Abstract
Small noncoding RNAs (sRNAs) regulate the response of bacteria to environmental stress in conjunction with the Sm-like RNA binding protein Hfq. DsrA sRNA stimulates translation of the RpoS stress response factor in Escherichia coli by base-pairing with the 5′ leader of the rpoS mRNA and opening a stem–loop that represses translation initiation. We report that rpoS leader sequences upstream of this stem–loop greatly increase the sensitivity of rpoS mRNA to Hfq and DsrA. Native gel mobility shift assays show that Hfq increases the rate of DsrA binding to the full 576 nt rpoS leader as much as 50-fold. By contrast, base-pairing with a 138-nt RNA containing just the repressor stem–loop is accelerated only twofold. Deletion and mutagenesis experiments showed that sensitivity to Hfq requires an upstream AAYAA sequence. Leaders long enough to contain this sequence bind Hfq tightly and form stable ternary complexes with Hfq and DsrA. A model is proposed in which Hfq recruits DsrA to the rpoS mRNA by binding both RNAs, releasing the self-repressing structure in the mRNA. Once base-pairing between DsrA and rpoS mRNA is established, interactions between Hfq and the mRNA may stabilize the RNA complex by removing Hfq from the sRNA.
Keywords: Hfq, RNA chaperone, sRNA, rpoS, noncoding RNA, translation regulation
INTRODUCTION
Control of gene expression in response to environmental factors is essential for bacterial survival. Small noncoding RNAs (sRNAs) are critical components in many bacterial environmental response pathways such as iron metabolism, osmotic shock, and pathogen virulence (Repoila et al. 2003; Valentin-Hansen et al. 2007). About 140 bacterial sRNAs have been proposed through bioinformatics and biochemical screens (Altuvia 2007). They are typically 60–120 nucleotides (nt) long, and the majority of them repress or activate the translation of target genes by base-pairing with complementary sequences in target mRNAs (Lease and Belfort 2000; Storz et al. 2004).
Overlapping networks of RNA interactions are an important feature of regulation by noncoding RNA: a single mRNA can be the target of multiple sRNAs, while individual sRNAs can target more than one mRNA (Repoila et al. 2003). An example of a gene that is regulated by multiple sRNAs is rpoS, which encodes the stress response transcription factor σS (Hengge-Aronis 2002). The expression of rpoS is repressed by the sRNA OxyS (Zhang et al. 1998) and activated by the sRNAs RprA and DsrA (Majdalani et al. 1998; Majdalani et al. 2001). The 5′-leader of the rpoS mRNA forms a self-inhibitory stem–loop that occludes the Shine–Dalgarno ribosome binding site and prevents translation (Fig. 1A; Brown and Elliott 1997). DsrA sRNA, which is the focus of this article, binds the top strand of this stem–loop and opens it, freeing the ribosome binding site and allowing the rpoS mRNA to be translated (Lease et al. 1998; Majdalani et al. 1998).
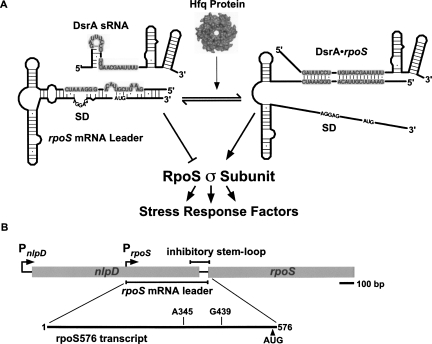
FIGURE 1.
Regulation of rpoS by DsrA sRNA and Hfq. (A) The rpoS gene encodes the σS subunit of RNA polymerase needed for the cell stress response. Translation is inhibited by a stem–loop at the 3′ end of the rpoS mRNA leader that masks the Shine–Dalgarno (SD) ribosome binding sequence (Brown and Elliott 1997). Base pairing with complementary sequences in DsrA sRNA (87 nt) releases rpoS self-inhibition in a process that requires Hfq (Lease et al. 1998; Majdalani et al. 1998). (B) The rpoS locus. Transcription mainly originates from the rpoS promoter within the nlpD coding sequence (Takayanagi et al. 1994). Upper bar indicates the inhibitory stem–loop (bp 439–576). Lower bar indicates the full 576-bp rpoS leader RNA used in these studies. Deletions past bp 345 resulted in loss of Hfq regulation (Cunning et al. 1998).
DsrA activation of rpoS translation requires the abundant RNA binding protein Hfq (Brown and Elliott 1996; Muffler et al. 1996; Sledjeski et al. 2001), a homo-hexameric ring protein first described as a host factor for Qβ phage replication (Franze de Fernandez et al. 1972). Hfq has sequence and structural homology with the archeal and eukaryotic Sm and Lsm proteins (Sauter et al. 2003). Hfq is a cofactor for many sRNAs (Zhang et al. 2003). It not only stabilizes sRNAs against decay, but promotes their association with mRNA targets (Valentin-Hansen et al. 2004; Brennan and Link 2007).
How Hfq promotes regulatory interactions between sRNAs and mRNAs is not understood. One model is that Hfq promotes annealing of complementary RNA sequences by bringing them into close proximity in a ternary complex (Storz et al. 2004). Zhang et al. (2002) showed that Hfq promoted the association of the sRNA OxyS with the fhlA mRNA, forming a stable ternary complex. Similarly, Hfq was found to enhance association of the Spot42 sRNA with galK mRNA (Møller et al. 2002). Alternatively or additionally, Hfq may promote annealing by refolding the mRNA, exposing nucleotides complementary to the sRNA (Geissmann and Touati 2004). Finally, Hfq can stimulate the association or exchange of RNA strands in double helices (Moll et al. 2003; Arluison et al. 2007; Rajkowitsch and Schroeder 2007).
Previous in vitro studies by Lease and Woodson (2004) on a 138-nt fragment of the rpoS leader containing just the inhibitory stem–loop showed that Hfq rapidly equilibrates among its binding sites on DsrA and rpoS mRNA, but annealing of these RNAs was optimal when there was just enough Hfq to saturate its binding site on DsrA. Surprisingly, however, Hfq increased the rate of RNA association and the stability of the rpoS•DsrA complex only twofold. This modest enhancement, which correlated with release of Hfq from the DsrA•rpoS mRNA complex, seemed inconsistent with the genetic requirement for Hfq in DsrA activation of rpoS translation (Sledjeski et al. 2001).
