that Ll.LtrB is more versatile in trans-splicing than expected from natural trans-splicing group II introns.
RESULTS
A pLE12-based conjugation assay to monitor Ll.LtrB splicing
We previously developed a sensitive splicing/conjugation assay in L. lactis (107-fold detection range) where conjugation of a relaxase-deficient chromosomal sex factor was used as a read-out for Ll.LtrB splicing. In that assay, the relaxase gene, interrupted by different cis- and trans-splicing Ll.LtrB variants, was provided from a non-mobilizable plasmid (Belhocine et al. 2007).
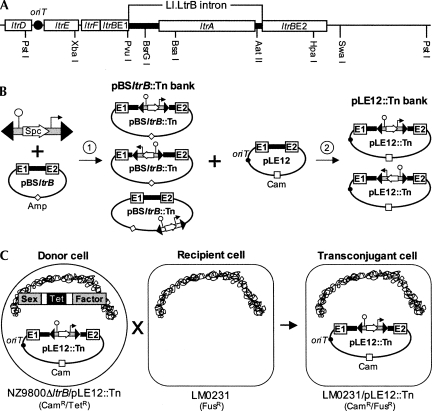
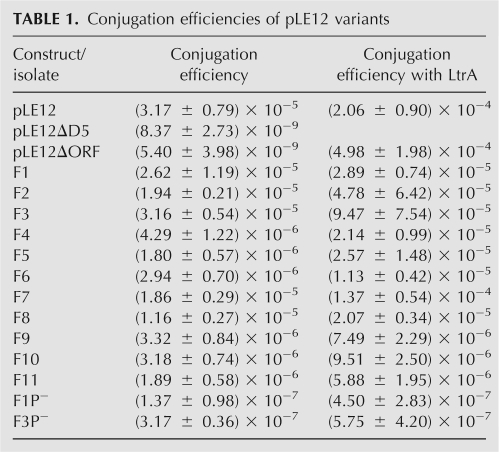
A similar splicing/conjugation assay was used here; however, Ll.LtrB splicing is monitored by the conjugation efficiency of its carrying mobilizable plasmid (pLE12; Belhocine et al. 2004). pLE12 harbors a portion of the pRS01 conjugative plasmid including the origin of transfer (oriT) and the ltrB relaxase gene, interrupted by Ll.LtrB (Fig. 3A, PstI fragment; Belhocine et al. 2004). Recognition of the oriT by the relaxase initiates pLE12 mobilization, which then allows its transfer by the chromosomally encoded sex factor conjugation machinery (Belhocine et al. 2004). The L. lactis strain NZ9800ΔltrB∷tet bears a defective relaxase gene and is used as the donor strain for pLE12 conjugation. In this strain, the Ll.LtrB intron and parts of its flanking exons were deleted from the chromosomal sex factor (Belhocine et al. 2004). The absence of relaxase in this strain prevents sex factor conjugation, as shown by the significant reduction in transfer efficiency from 10−3 (NZ9800) to 10−9 (NZ9800ΔltrB) (Belhocine et al. 2007). However, since pLE12 encodes its own relaxase, it is efficiently transferred from NZ9800ΔltrB to the LM0231 recipient strain (Table 1, pLE12, 3.17 × 10−5). To assess the dependence of pLE12 transfer on Ll.LtrB splicing, two plasmid variants carrying splicing defective introns were tested for conjugation. Either when pLE12 bears a catalytic mutant of Ll.LtrB (pLE12ΔDV) or when most of the LtrA coding region is deleted (pLE12ΔORF), the transfer efficiency drops significantly (∼104-fold) to background level (10−9) (Table 1). These data show that conjugation of pLE12 from NZ9800ΔltrB to LM0231 is initiated by the plasmid-borne relaxase, and that its conjugative transfer depends on splicing of Ll.LtrB from the ltrB transcript.
FIGURE 3.
