Abstract
The nucleus of spermatocytes provides during the first meiotic prophase an interesting model for investigating relationships of the nuclear envelope (NE) with components of the nuclear interior. During the pachytene stage, meiotic chromosomes are synapsed via synaptonemal complexes (SCs) and attached through both ends to the nuclear periphery. This association is dynamic because chromosomes move during the process of synapsis and desynapsis that takes place during meiotic prophase. The NE of spermatocytes possesses some peculiarities (e.g., lower stability than in somatic cells, expression of short meiosis-specific lamin isoforms called C2 and B3) that could be critically involved in this process. For better understanding of the association of chromosomes with the nuclear periphery, in the present study we have investigated the distribution of NE proteins in relation to SC attachment sites. A major outcome was the finding that lamin C2 is distributed in the form of discontinuous domains at the NE of spermatocytes and that SC attachment sites are embedded in these domains. Lamin C2 appears to form part of larger structures as suggested by cell fractionation experiments. According to these results, we propose that the C2-containing domains represent local reinforcements of the NE that are involved in the proper attachment of SCs.
INTRODUCTION
The nuclear envelope (NE)1 is composed of a double membrane, the pore complexes, and the nuclear lamina. The nuclear lamina is in intimate contact with the nuclear side of the inner nuclear membrane and belongs to the category of karyoskeletal structures. Available evidence indicates that the nuclear lamina provides mechanical stability to the nuclear periphery and that it is involved in the topological organization of chromatin. In somatic cells, the nuclear lamina is composed mostly of the lamins, a family of intermediate filament proteins. B-type lamins (lamins B1 and B2) are ubiquitous components of the nuclear lamina, whereas A-type lamins (lamins A and C) are expressed in differentiated but not in undifferentiated cells (for recent reviews see Krohne, 1998; Stuurman et al., 1998).
The inner nuclear membrane is distinct from the outer membrane and contains specific integral membrane proteins. These are, e.g., the protein p58 or lamin B-receptor (LBR) (Worman et al., 1990) and the lamina-associated polypeptides 1 (LAPs1A–C) (Senior and Gerace, 1988; Martin et al., 1995) and 2 (LAPs2 β and γ) (Foisner and Gerace, 1993; Harris et al., 1994; Furukawa et al., 1995; Dechat et al., 1998). These integral proteins of the inner nuclear membrane have affinity for lamins and chromatin and have been implicated in the attachment of the inner nuclear membrane to the nuclear lamina as well as in the structural organization of the nucleus (for reviews see Georgatos et al., 1994; Gerace and Foisner, 1994; Ye et al., 1998).
During the last few years, evidence has accumulated that indicates the existence of remarkable differences in the composition and organization of the NE between somatic and spermatogenic cells. In mammalian primary spermatocytes (meiotic prophase cells), the amount of lamins and LAPs2 per nucleus appears to be lower than in somatic cells (Vester et al., 1993; Alsheimer et al., 1998). Furthermore, lamin expression in spermatogenic cells shows a series of peculiarities. Somatic lamins A, C, and B2 are not detectable in spermatocytes. Instead, they express the lamins C2 and B3, which are meiosis-specific splicing variants of the lamin A and B2 genes, respectively. Of the lamins expressed in somatic cells, lamin B1 is the only one that could be detected in spermatocytes (Smith and Benavente, 1992; Furukawa and Hotta, 1993; Vester et al., 1993; Furukawa et al., 1994; Alsheimer and Benavente, 1996). During spermiogenesis (postmeiotic stages), a profound remodelling of the NE takes place, including the redistribution and progressive disappearance of lamin B1 as well as most of the LAPs2 (Alsheimer et al., 1998).
Sequencing of mammalian lamins C2 and B3 revealed that these proteins are shorter than the somatic members of the family. In both cases, the nonhelical N-terminus of the molecule and part of the helical domain that are typical for somatic lamins are substituted by a short nonhelical sequence (Furukawa and Hotta, 1993; Furukawa et al., 1994; Alsheimer and Benavente, 1996). Interestingly, from the investigations on somatic lamins (for review see Stuurman et al., 1998) it is known that the domains that are absent in lamins C2 and B3 are involved in the dimerization as well as the formation of more complex structures. According to this, it has been proposed that lamins C2 and B3 would supply a flexible condition to the NE (Furukawa and Hotta, 1993; Alsheimer and Benavente, 1996). If this is true, these findings would provide an explanation for the lower stability of nuclei observed in spermatocytes submitted to mechanical stress. Furthermore, they would also explain the breakdown of the nuclear periphery of pachytene spermatocytes treated with nonionic detergents (Stick and Schwarz, 1982).