In this article, we report that when the full-length rpoS leader is used as a substrate, Hfq stimulates the rate of anti-sense base pairing with DsrA sRNA 20- to 50-fold. The rpoS gene is primarily transcribed from a promoter within the nlpD coding sequence, 567 bp upstream of the rpoS start codon (Fig. 1B; Takayanagi et al. 1994; Lange et al. 1995). Cunning et al. (1998) compared the expression of rpoS∷lacZ fusions in wild-type and hfq null Salmonella typhimurium, and found that the effect of Hfq on DsrA-dependent upregulation of rpoS expression disappeared for translational fusions in which 345 nt were deleted from the 5′ end of the rpoS mRNA. This deletion produces a leader 100 nt longer than the minimal leader RNA used in previous in vitro studies, raising the possibility that rpoS sequences upstream of the inhibitory stem–loop are required for Hfq to promote DsrA annealing. Thus, we confirm that sequences upstream of the inhibitory stem–loop make the rpoS leader responsive to Hfq. We show that specific sequence elements within this upstream region recruit Hfq to the rpoS mRNA and enable protein-facilitated hybridization of DsrA sRNA.
RESULTS
DsrA and Hfq form a ternary complex with the full-length rpoS leader
If the ability of Hfq protein to stimulate base pairing between DsrA sRNA and rpoS mRNA was small in previous in vitro studies because the minimal rpoS leader RNA used in those experiments lacked important upstream sequences, then restoring those sequences should increase the effectiveness of Hfq in RNA hybridization reactions. To test this hypothesis, the full sequence of the rpoS mRNA-leader from Escherichia coli was cloned, and templates for in vitro transcription were prepared by PCR amplification (Fig. 1B). RNA transcripts were named according to their length; the full-length rpoS-leader is 576 nt and will be referred to as rpoS576.
Binding of DsrA and Hfq to the full-length rpoS mRNA was measured by a gel mobility shift assay as previously described (Lease and Woodson 2004). Radiolabeled DsrA RNA was incubated at 25°C with 0.2 μM rpoS576, 0.5 μM Hfq monomer, or both. Binary complexes of rpoS mRNA•DsrA (R•D) and rpoS mRNA•Hfq (R•H) and a ternary complex containing rpoS RNA, DsrA sRNA, and Hfq protein (Fig. 2A, R•D•H) were resolved in the gel, demonstrating that the full-length leader forms stable interactions with both Hfq protein and DsrA sRNA. Complexes with the same gel mobility as that of R•D and R•D•H were formed when the radiolabel was placed on rpoS mRNA instead of DsrA RNA, confirming the identities of these bands (Fig. 2A).
FIGURE 2.
Hfq accelerates annealing of DsrA to the full-length rpoS leader. (A) Resolution of complexes by native polyacrylamide gel mobility shift. Uniformly 32P-labeled rpoS576 (*)(R) was incubated at 25°C with 200 nM DsrA (D) and 500 nM Hfq monomer (H) as indicated above the lanes. The same R•D and R•D•H complexes were obtained with 32P-end labeled DsrA, 200 nM rpoS576 and 500 nM Hfq. (B) Time dependence of 200 nM DsrA binding to 32P-rpoS576 at 25°C with 500 nM Hfq (center lanes); R•D•H accumulates over 60 min. Unbound rpoS RNA migrates as two conformers (doublet). The gel was run continuously, so the last lane was electrophoresed a shorter time than the first. Standards containing one, two, or three components were prepared as in A and loaded at the start (left) or end (right) of the time course. (C) Kinetics of rpoS576•DsrA RNA association. No Hfq (dashed line), k obs = 0.03±0.008 min−1; 0.5 μM Hfq (solid line), k obs = 1.0 min−1. Data points represent the mean and standard deviation of two or three trials. (D) 32P-DsrA was incubated with 200 nM rpoS576 and 500 nM DsrA, as in B. No early R•D accumulation is observed, indicating that band R•H1 in B, which migrates similarly to R•D, is correctly assigned.
Hfq greatly increases the kinetics of RNA hybridization
We next asked whether Hfq protein could stimulate the rate of DsrA base pairing with the full-length rpoS leader RNA. The rate of DsrA binding to rpoS576 at 25°C was assayed by mixing the two RNAs, with or without 0.5 μM Hfq (monomer), and loading aliquots on a native gel at specific times (Fig. 2B). The fraction of 32P-rpoS576 bound by DsrA was plotted as a function of time and fit to rate equations to obtain the observed rate constants (Fig. 2C).
In the absence of Hfq, the two RNAs hybridized with an observed rate constant of 0.03±0.009 min−1, three times slower than DsrA binding with the minimal (138 nt) leader at 25°C (Lease and Woodson 2004). In the presence of Hfq, the RNA binding kinetics were best fit with a biphasic rate equation, suggesting that protein-dependent hybridization involves an intermediate or that the reaction occurs through two separate pathways. The latter might be due to the fact that the unbound rpoS323 RNA migrates as two conformational species in native gels, even after a variety of annealing conditions. The fast phase accounted for 70%–80% of the reaction, with an observed rate constant of 1.1±0.1 min−1 (Table 1). Similar results were obtained when DsrA was radiolabeled (Fig. 2D). Thus, Hfq increases the rate of DsrA association 30-fold (Table 1, k obs+Hfq / k obs−Hfq), an order of magnitude larger stimulation than observed for the minimal leader (Lease and Woodson 2004). These results show that upstream sequences in the rpoS mRNA leader are important for promotion of sRNA annealing by Hfq.
TABLE 1.
DsrA and Hfq binding properties of rpoS-leader RNAs
During the hybridization reaction, a stable ternary complex between rpoS RNA, DsrA, and Hfq (R•D•H) accumulated over time in the presence of 0.5 μM Hfq (Fig. 2B). This result suggested that when the full-length rpoS leader is present, Hfq remains bound to the functional complex for rpoS translational activation by DsrA. By contrast, the minimal rpoS leader accumulated as the R•D binary complex under these conditions, because Hfq binds less strongly to DsrA after it base-pairs with rpoS mRNA (Lease and Woodson 2004).
Deletion map of the rpoS leader
Having observed that Hfq greatly stimulates DsrA binding to the full-length rpoS leader, we next defined which sequence elements in the rpoS leader were required for this effect. The 576-nt rpoS leader RNA was progressively shortened from the 5′ end to give RNAs of length 430, 323, 227, and 176 nt, respectively (Fig. 3). These truncated rpoS leader RNAs were assayed for their rate of annealing to DsrA in the presence and absence of Hfq as described above.
FIGURE 3.
Deletions of the rpoS leader. The binding kinetics (k obs) (right) between the rpoS leader RNAs on the left and DsrA were measured as in Figure 2, with (dark gray) or without 0.5 μM Hfq (light gray). Leader sequences are numbered from the rpoS promoter as in Figure 1B. For leaders 227 nt and longer, Hfq significantly increased the rpoS•DsrA binding rate constant. The rpoS138 rate contants are interpolated from an Arrhenius plot (Lease and Woodson 2004).