Trans-splicing/conjugation assay. (A) Schematic of the pRS01 fragment subcloned within pLE12 (PstI). (B) Generation of saturated Tn5 insertion banks. The Tn5 transposon was mixed with the target plasmid (pBSltrB) in presence of the Tn5 transposase (step 1). This in vitro reaction created a bank of Tn5 insertions within the target plasmid (pBSltrB∷Tn) consisting of unique insertions between every nucleotide. To generate various pLE12∷Tn banks bearing Tn5 insertions in specific sections of Ll.LtrB, the corresponding sections were excised from the pBSltrB∷Tn bank and transferred to pLE12 (step 2). (C) Trans-splicing/conjugation assay. The pLE12∷Tn bank is introduced in the NZ9800ΔltrB donor strain and mated with the LM0231 recipient strain. This assay selects for plasmids harboring proficient Ll.LtrB trans-splicing variants. Every donor cell contains a different plasmid, only the pLE12∷Tn variants that harbor a trans-splicing proficient Ll.LtrB intron produce relaxase and are transferred by conjugation. Origin of conjugative transfer (oriT), black circle.
TABLE 1.
Conjugation efficiencies of pLE12 variants
Tn5-based genetic screen to study Ll.LtrB trans-splicing versatility
We previously demonstrated that Ll.LtrB splices in trans when fragmented independently within DI, DIII, or DIV, either upstream or downstream from ltrA (Fig. 4, sites labeled S1–S5; Belhocine et al. 2007). Interestingly, all fragmented Ll.LtrB variants, which were engineered to mimic natural trans-splicing introns, were able to trans-splice (Belhocine et al. 2007). We therefore decided to develop a more comprehensive approach to investigate the full trans-splicing potential of group II introns.
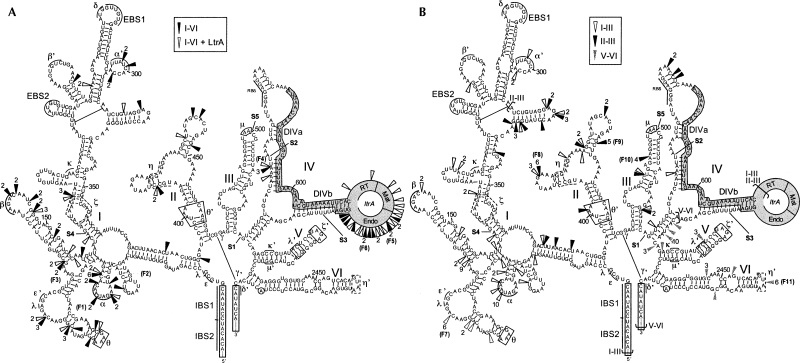
FIGURE 4.
Selected Tn5 insertion sites supporting Ll.LtrB trans-splicing. (A) Ll.LtrB full-length screen (Fig. 3A, PvuI/HpaI). The screen was performed with (open arrowheads) or without (black arrowheads) the expression of LtrA in trans (pDEltrA). (B) Screens of Ll.LtrB subdomains. The various sections of Ll.LtrB that were independently screened are delimited by brackets (I–III, open arrowheads; II–III, black arrowheads; V–VI, gray arrowheads). Isolates found multiple times (numbers) and the ones further analyzed are indicated (F1 to F11). The fragmentation points that were previously engineered to mimic natural trans-splicing group II introns are denoted (S1–S5, Belhocine et al. 2007). Exon binding sites 1 and 2, EBS1 and 2; intron binding sites 1 and 2, IBS1 and 2; exons, open boxes; ltrA, gray box; LtrA reverse transcriptase domain, RT; LtrA maturase domain, Mat; LtrA endonuclease domain, Endo; tertiary interactions, pairs of Greek letters.
We engineered a Tn5 transposon carrying the transcriptional terminator from pepN followed by the P23 L. lactis constitutive promoter (Fig. 3B). Insertion of this transposon into a target gene severs the RNA transcript into two independent fragments: one fragment extending from the natural promoter of the gene up to the Tn5 insertion site (transcriptional terminator) and the second fragment extending from the Tn5 insertion site (P23 promoter) to the end of the transcript (Fig. 1C, step 1). We first used this randomly inserting transposon (Reznikoff et al. 2004) to generate a saturated bank of transposon-containing pBSltrB plasmids (pBSltrB∷Tn) (Fig. 3B, step 1). Next, the section of pBSltrB∷Tn containing Ll.LtrB (Fig. 3A, PvuI/HpaI fragment) was transferred by subcloning to pLE12 (pLE12∷Tn) (Fig. 3B, step 2) so that only the intron and portions of exon 1 and 2 were saturated with Tn5 insertions. Statistically, the pLE12∷Tn bank contains independent plasmids, each carrying a single Tn5 insertion between each nucleotide of Ll.LtrB.