An additional interesting peculiarity of the nuclear periphery of spermatocytes concerns its relationship with components of the nuclear interior. For example, during the pachytene stage of meiotic prophase, chromosomal bivalents are synapsed via synaptonemal complexes (SCs). The SC is a tripartite, ribbon-like structure composed of two lateral elements and a central region that extends all along the chromosomal bivalent (for a recent review see Moens et al., 1998) and is attached only at both ends to the NE (Wettstein and Sotelo, 1971; Esponda and Giménez-Martín, 1972). Chromatin loops of the bivalents, for their part, are anchored at the lateral elements of SCs (Rattner et al., 1980). Despite being attached at the nuclear periphery, chromosomes actively move in meiotic prophase nuclei during the process of synapsis and desynapsis (Wilson, 1925; Hiraoka, 1952; Moses, 1968; Solari, 1970; Moens, 1973; Parvinen and Söderström, 1976; Loidl, 1990; Scherthan et al., 1996; Bass et al., 1997). Therefore, it has been postulated that chromosome movements during meiotic prophase would require a modified nuclear periphery with properties that differ from that of somatic cells (for an overview see Alsheimer and Benavente, 1996).
The peculiarities of the nuclear periphery of meiotic cells summarized above and their probable relevance for the meiotic process prompted us to investigate in detail the distribution and behavior in cell fractionation experiments of NE proteins in pachytene spermatocytes of the rat.
MATERIALS AND METHODS
Cells
Enriched populations of Wistar rat pachytene spermatocytes were obtained by centrifugal elutriation according to standard procedures (Meistrich, 1977; Heyting and Dietrich, 1991). Cell line RV-SMC (vascular smooth muscle cells) of the rat was cultured as described (Franke et al., 1980).
Antibodies and Immunocytochemistry
The following primary antibodies against nuclear protein components were used: 1) monoclonal antibody (mAb) 13d4 (LAPs2 α, β, and γ; Alsheimer et al., 1998); 2) mAb R27 (lamins A, C, and C2; Höger et al., 1991; Smith and Benavente, 1992); 3) mAb PKB8 (lamins A, B1, and C; Krohne et al., 1984); 4) mAb against pore complex protein p62 (Benavente et al., 1989); 5) guinea pig serum specific for SC-protein SCP3 (Alsheimer and Benavente, 1996); and 6) human autoimmune serum MAN against LAPs2 (Paulin-Levasseur et al., 1996; Lang et al., 1999).
After elutriation, aliquots containing 105 pachytene spermatocytes were suspended in 100 μl PBS (140 mM NaCl, 2.6 mM KCl, 6.4 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.4). The cells were fixed by adding 100 μl of PBS containing 2% formaldehyde (freshly prepared from paraformaldehyde). After 10 min, the cell suspension was centrifuged for 2 min at 800 rpm (Cytospin 2, Shandon, Frankfurt, Germany) onto a slide that had been covered with 0.1% poly-l-lysine 6000.HBr (Serva, Heidelberg, Germany). Immediately after centrifugation, the cells were incubated in PBS containing 0.05% Triton X-100 for 1 min followed by three washes in PBS (5 min each). Then the cells were incubated for 20 min with one of the primary antibodies. After being washed in PBS (15 min), the slides were incubated for 10 min with the appropriate secondary antibodies conjugated to dichloro-triazimyl-amino-fluoresceine or Texas Red (Dianova, Hamburg, Germany). The DNA-specific fluorochrome Hoechst 33258 (Hoechst, Frankfurt, Germany; final concentration 5 μg/ml in PBS) was added to the secondary antibodies. After being washed in PBS, followed by a 2 min incubation in 96% ethanol, the slides were air-dried, embedded in Mowiol, and covered with a coverslip. For double-label immunofluorescence, essentially the same protocol was used, except that the slides were incubated first with mixtures of two different primary antibodies. Appropriate secondary antibodies were also mixed before incubation (Kralewski and Benavente, 1997).