For rpoS430, rpoS323, and rpoS227, the dominant product was the R•D•H ternary complex, and the rate of rpoS•DsrA association was increased 20- to 50-fold in the presence of Hfq (Fig. 3; Supplemental Fig. S1), similar to the full-length rpoS leader (Table 1). For rpoS176, however, the dominant product was the R•D binary complex, and the presence of Hfq increased the association rate roughly twofold, similar to its effect on the minimal rpoS leader (Table 1). The results of these deletions are consistent with in vivo studies of the activity of translational lacZ fusions to the rpoS leader in Salmonella, in which only leaders longer than 230 nt were sensitive to Hfq (Cunning et al. 1998). The 227-nt leader, however, still responds to Hfq in vitro. This difference with the in vivo deletion studies may be due to perturbations to the structure of rpoS227 RNA.
Hfq stabilizes the rpoS•DsrA complex
The stability of the rpoS RNA•DsrA complex was also found to depend on the length of the rpoS-leader sequence. 32P-labeled rpoS leader RNAs were titrated with DsrA in the presence and absence of 0.5 μM Hfq (Fig. 4). In the absence of Hfq, rpoS176, rpoS227, and rpoS323 bound DsrA 10- to 20-fold less tightly than the minimal rpoS138, which has a KD of 3.7 nM (Table 1). Therefore, as few as 40 additional nucleotides 5′ of the inhibitory stem–loop are sufficient to antagonize base pairing with DsrA, suggesting that the upstream sequences stabilize the repressive secondary structure in the leader.
FIGURE 4.
Hfq stabilizes DsrA•rpoS mRNA complexes. DsrA binding to 32P-labeled rpoS176 (gray circles) and rpoS323 (black squares). In the absence of Hfq (filled symbols, solid line), both leaders have a nearly 10-fold reduction in affinity for DsrA compared to rpoS138 (Table 1). In the presence of 0.5 μM Hfq (open symbols, dashed line), the affinity rpoS176 for DsrA is increased only threefold, while the affinity of rpoS323 for DsrA is restored to the value observed for rpoS138 in the presence of 0.5 μM Hfq (Table 1).
Hfq protein stabilized all the rpoS leader RNA complexes with DsrA. However, the extent of stabilization depended on the length of the rpoS RNA. For rpoS176, Hfq stabilized its binding to DsrA about threefold (Fig. 4), from a K D of 34 nM to a K D of 12 nM, similar to the effect of Hfq on the rpoS138•DsrA K D (Table 1; Lease and Woodson 2004). However, Hfq stabilized the complex with rpoS323 significantly more (Fig. 4), lowering the K D ∼20-fold, from 30 nM in the absence of Hfq to 1.4 nM in the presence of the protein. A similar result was observed for rpoS227 (Table 1).
The results above show that in the absence of Hfq, long rpoS RNAs are significantly less able to base-pair with DsrA sRNA than minimal rpoS leader RNAs containing just the inhibitory stem–loop. For rpoS leaders ≥227 nt, however, Hfq greatly increases both the stability of the rpoS RNA•DsrA complex and its rate of formation, so that in the presence of protein, DsrA binds long rpoS mRNAs more rapidly than short mRNAs. We next wanted to determine what sequence elements in the longer rpoS leader sequences allowed Hfq to so significantly promote RNA•RNA association compared to shorter leader sequences.
Long rpoS-leaders contain a strong Hfq binding site
Since long rpoS leader RNAs form more stable ternary complexes with DsrA and Hfq than short leader RNAs, we reasoned that the long leaders might contain an additional strong binding site for Hfq. We previously found that the minimal rpoS leader RNA binds Hfq 4–5 times less strongly than DsrA (Lease and Woodson 2004). To test this hypothesis, the affinity of Hfq for long and short rpoS leaders was compared.
Uniformly labeled rpoS176 and rpoS323 were titrated with Hfq (Fig. 5), and the RNA•protein complexes resolved by native gel electrophoresis. As previously described for rpoS138 (Lease and Woodson 2004), the rpoS leader binds multiple Hfq multimers with increasing Hfq concentration (Fig. 5). Complexes with one, two, or three Hfq multimers were resolved by native gel electrophoresis, and the fraction of each RNP as a function of Hfq concentration was fit with a partition function to obtain the binding constant for each complex (see Materials and Methods).
FIGURE 5.
A strong Hfq binding site on the rpoS leader. (A) Hfq titrations of uniformly 32P-labeled rpoS176 at 25°C. With increasing [Hfq], additional Hfq multimers bind to the rpoS leader and are resolved as more slowly migrating bands on a native gel (R•H1, R•H2, R•H3). (R) free rpoS RNA. The data were fit best with a partition function that assumed three equivalent binding sites with dissociation constant K ns = 1.0 μM (see Materials and Methods). R•H3 is the sum of several high molecular weight complexes. (B) Hfq binding to rpoS323. The data were fit with three nonspecific binding sites as in A, plus an additional high affinity binding site (R•HT), with K T = 0.28 μM. The unliganded rpoS323 RNA (R) migrates as two distinct conformers.
The model that best fit the data for rpoS176 and other short leader RNAs assumed three equal and independent Hfq binding sites, with a dissociation constant K ns=1 μM (Fig. 5A). A cooperativity factor for complexes with three or more bound Hfq multimers was included to account for the fact that the large molecular weight RNPs were not completely resolved in the gel. By contrast, the data for rpoS323 were best fit with a model that included an additional tight Hfq binding site with K T = 0.28 μM Hfq monomer (Fig. 5B). Assuming an apparent stoichiometry of 12:1 Hfq:RNA obtained from previous titrations (Lease and Woodson 2004), the dissociation constant for each independently bound Hfq multimer is 23 nM Hfq12. This result confirmed the hypothesis that the long rpoS leaders bind Hfq more tightly than the minimal leader RNA. Interestingly, Hfq has similar affinity for rpoS323 as for DsrA (K D = 0.22 μM or 18 nM Hfq12) (Lease and Woodson 2004). This suggests a mechanism of action in which Hfq binds both RNAs at the same time.