The pLE12∷Tn bank was transformed into L. lactis NZ9800ΔltrB, the transformants were pooled and used as donor cells for conjugation (Fig. 3C). Since relaxase production is needed to initiate pLE12 transfer, a pLE12∷Tn variant can only be transferred if the ltrB transcript tolerates fragmentation at the Tn5 insertion site. More specifically, the plasmid will only be transferred if it carries a Tn5-fragmented Ll.LtrB that can trans-splice and ligate its flanking exons, allowing for relaxase production. Plasmids were recovered from 66 independent transconjugant cells, i.e., recipient cells that acquired a pLE12∷Tn variant, and the transposon insertion sites were localized by sequence analysis. As seen in Figure 4A (black arrowheads), the Tn5 insertions are not randomly distributed within Ll.LtrB following the screen. Instead, the great majority of insertions (56/66) are found at the 5′ end of DI (42/66) and within DIV (14/66) either upstream of the LtrA start codon or within the last 300 nucleotides (nt) of ltrA downstream from the maturase domain. Interestingly, these three locations correspond to fragmentation sites observed in trans-splicing group II introns found in organelles (Bonen 1993). The rest of the Tn5 insertion sites (10/66) are novel fragmentation sites within DI and DII, which were never previously observed in characterized trans-splicing group II introns. No Tn5 insertions were isolated within DIII, DV, or DVI.
The presence of multiple Tn5 insertions within the endonuclease domain of ltrA, downstream from the maturase domain, suggests that these trans-splicing introns express truncated versions of active maturases. We repeated the conjugation screen in the presence of LtrA expressed from a second plasmid (pDEltrA). Interestingly, when the pressure to produce an active maturase from the intron was relieved, numerous Tn5 insertions were found randomly distributed within ltrA, including insertions upstream and within the maturase domain (Fig. 4A, open arrowheads).
These genetic screens demonstrate that the 5′ end of DI and DIV correspond to the best fragmentation regions to create proficient Ll.LtrB trans-splicers. In addition, these experiments confirm the importance of the maturase function of LtrA for Ll.LtrB trans-splicing in L. lactis.
Trans-splicing efficiency of some Tn5 fragmented Ll.LtrB variants
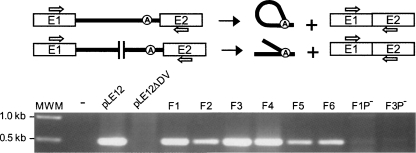
The transfer rate of conjugative elements between L. lactis strains was shown to be directly proportional to the Ll.LtrB splicing efficiency from the ltrB transcript (Belhocine et al. 2007). We thus assessed the trans-splicing efficiency of some selected Tn5-fragmented Ll.LtrB introns by conjugation of their pLE12 carriers (Fig. 4A, F1–F6). The six isolates tested showed significantly higher conjugation efficiencies (10−5–10−6) compared to the splicing-deficient introns (10−9); some of these isolates (Fig. 4A, F1–F3) supported conjugation at a similar rate as pLE12 harboring the cis-splicing intron (Table 1). Moreover, detection of ligated exons by RT-PCR confirmed that both Ll.LtrB fragments are able to fold correctly, assemble, and trans-splice in order to ligate their flanking exons (Fig. 5, F1–F6). As expected from our functional analyses, sequencing of the RT-PCR products (Fig. 5, F1–F6) confirmed that removal of Ll.LtrB by trans-splicing is accurate, precisely joining the flanking exons.
FIGURE 5.
RT-PCR analysis of ltrB ligated exons. The primers used to amplify ligated exons are represented as arrows on the Ll.LtrB splicing schematics. The agarose gel shows the RT-PCR amplification products across the junction of ltrB ligated exons. Amplifications were performed on total RNA extracts of NZ9800ΔltrB-containing plasmids harboring either continuous (pLE12, pLE12ΔD5) or fragmented introns (F1–F6). Variants of the F1 and F6 constructs missing the P23 promoter were also analyzed (F1P− and F3P−).
Ll.LtrB trans-splicing variants fragmented both upstream of (Fig. 4A, F1–F4) and downstream (Fig. 4A, F5,F6) from ltrA were selected. We assumed that insertion of a constitutive promoter upstream of the ORF leads to higher expression levels of LtrA than when expressed from its native promoter. Indeed, Western blot analyses confirmed that significantly more LtrA is present in cells that contain an Ll.LtrB intron bearing a Tn5 insertion upstream of ltrA (data not shown). Nevertheless, when LtrA was provided in trans, all fragmented introns trans-spliced more efficiently (Table 1, 1.8- to 14.2-fold). The increase in trans-splicing efficiency parallels the increase of Ll.LtrB cis-splicing from pLE12 in the same conditions (Table 1, 6.5-fold). These results suggest that, when expressed from its natural promoter, the level of LtrA is suboptimal for both cis- and trans-splicing of Ll.LtrB.