Confocal laser scanning microscopy was performed as described previously (Hock et al., 1998) with a Leica microscope TCS-NT (Leica, Bensheim, Germany) equipped with a 63×/1.30 Neofluar oil immersion objective. Single optical sections were taken with zoom 2 and 4× accumulation. Fluorescent signals of both fluorochromes were recorded simultaneously at one scan. To merge the pictures, Adobe Photoshop (Adobe Systems, San Jose, CA) software was used.
Electron microscopical immunolocalization was performed according to the method described by Graham and Karnovsky (1966). Pachytene spermatocytes were fixed and incubated with antibodies as described above, except that secondary antibodies were conjugated to peroxidase (Dianova). After incubation for 7–20 min in 3,3′-diaminobenzidine tetrahydrochloride (DAB; Serva) and 30% hydrogen peroxide (Merck, Darmstadt, Germany), the cells were fixed with 2.5% glutaraldehyde (30 min; 4°C) and 1% osmium tetroxide (30 min; 4°C). The cells were dehydrated and embedded in Epon according to conventional procedures. Electron micrographs of unstained sections were taken at 80 kV.
Cell Fractionation
The fate of NE proteins was investigated in cell fractionation experiments using protocols similar to those described previously (Senior and Gerace, 1988; Foisner and Gerace, 1993; Dechat et al., 1998). Cells were lysed in a 10 mM Tris/HCl buffer (pH 7.4) containing 1 mM phenylmethylsulfonyl fluoride, 4 mM EDTA, 0.5 mM dithiothreitol, 0.1 mg/ml trypsin inhibitor SI (Sigma, Deisenhofen, Germany), and 1% Triton X-100. After incubation for 10 min on ice, the suspension was centrifuged for 10 min (1600 × g at 4°C). The pellet was resuspended in a buffer containing 10 mM Tris/HCl (pH 7.8), 1 mM phenylmethylsulfonyl fluoride, 4 mM MgCl2, 0.5 mM dithiothreitol, 0.1 mg/ml trypsin inhibitor, and 10 U/ml DNase I (Boehringer Mannheim, Mannheim, Germany). After the DNA digestion step (10 min on ice), NaCl was added (final concentrations 250 mM or 2 M), and the suspensions were incubated for another 10 min on ice. Nonsoluble proteins were pelleted by centrifugation at 13,000 × g (4°C) for 10 min. The supernatants and the pellets were then analyzed by PAGE.
SDS-PAGE and Immunoblotting
One-dimensional SDS-PAGE was performed on 10% polyacrylamide gels (Laemmli, 1970). The proteins were transferred to nitrocellulose membranes by using the semi-dry Western blotting system described by Matsudaira (1987). The membranes were blocked for 2 h at room temperature with TBST buffer (10 mM Tris/HCl, pH 7.4, 150 mM NaCl, 0.1% Tween 20) containing 10% milk powder. After being washed with TBST, membranes were incubated for 2 h at room temperature with hybridoma supernatant containing mAb 13d4, PKB8, or R27. Bound antibodies were detected with the enhanced chemiluminescence system (Amersham, Braunschweig, Germany). Two-dimensional SDS-PAGE was performed essentially as described by O’Farrell (1975), with one exception. In the case of the LAPs2, the following ampholine concentrations were used in the first dimension: pH 5–7, 1.8%; pH 7–9, 1.8%; pH 9–11, 0.9%; and pH 2–11, 1.8%.
RESULTS
Distribution of Spermatocyte NE Proteins
We have investigated the distribution of NE proteins of pachytene spermatocytes using confocal laser scanning microscopy. In a first set of experiments, the cells were incubated with antibodies to different protein components of the NE (Figure 1). To establish the spatial relationship between NE proteins and the attachment sites of SCs, in a second set of experiments (Figures 2 and 3) pachytene spermatocytes were double-labeled with NE antibodies and antibodies against SCP3, a major structural protein component of the lateral elements of the SC (Lammers et al., 1994; Yuan et al., 1998).
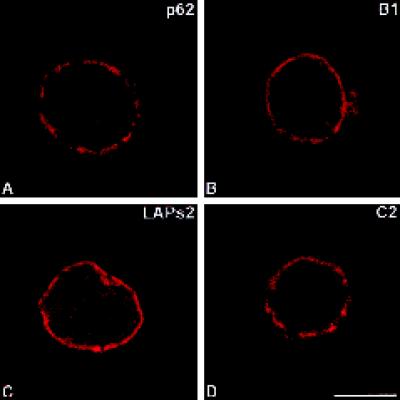
Figure 1.