Structure of the rpoS leader
To further determine what features of the rpoS-leader were important for Hfq-dependent enhancement of rpoS•DsrA complex formation, the structure of rpoS323 RNA was probed by DMS base methylation or acylation of ribose 2′ hydroxyl with NMIA (Merino et al. 2005). rpoS323 was chosen for these experiments because it was the shortest substrate that could explain both in vitro and in vivo results. Modifications were detected by primer extension (Fig. 6A; Supplemental Fig. S4). A secondary structure model was obtained using nucleotides strongly modified by NMIA as constraints in the structure prediction program RNAstructure (Mathews et al. 2004). The sequence and thus secondary structure of the rpoS leader, which overlaps the nlpD coding region, is highly conserved among gram-negative bacteria (see Supplemental Fig. S3). The predicted structure of rpoS323 is shown in Figure 6B with the sites of NMIA and DMS modification. Although this model is preliminary, it suggested several functionally important features.
FIGURE 6.
Structure of rpoS323. The secondary structure of rpoS323 was probed with DMS and NMIA modification in annealing buffer and analyzed by primer extension as detailed in Materials and Methods. (A) Representative sequencing gels. (P) untreated RNA in TE; (G-U) dideoxy sequencing reactions; (UM) unmodified RNA in annealing buffer. Remaining lanes show modified RNA, with or without 6 μM Hfq. (B) Secondary structure model of rpoS323 using modification data as constraints. (Open circles) NMIA modification; (solid circles) strong NMIA modification; (triangle) DMS methylation. The U4, A6, and AAYAA candidate Hfq sites are indicated. Gray letters represent nucleotides for which no data was collected. (C) NMIA and DMS modification in 6 μM Hfq. Symbols as in B, except arrows marked H indicate the range of data collection and open and closed arrowheads indicate 5- and 10-fold enhancement of modification, respectively.
First, the modification data and folding program predicted the same structure for the inhibitory stem–loop in the 3′ half of rpoS323 as previous models of the rpoS leader RNA (Brown and Elliott 1997; Lease and Woodson 2004). Second, the 5′ upstream region is predicted to form three helices (Fig. 6B). Residues at the interface between the inhibitory and upstream regions were predicted to be base paired, with weak modification by NMIA indicating that the base of this helix was dynamic (Fig. 6B). These residues may be less stably paired than the rest of the rpoS323 structure because they are capable of making different interactions in the full-length leader.
Third, the model predicts two A-rich single-stranded sequences in the upstream region. As Hfq binds preferentially to single-stranded A/U rich RNA (Senear and Steitz 1976), these sequences were attractive candidates for Hfq binding sites. The first candidate is a conserved stretch of six unpaired A's from A393 to A398, labeled A6 in Figure 6B. The second is a stretch of 11 single-stranded nucleotides (A369–A379) that contain AAYAA repeats, which are slightly less conserved (Fig. 6B). Interestingly, a AACAAC sequence was recently found in the leaders of several Salmonella mRNAs regulated by small RNAs and Hfq (Sharma et al. 2007). A third potential Hfq binding site is the run of four U's (U442–U445) at the junction between the 5′ and 3′ halves of the leader (U4 in Fig. 6B), which were weakly protected from modification.
Modification of the rpoS leader in the presence of Hfq
To test whether Hfq interacts with these candidate binding sites or changes the structure of the rpoS leader, the rpoS323 was also modified with NMIA and DMS in the presence of 6 μM Hfq (Fig. 6A). Hfq increased the extent of NMIA modification of many nucleotides over the region tested (G496–A324), consistent with a more dynamic or less tightly folded RNA structure (Fig. 6C). Perturbation of the RNA structure was most apparent at the junction between the upstream and downstream halves of the leader, between residues 430 and 450 (Supplemental Fig. S4). Interestingly, Hfq enhanced 10- to 15-fold the NMIA modification of the AAYAA and a U-rich sequence within the inhibitory stem–loop that was previously proposed as a binding site for Hfq (Fig. 6C; Supplemental Fig. S4).
Very specific changes in the modification pattern of the AAYAA motif suggest either a conformational switch or, more attractively, direct interactions of this sequence with Hfq. The fourth A of each repeat (A372, A375, and A378) was 2′ acylated more strongly with Hfq, while A378, A379 were protected from base methylation by DMS (Fig. 6A). There was relatively little change in the relative modification of the A6 motif.
Hfq response element in the rpoS leader
To test whether Hfq tightly binds specific sequences in the upstream half of the rpoS leader, the candidate Hfq binding sites were disrupted by site-directed mutagenesis. We first measured the binding of Hfq to an RNA containing only the upstream half of the leader (Fig. 7). An RNA including the U4 element (rpoS323254–457) bound tightly to Hfq, but an RNA lacking these U's (rpoS323254–440) did not (Table 1). While this result seemed to implicate the U4 element as the strong Hfq binding site, substitution of these U's in the long leader RNA (rpoS323ΔU4) had no effect on Hfq binding or enhancement of DsrA hybridization (Table 1). Thus, the U4 element is likely not required for Hfq responsiveness. These results did suggest that the structural context of individual Hfq binding sites is important.
FIGURE 7.
Mutagenesis of potential Hfq binding sites in rpoS323. The U4, A6, and AAYAA elements were changed by site-directed mutagenesis of rpoS323 as indicated. All three mutants retained a tight Hfq binding site, but mutagenesis of AAYAA compromised acceleration of DsrA binding by Hfq (Table 1). RNAs containing just the upstream sequences were rpoS323254–440, nt 254–440; rpoS323254–457, nt 254–457 (Table 1). Residues are numbered from the rpoS promoter.
Next, we used site-directed mutagenesis to individually change the upstream A6 element to a BsaAI restriction site (rpoS323ΔA6) or the AAYAA element into a GC-rich sequence (Fig. 7, rpoS323ΔAAYAA). Mutagenesis of the A6 sequence had little effect on Hfq binding or on its ability to increase the initial rate of DsrA binding (Table 1). Like wild-type rpoS323, rpoS323ΔA6 accumulated as an R•D•H ternary complex. However, the fraction bound in the ternary complex saturated at 55% of the total and the remaining RNA accumulated as an R•D complex with k obs = 0.05 min−1 (Supplemental Fig. S1).
Mutation of the AAYAA motif had a much larger effect on Hfq sensitivity (Fig. 7). While mutation of this site decreased the affinity of Hfq only slightly (K T = 0.36 μM), the RNA lacking the AAYAA motif (rpoS323ΔAAYAA) accumulated only the R•D binary complex. Hfq was no longer able to strongly stimulate DsrA hybridization, increasing the binding rate to rpoS323ΔAAYAA only fourfold, from 0.01 min−1 to 0.04 min−1 (Table 1). DMS footprinting indicates that the ΔAAYAA mutation, which includes a 2-nt deletion, does not alter the secondary structure of the leader (data not shown). However, other structural changes in the RNA may contribute to the results observed for rpoS323ΔAAYAA. While neither mutation eliminated tight Hfq binding to rpoS mRNA, these results indicate that the AAYAA motif is critically important for the ability of Hfq to promote the assembly of the rpoS RNA•DsrA regulatory complex.