As a control, we deleted the P23 promoter from the transposon of two selected Ll.LtrB trans-splicers (Fig. 4A, F1,F3). This resulted in a significant reduction of their respective conjugation efficiency (Table 1, ∼100-fold decrease) and a drastic reduction of the RT-PCR signal corresponding to ligated exons (Fig. 5, F1P−,F3P−). These results demonstrate that expression of the second intron fragment is required to produce active relaxase. However, since the conjugation efficiencies of both controls are slightly over background (Table 1, cf. F1P− and F3P− and pLE12ΔD5 and pLE12ΔORF) and that a faint RT-PCR signal can be detected for F1P− (Fig. 5), we cannot completely rule out the possibility that some Tn5-interrupted Ll.LtrB variants splice in cis following a transcriptional read-through of the terminator present within the transposon.
Screening Ll.LtrB subdomains for functional fragmentation sites
The full-length intron screen yielded a majority of fragmentation sites in a section of DI and within DIV (Fig. 4A). To facilitate the identification of novel fragmentation sites outside these regions, subdomains of the intron were subjected to the Tn5 screen (Fig. 4B; see brackets, I–III,II–III,V–VI). We first subjected domains I to III of Ll.LtrB to our screen, excluding most of ltrA (Fig. 3A, PvuI/BsaI fragment; Fig. 4B, I–III brackets). Following conjugation, the transposon insertion sites from 68 transferred plasmids were identified (Fig. 4B, open arrowheads). As we found in the original full-length intron screen (Fig. 4A), almost two thirds of the Tn5 insertions (41/68) were located in DI within a 120-nt section near the 5′ end of the intron. Moreover, numerous fragmentation sites that were selected within DI in the original full-length screen were isolated again (Fig. 4, cf. A and B). Nevertheless, the strategy of excluding most of DIV from the screen allowed the identification of more fragmentation sites within DII and two novel sites within DIII. These results confirm that the region located between the basal stem of DI and the ζ region constitutes a region of predilection for Ll.LtrB fragmentation.
We also screened the DII–III region of Ll.LtrB, excluding most of ltrA as well as the beginning of DI (Fig. 3A, fragment BsrGI/BsaI; Fig. 4B, II–III brackets). The transposon insertion sites from 41 transconjugants were sequenced (Fig. 4B, black arrowheads). Twelve transposon insertions were found within DI, concentrated in a 30-nt stretch located upstream of the ζ acceptor site. The majority of fragmentations (15/41) occurred within DII while seven insertions were found in DIII. Finally, four isolates harbored the Tn5 transposon within DIV, upstream of ltrA. These results suggest that DII is more flexible and tolerant to fragmentation than the 3′ portion of DI and DIII.
Domains V and VI are the most conserved domains among group II introns and both play key roles in splicing (Michel and Ferat 1995). DV is the catalytic domain while DVI contains the branch point nucleotide (A) that initiates splicing. We subjected the DV–VI region of Ll.LtrB to the transposon screen (Fig. 3A, fragment AatII/HpaI; Fig. 4B, V–VI brackets). The transposon insertion sites were obtained from 30 transconjugants (Fig. 4B, gray arrowheads). Half of the insertions were located within the short 10-nt stretch of DIV that was included in the screen. Four insertions occurred in DV, with three isolates mapping to the same position. However, these four insertion sites produced largely intact DV and are thus equivalent to the insertions found at the end of DIV. Insertion of the Tn5 transposon creates a 9-nt direct repeat of the target sequence (Goryshin and Reznikoff 1998). Therefore, the second ltrB fragment, initiated from the P23 promoter, starts 9 nt upstream of the indicated arrowheads in Figure 4. The remaining 11 insertions were found in DVI, with six mapping to the same position in the middle of the GUAA tetraloop.
We compared the conjugation efficiencies of selected samples harboring transposon insertions within different domains of Ll.LtrB (Fig. 4B, F7–F11). These conjugation efficiencies are similar to the ones previously observed (Fig. 4B, F1–F6). When LtrA is expressed from a second plasmid, slightly higher conjugation efficiencies were observed, which were significantly higher than the background (Table 1), therefore indicating that Ll.LtrB trans-splices efficiently in all cases.