Immunolocalization of pore complex protein p62 (A), lamin B1 (B), LAPs2 (C) as well as lamin C2 (D). Labeled rat pachytene spermatocytes were investigated by confocal laser scanning microscopy. Bar, 10 μm.
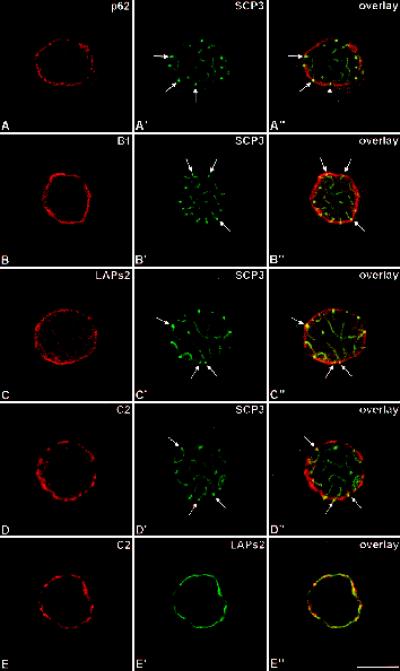
Figure 2.
Double-label immunolocalization with antibodies against pore complex protein p62 and SC-protein SCP3 (A–A′), lamin B1 and SCP3 (B–B′), LAPs2 and SCP3 (C–C′), lamin C2 and SCP3 (D–D′), and C2 and LAPs2 (E–E′). Labeled rat pachytene spermatocytes were investigated by confocal laser scanning microscopy. Overlays are shown in A"–E". Some of the attachment sites of the SCs are denoted by arrows. Bar, 10 μm.
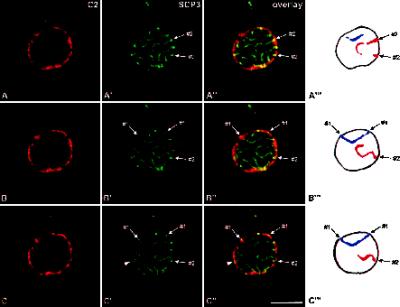
Figure 3.
Double-label immunolocalization with antibodies against lamin C2 (A–C) and protein SCP3 (A′–C′). The labeled rat pachytene spermatocyte was investigated by confocal laser scanning microscopy. Three consecutive optical sections are shown (A–A′, B–B′, C–C′). Overlays are presented in A"–C". A‴–C‴, Schematic representation of the arcs displayed by SCs #1 (blue) and #2 (red). The arrowheads in C′–C" point at an XY body. Bar, 10 μm.
From earlier electron microscopical studies it is known that pore complexes are not randomly distributed at the surface of spermatocyte nuclei. Rather, they occur in clusters and are excluded from the SC attachment sites (Fawcett and Chemes, 1979). Therefore, we used an antibody against pore complex protein p62 to test the level of detail provided by the confocal microscope in our spermatocyte preparations. We observed that the antibody labeled several discrete domains at the nuclear periphery of spermatocytes that most probably correspond to the clusters of pore complexes described at the electron microscopical level (Figures 1A and 2A). In Figure 2A–A" we show that these domains do not overlap with SC attachment sites.
When compared with the p62 antibody, antibodies against lamin B1 and LAPs2 labeled the nuclear periphery of spermatocytes in a different way (Figures 1, B and C, and 2, B and C). In both cases, the fluorescence signal at the nuclear periphery was continuous, which is consistent with the ring-like pattern of somatic cells. As expected, in the overlay the signals corresponding to these NE proteins merge with that of SC attachment sites (Figure 2, B" and C").