DISCUSSION
Stable ternary complex with Hfq promotes sRNA hybridization
An important function of Hfq in noncoding RNA regulation is to promote the association of sRNAs with their complementary binding sites in target mRNAs. Like Sm proteins, Hfq preferentially binds single-stranded U or A nucleotides adjacent to a double helix (Brescia et al. 2003). However, Hfq can also form stable ternary complexes with two RNA strands. Such complexes were observed to form on OxyS sRNA (Zhang et al. 2002) and in looped replication intermediates of phage Qβ (Schuppli et al. 1997). The complementary RNAs remain associated when Hfq is removed, suggesting that Hfq facilitates base pairing between them (Zhang et al. 2002). In addition to chaperoning sRNAs, Hfq is thought to recruit ribosomes, RNase E, polyA polymerase, and other enzymes to the mRNA (Brennan and Link 2007).
Our results show that Hfq forms stable ternary complexes with DsrA sRNA and the full rpoS leader, and that the accumulation of these complexes correlates with a 20- to 50-fold increase in the rate of DsrA binding. The formation of stable rpoS•DsrA•Hfq complexes and the enhancement of DsrA and rpoS RNA hybridization requires rpoS sequences far upstream of the DsrA binding site, as minimal RNAs respond very little to Hfq. Our observation that upstream leader sequences are needed for Hfq responsiveness in vitro is consistent with genetic deletions in Salmonella showing that the corresponding sequences are required for post-transcriptional regulation of rpoS expression by Hfq (Cunning et al. 1998).
Together these results support a model for Hfq action in which Hfq facilitates the interactions between sRNA and their targets by recruiting both RNAs to a stable ternary complex (Fig. 8). The long rpoS leader accomplishes this by providing a strong binding site for Hfq that keeps the protein tethered to the mRNA. By contrast, we propose that Hfq must be displaced from its binding site on DsrA in order for the complementary sequences in DsrA and rpoS mRNA to become fully base paired (Lease and Woodson 2004). Hfq preferentially binds single-stranded RNA, such as the unpaired U's in DsrA, and thus would not be expected to associate tightly with the anti-sense duplex. The idea that Hfq is recycled from the minimal anti-sense complex is supported by the observations that Hfq binds the minimal DsrA•rpoS138 complex less tightly than it binds DsrA alone and that very high Hfq concentrations inhibit RNA association (Lease and Woodson 2004). Thus, an additional function of the Hfq binding sites in the full-length rpoS leader may be to facilitate transfer of Hfq away from DsrA after base pairing with rpoS mRNA is initiated, thereby allowing complete hybridization of the two RNAs.
FIGURE 8.
Model for Hfq-dependent regulation of rpoS. In the absence of Hfq, the folded rpoS leader represses translation initiation and disfavors base pairing with DsrA sRNA. Binding of Hfq to the upstream leader and interactions with the AAYAA sequence relieve autorepression and recruits DsrA sRNA. A stable ternary complex between DsrA, rpoS mRNA, and one or more Hfq hexamers facilitates base pairing between complementary sequences in rpoS mRNA and DsrA. When anti-sense pairing is complete, Hfq cycles off its DsrA binding site but remains bound to upstream sequences in the rpoS mRNA leader.
Hfq binding sites in rpoS
In our experiments, the kinetics of DsrA and rpoS RNA association correlate with the ability of Hfq to bind both RNAs. Intriguingly, the rpoS leader contains two A-rich sequences, which raise the affinity for Hfq so that it is similar to the U-rich Hfq binding site in DsrA. Hfq is proposed to have distinct binding sites for A-rich and U-rich sequences (Mikulecky et al. 2004; Sun and Wartell 2006), allowing a single hexamer to engage the U-rich sequence in DsrA and the A-rich sequence in rpoS mRNA. However, formation of the ternary complex may involve association of two or more hexamers or conformational changes in the flexible C-terminal domain of Hfq (Vecerek et al. 2008). Regardless of the binding mechanism, there is evidence that both U-rich and A-rich sequences are needed to form a functional Hfq complex (Vecerek et al. 2005). Because DsrA and rpoS mRNA bind Hfq with similar affinity, the amount of ternary complex will depend on the relative concentrations of each RNA and the presence of other RNAs that bind Hfq in the cell.
Hfq relieves self-repression in the rpoS leader
In addition to bringing the two RNAs into close proximity, the footprinting results suggest that Hfq may make rpoS translation more responsive to sRNA regulation by destabilizing the folded structure of the leader. In the absence of Hfq, DsrA binds long rpoS leaders 8–15 times less tightly than it binds the minimal leader (Table 1), suggesting that the upstream sequences contribute interactions that stabilize the inhibitory stem–loop and make the rpoS leader intrinsically less accessible to DsrA or to the 30S ribosome. The presence of tertiary structure within the rpoS leader is an attractive explanation for such additional interactions.
Recruitment of Hfq to the rpoS mRNA completely overcomes this additional repression (Fig. 8). Thus, the sensitivity to Hfq is achieved by tighter repression in the absence of the protein and more facile recruitment of DsrA in the presence of Hfq. These results are consistent with the expression of β-galactasidase from rpoS∷lacZ fusions in Salmonella, in which the loss of Hfq sensitivity was due as much to increased translation in Hfq− cells as decreased translation in Hfq+ cells (Cunning et al. 1998).
A distinct role for Hfq binding DsrA
Hfq has been shown in these and previous results to confer a twofold increase on the rate of formation and the stability of the complex between DsrA and the minimal rpoS leader. This modest rate enhancement is distinct from the much larger effects of Hfq seen with the longer rpoS leaders and probably is the result of Hfq's interaction with DsrA. Alternative structures have been proposed for DsrA (Rolle et al. 2006), and it is possible that, analogous to what we propose for the Hfq•rpoS interaction, Hfq stabilizes DsrA in a conformation more amenable to binding the rpoS leader.
Dual roles of Hfq in promoting DsrA annealing to the rpoS leader
In summary, we propose a model in which both DsrA and the rpoS leader RNA bind Hfq at separate binding sites (Fig. 8). Hfq binding has the dual effects of stabilizing each RNA in a conformation more receptive to annealing as well as bringing the RNAs into close proximity and thus increasing their rate of annealing. Hfq is displaced from DsrA upon RNA annealing because the Hfq and rpoS binding sites on DsrA are mutually exclusive, but Hfq remains bound to the rpoS leader to form a final ternary complex. This complex may have additional functions in translational regulation, via interactions with ribosomal protein S1 and the 30S ribosome (Kajitani et al. 1994; Sukhodolets and Garges 2003) or even initiator tRNAs (Lee and Feig 2008).