DISCUSSION
In this study, we describe the use of a Tn5-based genetic screen to explore the versatility of group II introns to splice in trans. The Tn5-fragmented Ll.LtrB variants were selected for proficient trans-splicing introns using a highly sensitive splicing/conjugation assay (Fig. 3C). This experimental approach is both powerful and reliable. First, we identified the Tn5 insertion sites in 43 independent plasmids from the initial pLE12∷Tn bank. Insertions were randomly distributed in both the intron and the ltrB exons, and occurred in both orientations. Conversely, the 247 plasmids selected by conjugation did not display a random distribution of Tn5 insertions (Fig. 4). Moreover, all Tn5 insertions were found in the same orientation as the relaxase gene such that the terminator and promoter are functional and result in the creation of a bipartite intron. In addition, fragmented introns that were selected with our screen harbored either a full-length or truncated version of ltrA that contained the maturase domain, which is essential for Ll.LtrB trans-splicing in vivo (Belhocine et al. 2007). However, when LtrA was provided in trans during selection, the pressure to express a maturase from the intron was relieved, and introns containing Tn5 insertions throughout the whole ltrA gene were selected. As well, removal of the P23 promoter from two Ll.LtrB trans-splicing variants severely reduced the transfer efficiency of their corresponding plasmid carrier. Taken together, these results confirm the reliability of our screen to identify functional fragmentation sites within Ll.LtrB.
The first two Tn5-based genetic screens that we performed, covering the entire intron, revealed that the majority of the fragmentation sites selected were found in DIB-C and DIV of Ll.LtrB (Fig. 4A). These trans-splicing variants of Ll.LtrB are very proficient, some of them splicing almost as efficiently as the non-fragmented Ll.LtrB splicing in cis (Table 1, cf. F1–F6 and pLE12). Interestingly, these sites correspond to natural break points identified in organellar trans-splicing group II introns (Bonen 1993). Then, by targeting subdomains of Ll.LtrB for fragmentation, we demonstrated that group II introns are considerably more versatile than expected from previously described natural trans-splicing group II introns. Indeed, fragmentation sites supporting efficient trans-splicing were found not only in DI and DIV but rather distributed throughout Ll.LtrB. As expected, fragmentations are not tolerated in functionally important regions of Ll.LtrB such as EBS1, EBS2, DV, the 5′ and 3′ splice sites, and the area surrounding the branch point in DVI (Fig. 4). This is also generally true for the structurally important long-range tertiary interactions (Fig. 4, pairs of Greek letters). One exception is the α–α′ long-range interaction where both counterparts of this motif were independently interrupted numerous times. We assume that the α–α′ interaction may still be maintained in these Ll.LtrB trans-splicing variants since insertion of the Tn5 transposon creates a direct repeat of the target sequence which causes the second ltrB fragment to start 9 nt upstream of the fragmentation site, therefore allowing the transcription of an intact α region. However, it is unclear why this small sequence motif is more susceptible to fragmentation than the other small structural motifs present throughout the Ll.LtrB structure.
Our data also confirmed the importance of the maturase function of LtrA for Ll.LtrB trans-splicing in vivo (Belhocine et al. 2007). All trans-splicing variants selected encoded either a full-length or truncated version of LtrA harboring the RT and maturase domains. Also, taking into consideration the Tn5 target site duplication of 9 nt, the high-affinity binding site for LtrA, DIVa, is intact in almost all cases. It is interesting to note that the high-affinity binding site for LtrA, and ltrA, can be present within either the 3′ or the 5′ intron fragment. LtrA may thus be involved in the assembly of the two intron fragments by binding first to its high-affinity binding site in one fragment, and subsequently making secondary contacts with either DI, DII, or DVI (Wank et al. 1999; Matsuura et al. 2001) in the second fragment, therefore contributing to functional trans-splicing of the intron. Potential base pairs and long-range interactions (Fig. 4, pairs of Greek letters) between intron fragments could also be involved in the assembly and trans-splicing of Ll.LtrB.