Remarkably, we noted significant differences in the distribution of lamin C2 when compared with that of the other NE proteins described here. In fact, the antibodies against lamin C2 labeled domains of the NE (Figures 1D, 2D, and 3). This nonhomogeneous distribution of lamin C2 is more clearly documented in double-label immunofluorescence experiments in which spermatocytes were also incubated with LAPs2 antibodies. The ring-like fluorescence pattern obtained with the LAPs2 antibody contrasts with the apparently discontinuous distribution of lamin C2 in the same spermatocyte (Figure 2, E–E"); however, the spatial relationship between the lamin C2-positive domains and the SC attachment sites differed from that described for pore complexes. As shown in Figure 2, D–D", we observed that SC attachment sites are embedded in NE domains strongly labeled with the anti-C2 antibody. This is more clearly seen in Figure 3 where three consecutive optical sections through a pachytene spermatocyte are shown (Figure 3, A–A′, B–B′, and C–C′). Two of the SCs entirely contained in these sections were denoted by #1 and #2, respectively. (The denotations #1 and #2 are arbitrary and do not correspond to the karyotype number of these chromosomes.) The attachment sites of SC #1 are contained in section 3C′–C", whereas those of SC #2 are seen in 3A′–A". These observations could be confirmed at the electron microscopical level. The patchy distribution of lamin C2 (Figure 4, C and E) contrasts with the more continuous labeling of the nuclear periphery obtained with lamin B1 antibodies (Figure 4A). SC attachment sites, for their part, were observed in regions of the NE that are positive with the antibodies to B1 and C2 lamins (Figure 4, B, D, and F).
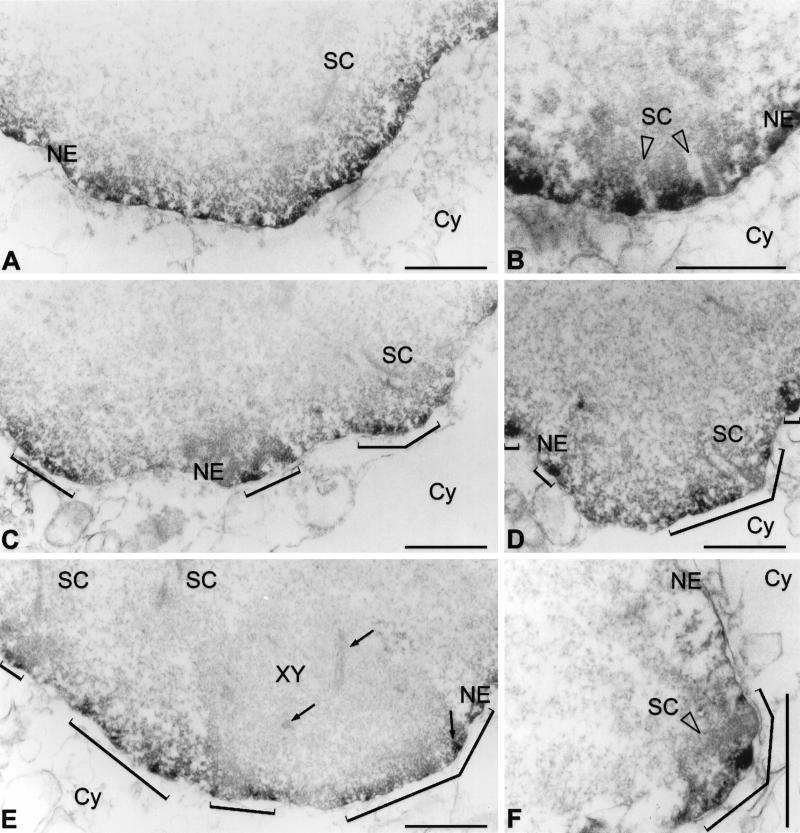
Figure 4.
Electron microscopical immunolocalization of lamins B1 (A, B) and C2 (C–F) in pachytene spermatocytes of the rat using peroxidase-conjugated secondary antibodies. The patchy pattern obtained with C2 antibodies is denoted by brackets (C–F). Frontal (B, D) and lateral (F) views of SC attachment sites at the NE are shown. XY, XY body; Cy, cytoplasm. XY body axial elements (including an insertion at the NE) are denoted by arrows (E). Bars, 1 μm.
A further interesting aspect was to investigate the spatial relationship between the lamin C2-containing domains and the XY body. This structure appears as a mass of distinctly condensed chromatin that is associated with the NE of spermatocytes. The XY body is formed of the partially synapsed sex chromosomes X and Y, and as in the case of autosomal bivalents, the ends of the axial structures of sex chromosomes are also attached to the NE (Solari, 1974, 1994). As shown in Figure 3C–C", the XY body was found closely associated with the nuclear periphery in a region in which several small C2-positive domains can be resolved. A corresponding patchy pattern is seen at the electron microscopical level after peroxidase immunoreaction (Figure 4E).