MATERIALS AND METHODS
Plasmids and transcription templates
The rpoS leader sequences were amplified by PCR from chromosomal DNA in E. coli M182 cells, cloned into a plasmid (pTM182FL) using a TOPO cloning kit (Invitrogen), and sequenced. The upstream PCR primer (5′-GCAACTAATACGACTCACTATAGGGTGAACAGAGTGCTAAC) contained a T7 promoter and the downstream primer (5′-GTGAATTCTGACTCATAAGGTGG) contained an EcoRI site.
The DNA templates for transcription of rpoS576 and its variants were prepared by PCR using a common downstream primer (5′-GTGAATTCTGACTCATAAGGTGG) and the following upstream primers: rpoS576: 5′-GCAACTAATACGACTCACTATAGGGTGAACAGAGTGCTAAC; rpoS430: 5′-GTAGTAATACGACTCACTATAGGCCGACTGAGGGCAAAG; rpoS323: 5′-GTAGTAATACGACTCACTATAGGCCGCGTTGTTTATGCTG; rpoS227: 5′-GTAGTAATACGACTCACTATAGACACAATGCTGGTCCGGG; and rpoS176: 5′-GTAGTAATACGACTCACTATAGCGACCATGGGTAGCACCG. Prior to their use in transcription reactions, PCR products were purified (GFX; GE Healthcare), extracted with phenol and chloroform, precipitated with salt and ethanol, dried, and resuspended in TE (10 mM TrisHCl at pH 7.5, 1 mM EDTA at pH 5).
RNA preparation
DsrA and minimal rpoS RNA (rpoS138) were transcribed from plasmids pUCT7DsrA and pUCT7RpoS2, respectively, and purified as previously described (Lease and Woodson 2004). Other RNAs were transcribed from PCR templates described above, according to standard protocols (1–3 mL total volume; 1 μg/mL plasmid DNA or 0.5 μg/mL PCR DNA). Transcripts were purified by denaturing PAGE as previously described (Zaug et al. 1988).
Radiolabeled RNA was obtained either by a small-scale transcription with α32P-ATP (40 μL total volume, 0.5 μg PCR DNA or 1 μg linearized plasmid, T7 RNAP) and purified by passing over a Clontech spin column or by 5′-end phosphorylation of a dephosphorylated RNA as previously described (Lease and Woodson 2004). Concentrations of uniformly labeled RNAs were estimated from the absorbance at 260 nm of RNA from a labeling reaction with α32P-ATP omitted.
Native gel mobility shift assays
The wild-type Hfq protein was overexpressed in E. coli and purified as previously described (Zhang et al. 2002). All binding reactions were carried out in annealing buffer (50 mM Tris-HCl at pH 7.5, 250 mM NaCl, 250 mM KCl) at 25°C unless otherwise specified. Prior to use, all rpoS and DsrA RNAs were renatured by heating 1 min at 75°C–80°C, followed by cooling 5 min at room temperature. Hfq storage buffer (50 mM Tris-HCl at pH 7.5, 1 mM EDTA, 250 mM NH4Cl, 10% [v/v] glycerol) was substituted for Hfq solution in no-protein reactions. For all reactions, 2-μL aliquots were loaded under power on native 6% polyacrylamide (29:1) gel in 1× TBE. Gels were dried and analyzed using a Molecular Dynamics PhosphorImager.
Equilibrium Hfq•rpoS binding experiments were carried out as previously described (Lease and Woodson 2004), except that 32P-rpoS mRNA was ∼10 nM, and reactions were incubated 10 min at 25°C before processing as above. Equilibrium rpoS•DsrA binding reactions (10 μL) contained DsrA, 1–2 nM 32P-labeled rpoS, and either 0 or 0.5 μM Hfq monomer (final concentrations) and were incubated 3 h.
Kinetic rpoS•DsrA binding experiments (30 μL) contained ∼10 nM uniformly labeled rpoS leader, 0.2 μM DsrA, and 0 or 0.5 μM Hfq monomer (final concentrations) in annealing buffer. Where stated, 32P-labeled DsrA was mixed with 0.2 μM rpoS RNA. The reaction was initiated by adding the buffer and protein to the RNAs, which do not bind in the absence of salt (R. Lease, pers. comm.), and mixed by gentle pipeting. Aliquots were loaded on native gels at various times (0.5–90 min) as described above.
Determination of binding constants
The fraction of each species in each lane was calculated by dividing the counts in each individual band by the sum of the counts in all bands in the lane. For equilibrium DsrA binding, the fraction of R•D or R•D•H complex versus DsrA concentration was fit to a single-site binding isotherm (KaleidaGraph). Where necessary, the concentration of free DsrA was corrected as previously described (Lease and Woodson 2004). For kinetic experiments, the fractions of R•D or R•D•H complex were individually plotted against time and fit to rate equations containing one or two exponential terms.
For equilibrium Hfq binding experiments the fraction of each R•H species versus Hfq concentration was fit to Equations 1 or 2 depending on the number of resolvable RNP complexes. Experiments with three complexes (RH1, RH2, RH3) were fit to a partition function for three identical independent binding sites with a binding constant Kns:
Plots with four complexes (RHT, RH1, RH2, RH3) were fit to a partition function for one tight (KT) and three identical (Kns) sites:
 |
In both cases the data were best fit assuming that RH3 was a combination of multiple RNP complexes (poorly resolved in the gel), with an apparent linkage of L = 1.5–2. Typical values of n ranged from 2.4 to 3.5.
Chemical footprinting
The secondary structure of the rpoS323 RNA was probed with SHAPE chemistry (Wilkinson et al. 2006). RNA (2.5 pmol) was modified in the presence or absence of 6 μM Hfq in a 10 μL reaction as described above but omitting glycerol dye and carrier tRNA. Modification was carried out with either 1 μL saturated NMIA solution for 1 h at room temperature or 1 μL DMS diluted 1:5 in 95% EtOH for 20 min on ice. The close agreement in modification patterns suggests that the secondary structure of the RNA does not change significantly with temperature. Modified RNA was analyzed by primer extention as previously described (Pan and Woodson 1998). The primer GTGTGAATTCTGACTCATAAGGTGG was used for downstream sequences and the primer GCAAGCGTGTTGAACTGGTTCCG for upstream sequences.
Individual modification experiments were analyzed by the program SAFA (Das et al. 2005) to obtain band intensities at each resolvable position. The unmodified control lane was subtracted from the modified lanes in each experiment and the average intensity of bands in the ddGTP sequencing lane was used to correct for the number of counts loaded. Corrected band intensities from individual experiments were combined and normalized to the highest peak (Supplemental Fig. S4). For NMIA data, positions ≥0.15 of the highest intensity were considered modified and positions ≥0.35 were considered strongly modified. For DMS data, positions ≥0.15 of the highest intensity were considered modified.