The discovery of group II introns fragmented into two or three pieces strongly supports the theories proposing that self-splicing group II introns are the progenitors of nuclear introns and that the five spliceosomal snRNAs were derived from fragments of group II introns (Sharp 1991). While numerous functional and/or structural parallels can be drawn between regions of group II introns and some snRNAs (Fig. 2), the specific group II intron segments that correspond to the RNA components of the spliceosome machinery are not well defined and were never precisely delineated. Nevertheless, the discovery that a group II intron can be functionally fragmented at multiple locations throughout its structure and between regions that are functionally and/or structurally analogous to the five snRNAs supports the theory suggesting that the snRNAs, which constitute the heart of the spliceosome machinery, originated from the fragmentation of an ancestral group II intron.
MATERIALS AND METHODS
Strains
L. lactis strains NZ9800ΔltrB∷tet (Belhocine et al. 2004) and LM0231 (Belhocine et al. 2007) were used as donor and recipient for conjugation, respectively. They were grown in M17 broth supplemented with 0.5% glucose (GM17) at 30°C without shaking. Escherichia coli strains DH10β and DH5α were used for cloning and plasmid amplification, respectively, and were grown in LB broth at 37°C with shaking. The following concentrations of antibiotics were used: chloramphenicol (Cam), 10 μg/mL; fusidic acid (Fus), 25 μg/mL; spectinomycin (Spc), 300 μg/mL; ampicillin (Amp), 100 μg/mL; erythromycin (Erm), 300 μg/mL.
Plasmids
The pBSltrB plasmid was constructed by cloning the XbaI/SwaI fragment from pLE12 (Fig. 3A; Belhocine et al. 2004) into the unique SmaI site of pBlueScript SK-. This plasmid was used as the target for the Tn5 in vitro transposition assay. The insertion bank generated (pBSltrB∷Tn) statistically contained 22 copies of each plasmid carrying a single unique Tn5 insertion between each nucleotide (22 insertions/position); every possible Tn5 insertion was represented 22 times. The statistical representation of the bank was calculated by dividing the number of Spc colonies recuperated following transformation of the transposition assay (Tn5-containing plasmids) by twice the size of the plasmid. This method of calculation takes into consideration that Tn5 can insert randomly on either strand of the target plasmid (Goryshin and Reznikoff 1998; Reznikoff et al. 2004). The pBSltrB∷Tn bank was used to construct the various pLE12∷Tn banks except the V–VI bank. For the latter, a variant of the target plasmid, pBSltrBAII, carrying an engineered AatII restriction site within DIV was used to create the pBSltrBAII∷Tn bank (1.9 insertions/position). The AatII site was engineered by site-directed mutagenesis changing intron nucleotides 2384–2387 from 5′-ACGA-3′ to 5′-CGTC-3′. We confirmed by conjugation that splicing of the resulting intron was not impaired (data not shown). The various pLE12∷Tn banks were built by subcloning the corresponding fragments from either the pBSltrB∷Tn (full-length, PvuI/HpaI; I–III, PvuI/BsaI; II–III, BsrGI/BsaI) or pBSltrBAII∷Tn bank (V–VI, AatII/HpaI) into the pLE12 plasmid, where the unique BamHI site present in the MCS was removed. The pMOD−2<MCS> Transposon Construction Vector from Epicentre (pMOD) was used to construct the Tn5 variant used in this study (Fig. 3B). The P23 promoter (two complementary oligos, Table 2) was cloned between the two Tn5 mosaic ends within pMOD (BamHI). The pepN terminator was PCR-amplified from pLEIItd+KR″ (Table 2, primers; Cousineau et al. 1998 and cloned upstream of P23 (HindIII). Finally, the aad9 gene conferring spectinomycin resistance (Spc), flanked by its promoter and terminator, was PCR-amplified from pDL278 (Table 2, primers; Belhocine et al. 2004) and cloned between the pepN terminator and P23 (Fig. 2B, PstI). pDEltrA was constructed by first cloning the PCR-amplified ltrA gene (Table 2, primers) in the pDL-P23 2 plasmid (Belhocine et al. 2007) under the control of P23 (NotI). The ermAM gene conferring erythromycin resistance (Erm) was PCR-amplified from pMSP3545 (Bryan et al. 2000) and cloned in the resulting plasmid (SmaI). Finally, the aad9 gene conferring Spc resistance was deleted (PacI/SwaI).
TABLE 2.