Biochemical Characterization of Spermatocyte NE Proteins
As summarized in the INTRODUCTION, the NE of spermatocytes presents a series of differences when compared with that of somatic cells. These differences refer to the amount of NE proteins per nucleus as well as to the complement of structural proteins (i.e., lamins) that are expressed. Here we provide the analysis of pachytene spermatocyte NE proteins by two-dimensional PAGE and in cell fractionation experiments.
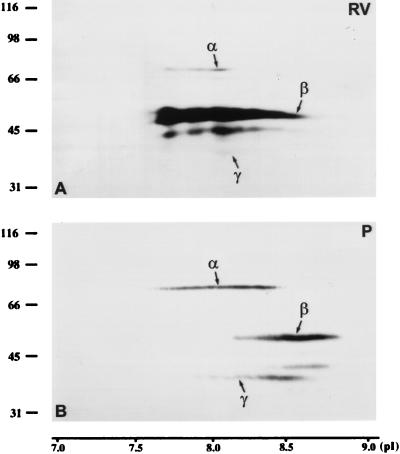
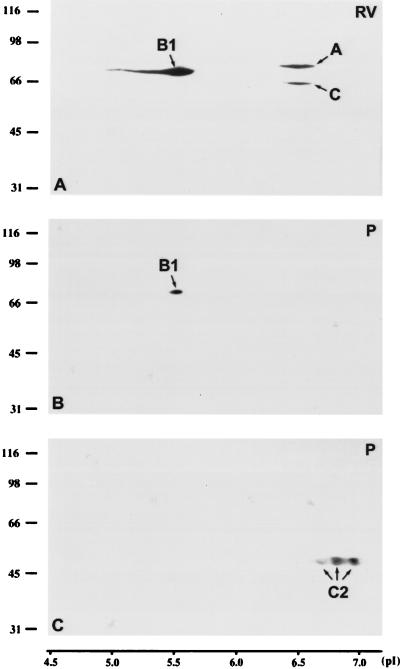
The mobility of LAPs2 of pachytene spermatocytes and RV-SMC somatic cells was compared after separation by two-dimensional PAGE and immunoblotting. The following pI values were obtained in RV-SMC cells: 7.7–8.2 for LAP2α, 7.6–8.7 for the very prominent LAP2β, and 8.0–8.2 for LAP2γ, which is weakly expressed in this cell line (for differences in the relative expression levels of LAPs2 in different cell types, see Alsheimer et al., 1998). In pachytene spermatocytes, the obtained pI values were quite similar, although slightly more basic: 7.6–8.5 for LAP2α, 8.1–8.8 for LAP2β, and 7.9–8.6 for LAP2γ (Figure 5). As shown in Figure 6, the mobility of lamin B1 of pachytene spermatocytes and somatic cells was virtually the same (pI 5.5). A-type lamins, for their part, showed nearly neutral pI values (lamins A and C: 6.4–6.6; lamin C2: 6.6–7.0).
Figure 5.
Two-dimensional PAGE of whole RV-SMC cells (A; 5 × 106 cells) and pachytene spermatocytes of the rat (B; 5 × 106 cells). After transfer to nitrocellulose, the proteins were incubated with mAb 13d4 against the LAPs2 α, β, and γ.
Figure 6.
Two-dimensional PAGE of whole RV-SMC cells (A; 5 × 106 cells) and pachytene spermatocytes of the rat (B, C; 5 × 106 cell/gel). After transfer to nitrocellulose, the proteins were incubated with mAb PKB8 against lamins A, B1, and C (A and B) or mAb R27 against lamins A, C, and C2 (C).