Secondary structure models of rpoS323 were obtained using the program RNAstructure with standard parameters (Mathews et al. 2004). Nucleotides that were strongly modified by NMIA were constrained to be single stranded and no other constraints were used.
SUPPLEMENTAL DATA
Supplemental material can be found at http://www.rnajournal.org.
ACKNOWLEDGMENTS
We thank R. Lease and J. Hopkins for gifts of plasmids, assistance with Hfq purification, and many helpful discussions. This work was supported by a grant from the NIH (GM46686).
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.1110608.
REFERENCES
- Altuvia S. Identification of bacterial small non-coding RNAs: Experimental approaches. Curr. Opin. Microbiol. 2007;10:257–261. doi: 10.1016/j.mib.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Arluison V., Hohng S., Roy R., Pellegrini O., Regnier P., Ha T. Spectroscopic observation of RNA chaperone activities of Hfq in post-transcriptional regulation by a small noncoding RNA. Nucleic Acids Res. 2007;35:999–1006. doi: 10.1093/nar/gkl1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan R.G., Link T.M. Hfq structure, function, and ligand binding. Curr. Opin. Microbiol. 2007;10:125–133. doi: 10.1016/j.mib.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Brescia C.C., Mikulecky P.J., Feig A.L., Sledjeski D.D. Identification of the Hfq-binding site on DsrA RNA: Hfq binds without altering DsrA secondary structure. RNA. 2003;9:33–43. doi: 10.1261/rna.2570803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L., Elliott T. Efficient translation of the RpoS σ factor in Salmonella typhimurium requires host factor I, an RNA-binding protein encoded by the hfq gene. J. Bacteriol. 1996;178:3763–3770. doi: 10.1128/jb.178.13.3763-3770.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L., Elliott T. Mutations that increase expression of the rpoS gene and decrease its dependence on hfq function in Salmonella typhimurium . J. Bacteriol. 1997;179:656–662. doi: 10.1128/jb.179.3.656-662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunning C., Brown L., Elliott T. Promoter substitution and deletion analysis of upstream region required for rpoS translational regulation. J. Bacteriol. 1998;180:4564–4570. doi: 10.1128/jb.180.17.4564-4570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R., Laederach A., Pearlman S.M., Herschlag D., Altman R.B. SAFA: Semi-automated footprinting analysis software for high-throughput quantification of nucleic acid footprinting experiments. RNA. 2005;11:344–354. doi: 10.1261/rna.7214405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franze de Fernandez M.T., Hayward W.S., August J.T. Bacterial proteins required for replication of phage Q ribonucleic acid. Purification and properties of host factor I, a ribonucleic acid-binding protein. J. Biol. Chem. 1972;247:824–831. [PubMed] [Google Scholar]
- Geissmann T.A., Touati D. Hfq, a new chaperoning role: Binding to messenger RNA determines access for small RNA regulator. EMBO J. 2004;23:396–405. doi: 10.1038/sj.emboj.7600058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge-Aronis R. Signal transduction and regulatory mechanisms involved in control of the σ(S) (RpoS) subunit of RNA polymerase. Microbiol. Mol. Biol. Rev. 2002;66:373–395. doi: 10.1128/MMBR.66.3.373-395.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajitani M., Kato A., Wada A., Inokuchi Y., Ishihama A. Regulation of the Escherichia coli hfq gene encoding the host factor for phage Q β. J. Bacteriol. 1994;176:531–534. doi: 10.1128/jb.176.2.531-534.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange R., Fischer D., Hengge-Aronis R. Identification of transcriptional start sites and the role of ppGpp in the expression of rpoS, the structural gene for the σ S subunit of RNA polymerase in Escherichia coli . J. Bacteriol. 1995;177:4676–4680. doi: 10.1128/jb.177.16.4676-4680.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lease R.A., Belfort M. Riboregulation by DsrA RNA: Trans-actions for global economy. Mol. Microbiol. 2000;38:667–672. doi: 10.1046/j.1365-2958.2000.02162.x. [DOI] [PubMed] [Google Scholar]
- Lease R.A., Woodson S.A. Cycling of the Sm-like protein Hfq on the DsrA small regulatory RNA. J. Mol. Biol. 2004;344:1211–1223. doi: 10.1016/j.jmb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Lease R.A., Cusick M.E., Belfort M. Riboregulation in Escherichia coli: DsrA RNA acts by RNA:RNA interactions at multiple loci. Proc. Natl. Acad. Sci. 1998;95:12456–12461. doi: 10.1073/pnas.95.21.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., Feig A.L. The RNA binding protein Hfq interacts specifically with tRNAs. RNA. 2008;14:514–523. doi: 10.1261/rna.531408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdalani N., Cunning C., Sledjeski D., Elliott T., Gottesman S. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc. Natl. Acad. Sci. 1998;95:12462–12467. doi: 10.1073/pnas.95.21.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdalani N., Chen S., Murrow J., St John K., Gottesman S. Regulation of RpoS by a novel small RNA: The characterization of RprA. Mol. Microbiol. 2001;39:1382–1394. doi: 10.1111/j.1365-2958.2001.02329.x. [DOI] [PubMed] [Google Scholar]
- Mathews D.H., Disney M.D., Childs J.L., Schroeder S.J., Zuker M., Turner D.H. Incorporating chemical modification constraints into a dynamic programming algorithm for prediction of RNA secondary structure. Proc. Natl. Acad. Sci. 2004;101:7287–7292. doi: 10.1073/pnas.0401799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino E.J., Wilkinson K.A., Coughlan J.L., Weeks K.M. RNA structure analysis at single nucleotide resolution by selective 2′-hydroxyl acylation and primer extension (SHAPE) J. Am. Chem. Soc. 2005;127:4223–4231. doi: 10.1021/ja043822v. [DOI] [PubMed] [Google Scholar]