Primers
Conjugation, RT-PCR, and Tn5 transposition assays
Conjugations were performed on milk plates as previously described (Belhocine et al. 2007). Conjugation efficiency was calculated as the ratio of transconjugant cells (Cam/Fus) to donor cells (Cam) for three independent assays. RT-PCR assays were performed as previously described (Belhocine et al. 2007). For the in vitro transposition assay, the Tn5 transposon was excised from pMOD (PvuII) and purified on agarose gel. The transposon was incubated with the pBSltrB or pBSltrBAII target plasmids (14:1 ratio) at 37°C for 2 h, in the presence of the EZ∷Tn5 transposase recombinant protein (Epicentre). A fraction of the transposition mix (10%) was then electroporated into DH10β ElectroMAX competent cells (Invitrogen) and plated on LB/Spc plates to select for transposon-containing plasmids. Notably, insertion of the Tn5 transposon creates a 9-nt direct repeat (Goryshin and Reznikoff 1998). Therefore, the second ltrB fragment that initiated from the P23 promoter within the transposon starts 9 nt upstream of the indicated arrowheads in Figure 4.
ACKNOWLEDGMENTS
We thank L. Bonen, L. Kronstad, F. Lang, R. Lease, C. Ritlop, A. Stern, and K.K. Yam for critical reading of the manuscript, and L. Kronstad for technical assistance. This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) to B.C. B.C. is a FRSQ Chercheur-Boursier Junior 2 and a McGill University William Dawson Scholar. K.B. was supported by a Postgraduate Studies Fellowship from NSERC.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.1083508.
REFERENCES
- Belhocine K., Plante I., Cousineau B. Conjugation mediates transfer of the Ll.LtrB group II intron between different bacterial species. Mol. Microbiol. 2004;51:1459–1469. doi: 10.1111/j.1365-2958.2004.03923.x. [DOI] [PubMed] [Google Scholar]
- Belhocine K., Mak A.B., Cousineau B. Trans-splicing of the Ll.LtrB group II intron in Lactococcus lactis . Nucleic Acids Res. 2007;35:2257–2268. doi: 10.1093/nar/gkl1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen L. Trans-splicing of pre-mRNA in plants, animals, and protists. FASEB J. 1993;7:40–46. doi: 10.1096/fasebj.7.1.8422973. [DOI] [PubMed] [Google Scholar]
- Bryan E.M., Bae T., Kleerebezem M., Dunny G.M. Improved vectors for nisin-controlled expression in gram-positive bacteria. Plasmid. 2000;44:183–190. doi: 10.1006/plas.2000.1484. [DOI] [PubMed] [Google Scholar]
- Byrd D.R., Matson S.W. Nicking by transesterification: The reaction catalysed by a relaxase. Mol. Microbiol. 1997;25:1011–1022. doi: 10.1046/j.1365-2958.1997.5241885.x. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Intron phylogeny: A new hypothesis. Trends Genet. 1991;7:145–148. [PubMed] [Google Scholar]
- Cech T.R. The generality of self-splicing RNA: Relationship to nuclear mRNA splicing. Cell. 1986;44:207–210. doi: 10.1016/0092-8674(86)90751-8. [DOI] [PubMed] [Google Scholar]
- Cousineau B., Smith D., Lawrence-Cavanagh S., Mueller J.E., Yang J., Mills D., Manias D., Dunny G., Lambowitz A.M., Belfort M. Retrohoming of a bacterial group II intron: Mobility via complete reverse splicing, independent of homologous DNA recombination. Cell. 1998;94:451–462. doi: 10.1016/s0092-8674(00)81586-x. [DOI] [PubMed] [Google Scholar]
- Fedorova O., Zingler M. Group II introns: Structure, folding and splicing mechanism. Biol. Chem. 2007;388:665–678. doi: 10.1515/BC.2007.090. [DOI] [PubMed] [Google Scholar]
- Goryshin I.Y., Reznikoff W.S. Tn5 in vitro transposition. J. Biol. Chem. 1998;273:7367–7374. doi: 10.1074/jbc.273.13.7367. [DOI] [PubMed] [Google Scholar]
- Hetzer M., Wurzer G., Schweyen R.J., Mueller M.W. Trans-activation of group II intron splicing by nuclear U5 snRNA. Nature. 1997;386:417–420. doi: 10.1038/386417a0. [DOI] [PubMed] [Google Scholar]