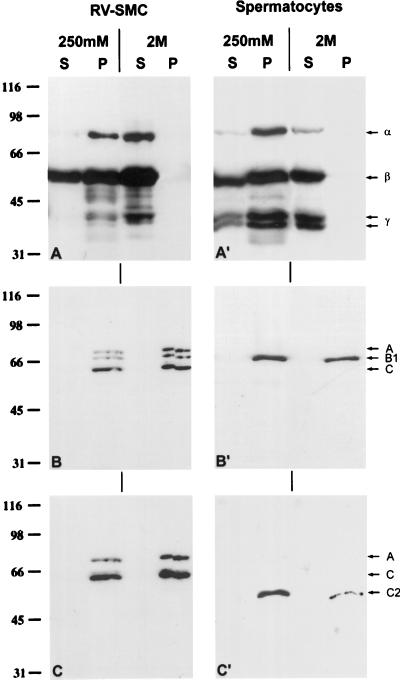
In an additional experiment, RV-SMC cells and pachytene spermatocytes were extracted with buffers containing 1% Triton X-100 and 250 mM NaCl, and the fate of the LAPs2 and lamins was followed by immunoblotting of the different fractions (Figure 7). As in somatic cells (Foisner and Gerace, 1993; Dechat et al., 1998), most of the LAPs2 of spermatocytes was found in the pellet fraction together with lamin B1. Under these experimental conditions lamin C2 was recovered in the pellet fraction. The lamins B1 and C2 remained insoluble even after extractions with higher salt concentrations (up to 2 M NaCl in the presence of 1% Triton X-100). In contrast, the three LAPs2 were solubilized completely under these conditions (Figure 7).
Figure 7.
Immunoblotting of NE proteins of RV-SMC cells (A–C; 2.5 × 104 cell equivalents/lane) and pachytene spermatocytes of the rat (A′–C′; 2.5 × 104 cell equivalents/lane) that were fractionated with buffers containing 1% Triton X-100 and 250 mM or 2 M salt. mAbs 13d4 (A–A′; against LAPs2 α, β, and γ), PKB8 (B–B′; against lamins A, B1, and C) as well as R27 (C–C′; against lamins A, C, and C2) were used to detect the respective antigens. S, Supernatant fraction; P, pellet fraction.
DISCUSSION
Peculiarities of the Nuclear Lamina of Rat Pachytene Spermatocytes
As summarized in the INTRODUCTION, a characteristic of the nuclear periphery of primary spermatocytes is the relative lower mechanical stability in comparison with that of somatic cells. Major differences in the composition of the nuclear periphery of spermatocytes that may account for this overall reduced stability have been characterized: 1) the peculiarities in the structure of meiosis-specific lamins (Smith and Benavente, 1992; Furukawa and Hotta, 1993; Furukawa et al., 1994; Alsheimer and Benavente, 1996) and 2) the lower amount of lamin B1 and LAP2β (Vester et al., 1993; Alsheimer et al., 1998). A further aspect of relevance for the present discussion is that pachytene spermatocytes are cells in prophase. As known from somatic cells, the process of mitotic nuclear disassembly requires the phosphorylation of lamins and integral proteins of the inner nuclear membrane. As a consequence of phosphorylation of lamins in mitotic cells, the structure of the nuclear lamina breaks down and the lamins disperse in the cytoplasm (Gerace et al., 1984; Heald and McKeon, 1990). Mitotic phosphorylation of LAPs2 leads to a reduced affinity for other components of the NE and chromatin (Foisner and Gerace, 1993; Dechat et al., 1998; for reviews see Gerace and Burke, 1988; Gerace and Foisner, 1994; Georgatos et al., 1994). Therefore, for the present study we were interested in obtaining biochemical information on major NE proteins of pachytene spermatocytes. The general conclusion that can be drawn from the experiments of Figures 5–7 is that lamins B1 and LAPs2 of pachytene spermatocytes behave as in interphase somatic cells. 1) Lamin B1 of spermatocytes remained in the pellet fraction after extractions with high salt buffers and nonionic detergents. The LAPs2, for their part, are extractable with buffers containing 2 M NaCl and nonionic detergents, but were mostly recovered in the pellet after incubation of cells with 250 mM NaCl and 1% Triton (Gerace et al., 1984; Foisner and Gerace, 1993; Dechat et al., 1998). 2) Lamin B1 of spermatocytes and somatic cells comigrated after separation in two-dimensional PAGE. In pachytene spermatocytes, we found no indication of an acidic charge shift of lamin B1, as previously described for lamins of dividing somatic cells and that would reflect the hyperphosphorylated state of these macromolecules (Gerace et al., 1984). The situation is similar for the LAPs2, which are even slightly more basic in spermatocytes than in somatic cells. In conclusion, we found no indication that the peculiarities of the NE of pachytene spermatocytes are due to a cell cycle-related modification (i.e., phosphorylation status) of lamin B1 and LAPs2. Interestingly, mouse pachytene spermatocytes cultured in the presence of okadaic acid (a phosphatase inhibitor) are able to complete prophase and to reach metaphase I within a few hours, a process that normally takes 2–4 d (Wiltshire et al., 1995). These observations would suggest that in pachytene stage the phosphorylation status of lamin B1 and LAPs2 as well as of other NE proteins is maintained by an interplay between kinases and phosphatases. This seems to be the case according to our recent preliminary data (von Glasenapp and Benavente, unpublished observations) obtained by using the model system described by Wiltshire et al. (1995).