- Mikulecky P.J., Kaw M.K., Brescia C.C., Takach J.C., Sledjeski D.D., Feig A.L. Escherichia coli Hfq has distinct interaction surfaces for DsrA, rpoS and poly(A) RNAs. Nat. Struct. Mol. Biol. 2004;11:1206–1214. doi: 10.1038/nsmb858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll I., Leitsch D., Steinhauser T., Blasi U. RNA chaperone activity of the Sm-like Hfq protein. EMBO Rep. 2003;4:284–289. doi: 10.1038/sj.embor.embor772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller T., Franch T., Hojrup P., Keene D.R., Bachinger H.P., Brennan R.G., Valentin-Hansen P. Hfq: A bacterial Sm-like protein that mediates RNA–RNA interaction. Mol. Cell. 2002;9:23–30. doi: 10.1016/s1097-2765(01)00436-1. [DOI] [PubMed] [Google Scholar]
- Muffler A., Fischer D., Hengge-Aronis R. The RNA-binding protein HF-I, known as a host factor for phage Qβ RNA replication, is essential for rpoS translation in Escherichia coli . Genes & Dev. 1996;10:1143–1151. doi: 10.1101/gad.10.9.1143. [DOI] [PubMed] [Google Scholar]
- Pan J., Woodson S.A. Folding intermediates of a self-splicing RNA: Mispairing of the catalytic core. J. Mol. Biol. 1998;280:597–609. doi: 10.1006/jmbi.1998.1901. [DOI] [PubMed] [Google Scholar]
- Rajkowitsch L., Schroeder R. Dissecting RNA chaperone activity. RNA. 2007;13:2053–2060. doi: 10.1261/rna.671807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repoila F., Majdalani N., Gottesman S. Small noncoding RNAs, co-ordinators of adaptation processes in Escherichia coli: The RpoS paradigm. Mol. Microbiol. 2003;48:855–861. doi: 10.1046/j.1365-2958.2003.03454.x. [DOI] [PubMed] [Google Scholar]
- Rolle K., Zywicki M., Wyszko E., Barciszewska M.Z., Barciszewski J. Evaluation of the dynamic structure of DsrA RNA from E. coli and its functional consequences. J. Biochem. 2006;139:431–438. doi: 10.1093/jb/mvj045. [DOI] [PubMed] [Google Scholar]
- Sauter C., Basquin J., Suck D. Sm-like proteins in Eubacteria: The crystal structure of the Hfq protein from Escherichia coli . Nucleic Acids Res. 2003;31:4091–4098. doi: 10.1093/nar/gkg480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuppli D., Miranda G., Tsui H.C., Winkler M.E., Sogo J.M., Weber H. Altered 3′-terminal RNA structure in phage Qβ adapted to host factor-less Escherichia coli . Proc. Natl. Acad. Sci. 1997;94:10239–10242. doi: 10.1073/pnas.94.19.10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senear A.W., Steitz J.A. Site-specific interaction of Qβ host factor and ribosomal protein S1 with Qβ and R17 bacteriophage RNAs. J. Biol. Chem. 1976;251:1902–1912. [PubMed] [Google Scholar]
- Sharma C.M., Darfeuille F., Plantinga T.H., Vogel J. A small RNA regulates multiple ABC transporter mRNAs by targeting C/A-rich elements inside and upstream of ribosome-binding sites. Genes & Dev. 2007;21:2804–2817. doi: 10.1101/gad.447207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sledjeski D.D., Whitman C., Zhang A. Hfq is necessary for regulation by the untranslated RNA DsrA. J. Bacteriol. 2001;183:1997–2005. doi: 10.1128/JB.183.6.1997-2005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz G., Opdyke J.A., Zhang A. Controlling mRNA stability and translation with small, noncoding RNAs. Curr. Opin. Microbiol. 2004;7:140–144. doi: 10.1016/j.mib.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Sukhodolets M.V., Garges S. Interaction of Escherichia coli RNA polymerase with the ribosomal protein S1 and the Sm-like ATPase Hfq. Biochemistry. 2003;42:8022–8034. doi: 10.1021/bi020638i. [DOI] [PubMed] [Google Scholar]
- Sun X., Wartell R.M. Escherichia coli Hfq binds A18 and DsrA domain II with similar 2:1 Hfq6/RNA stoichiometry using different surface sites. Biochemistry. 2006;45:4875–4887. doi: 10.1021/bi0523613. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y., Tanaka K., Takahashi H. Structure of the 5′ upstream region and the regulation of the rpoS gene of Escherichia coli . Mol. Gen. Genet. 1994;243:525–531. doi: 10.1007/BF00284200. [DOI] [PubMed] [Google Scholar]
- Valentin-Hansen P., Eriksen M., Udesen C. The bacterial Sm-like protein Hfq: A key player in RNA transactions. Mol. Microbiol. 2004;51:1525–1533. doi: 10.1111/j.1365-2958.2003.03935.x. [DOI] [PubMed] [Google Scholar]
- Valentin-Hansen P., Johansen J., Rasmussen A.A. Small RNAs controlling outer membrane porins. Curr. Opin. Microbiol. 2007;10:152–155. doi: 10.1016/j.mib.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Vecerek B., Moll I., Blasi U. Translational autocontrol of the Escherichia coli hfq RNA chaperone gene. RNA. 2005;11:976–984. doi: 10.1261/rna.2360205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecerek B., Rajkowitsch L., Sonnleitner E., Schroeder R., Blasi U. The C-terminal domain of Escherichia coli Hfq is required for regulation. Nucleic Acids Res. 2008;36:133–143. doi: 10.1093/nar/gkm985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson K.A., Merino E.J., Weeks K.M. Selective 2′-hydroxyl acylation analyzed by primer extension (SHAPE): Quantitative RNA structure analysis at single nucleotide resolution. Nat. Protoc. 2006;1:1610–1616. doi: 10.1038/nprot.2006.249. [DOI] [PubMed] [Google Scholar]
- Zaug A.J., Grosshans C.A., Cech T.R. Sequence-specific endoribonuclease activity of the Tetrahymena ribozyme: Enhanced cleavage of certain oligonucleotide substrates that form mismatched ribozyme-substrate complexes. Biochemistry. 1988;27:8924–8931. doi: 10.1021/bi00425a008. [DOI] [PubMed] [Google Scholar]
- Zhang A., Altuvia S., Tiwari A., Argaman L., Hengge-Aronis R., Storz G. The OxyS regulatory RNA represses rpoS translation and binds the Hfq (HF-I) protein. EMBO J. 1998;17:6061–6068. doi: 10.1093/emboj/17.20.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A., Wassarman K.M., Ortega J., Steven A.C., Storz G. The Sm-like Hfq protein increases OxyS RNA interaction with target mRNAs. Mol. Cell. 2002;9:11–22. doi: 10.1016/s1097-2765(01)00437-3. [DOI] [PubMed] [Google Scholar]
- Zhang A., Wassarman K.M., Rosenow C., Tjaden B.C., Storz G., Gottesman S. Global analysis of small RNA and mRNA targets of Hfq. Mol. Microbiol. 2003;50:1111–1124. doi: 10.1046/j.1365-2958.2003.03734.x. [DOI] [PubMed] [Google Scholar]