- Jacquier A. Self-splicing group II and nuclear pre-mRNA introns: How similar are they? Trends Biochem. Sci. 1990;15:351–354. doi: 10.1016/0968-0004(90)90075-m. [DOI] [PubMed] [Google Scholar]
- Jarrell K.A., Dietrich R.C., Perlman P.S. Group II intron domain 5 facilitates a trans-splicing reaction. Mol. Cell. Biol. 1988;8:2361–2366. doi: 10.1128/mcb.8.6.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambowitz A.M., Zimmerly S. Mobile group II introns. Annu. Rev. Genet. 2004;38:1–35. doi: 10.1146/annurev.genet.38.072902.091600. [DOI] [PubMed] [Google Scholar]
- Matsuura M., Saldanha R., Ma H., Wank H., Yang J., Mohr G., Cavanagh S., Dunny G.M., Belfort M., Lambowitz A.M. A bacterial group II intron encoding reverse transcriptase, maturase, and DNA endonuclease activities: Biochemical demonstration of maturase activity and insertion of new genetic information within the intron. Genes & Dev. 1997;11:2910–2924. doi: 10.1101/gad.11.21.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura M., Noah J.W., Lambowitz A.M. Mechanism of maturase-promoted group II intron splicing. EMBO J. 2001;20:7259–7270. doi: 10.1093/emboj/20.24.7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel F., Ferat J.L. Structure and activities of group II introns. Annu. Rev. Biochem. 1995;64:435–461. doi: 10.1146/annurev.bi.64.070195.002251. [DOI] [PubMed] [Google Scholar]
- Mills D.A., McKay L.L., Dunny G.M. Splicing of a group II intron involved in the conjugative transfer of pRS01 in lactococci. J. Bacteriol. 1996;178:3531–3538. doi: 10.1128/jb.178.12.3531-3538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman A.J., Norman C. U5 snRNA interacts with exon sequences at 5′ and 3′ splice sites. Cell. 1992;68:743–754. doi: 10.1016/0092-8674(92)90149-7. [DOI] [PubMed] [Google Scholar]
- Nilsen T.W. RNA–RNA interactions in the spliceosome: Unraveling the ties that bind. Cell. 1994;78:1–4. doi: 10.1016/0092-8674(94)90563-0. [DOI] [PubMed] [Google Scholar]
- Patel A.A., Steitz J.A. Splicing double: Insights from the second spliceosome. Nat. Rev. Mol. Cell Biol. 2003;12:960–970. doi: 10.1038/nrm1259. [DOI] [PubMed] [Google Scholar]
- Reznikoff W.S., Goryshin I.Y., Jendrisak J.J. Tn5 as a molecular genetics tool: In vitro transposition and the coupling of in vitro technologies with in vivo transposition. Methods Mol. Biol. 2004;260:83–96. doi: 10.1385/1-59259-755-6:083. [DOI] [PubMed] [Google Scholar]
- Seetharaman M., Eldho N.V., Padgett R.A., Dayie K.T. Structure of a self-splicing group II intron catalytic effector domain 5: Parallels with spliceosomal U6 RNA. RNA. 2006;12:235–247. doi: 10.1261/rna.2237806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P.A. On the origin of RNA splicing and introns. Cell. 1985;42:397–400. doi: 10.1016/0092-8674(85)90092-3. [DOI] [PubMed] [Google Scholar]
- Sharp P.A. Five easy pieces. Science. 1991;254:663. doi: 10.1126/science.1948046. [DOI] [PubMed] [Google Scholar]
- Shearman C., Godon J.J., Gasson M. Splicing of a group II intron in a functional transfer gene of Lactococcus lactis . Mol. Microbiol. 1996;21:45–53. doi: 10.1046/j.1365-2958.1996.00610.x. [DOI] [PubMed] [Google Scholar]
- Shukla G.C., Padgett R.A. A catalytically active group II intron domain 5 can function in the U12-dependent spliceosome. Mol. Cell. 2002;9:1145–1150. doi: 10.1016/s1097-2765(02)00505-1. [DOI] [PubMed] [Google Scholar]
- Valadkhan S. The spliceosome: A ribozyme at heart? Biol. Chem. 2007;388:693–697. doi: 10.1515/BC.2007.080. [DOI] [PubMed] [Google Scholar]
- Wank H., SanFilippo J., Singh R.N., Matsuura M., Lambowitz A.M. A reverse transcriptase/maturase promotes splicing by binding at its own coding segment in a group II intron RNA. Mol. Cell. 1999;4:239–250. doi: 10.1016/s1097-2765(00)80371-8. [DOI] [PubMed] [Google Scholar]