It has been proposed that meiotic lamins C2 and B3 would provide a flexible condition to the nuclear periphery of spermatocytes (Smith and Benavente, 1992; Furukawa and Hotta, 1993; Alsheimer and Benavente, 1996). This assumption is based on the differences that have been found in the primary structure of these two lamins in comparison with the somatic members of the family. Lamins C2 and B3 lack certain domains that, from investigations on the somatic isoforms, are known to be involved in dimerization and head-to-tail association of the molecules. Furthermore, in transfected somatic cells expressing lamin B3, nuclei adopt abnormal configurations (Furukawa and Hotta, 1993). The observation in this study, that lamin C2 is not distributed as a continuous layer but rather in apparently discontinuous domains, is remarkable and would provide additional evidence that this protein plays a role in providing flexibility to the nuclear periphery of spermatocytes; however, despite these differences, lamin C2 appears to retain at least part of the capability to form large structures because it remained together with lamin B1 insoluble after extraction of cells with high salt buffers and nonionic detergents. (The localization of lamin B3 and its behavior in cell fractionation experiments are not known.) Reports on a discontinuous distribution of lamins are scarce in the literature, and most of them deal with spermatogenic cells (for the situation in somatic cells see Belmont et al., 1993 and references therein). For example, uneven distribution over the NE of lamin B1 and LAPs2 has been described recently during rat spermiogenesis (Alsheimer et al., 1998). These findings are most probably not restricted to the rat, because uneven distribution of NE proteins has been also reported in late spermatids and/or mature sperms of evolutionary distant species, as for example, sea urchin (Collas et al., 1996), Xenopus (Benavente and Krohne, 1985), and mouse (Moss et al., 1987).
Taken together, the present study provides further support for the notion that spermatocytes contain a nuclear lamina structure that shows important differences in composition and organization when compared with that of somatic cells.
Attachment of the SC to the NE
As described previously at the electron microscopical level, SCs are attached at both ends to the NE. This association involves terminal morphological specializations of the SCs called attachment plaques (Esponda and Giménez-Martín, 1972, and references therein). At the level of the attachment plaques, the lateral elements are characteristically thicker (which would explain the stronger labeling of the SC ends often seen with anti-SCP3 antibodies [e.g., Figures 2 and 3]) and appear to have a reduced affinity for DNA than in other regions (Vázquez-Nin et al., 1993). In addition, the association of the attachment plaques with the NE appears to be mechanically stable because pieces of membranes have been observed repeatedly being attached to the tips of SCs isolated from spermatocyte preparations. On the other hand, this association must be dynamic to allow the chromosome movements that take place during meiotic prophase. At the present time we have no knowledge regarding the molecules involved in the attachment of SCs to the NE.
In the present study we have shown that lamin C2-antibodies labeled discontinuous domains of the nuclear periphery of spermatocytes. This is in contrast to the ring-like fluorescence pattern obtained with antibodies against lamin B1 and LAPs2. Remarkably, SC attachment sites were observed only in lamin C2-containing domains of the NE. This is also the case for the XY body. Because lamin C2 appears to be able to form part of larger structures (Figure 7), we propose here to consider the C2-containing domains as local reinforcements of the NE that would give to the subjacent membrane a higher mechanical stability that for its part would be required for the proper attachment of the SC.
The relationship between the SCs and the C2-containing domains at the molecular level is still unclear at this time. Also unknown is whether the C2-containing domains are stable or rather dynamic structures. Therefore, we present here two different scenarios that describe possible forms of association between the SCs and the C2-containing domains. 1) The SCs are firmly attached to the C2-containing domains and do not move in relation to them. Movement of the SC would be achieved by the displacement of the C2-containing domains over the subjacent membrane. According to this possibility, the SC and the associated C2-containing domains would form a functional unit. 2) C2-containing domains are highly dynamic structures that are able to move, fragment, and fuse with other domains. The association of the SCs with the C2-containing domains is also dynamic, so that C2-containing domains would function as a kind of platform for SC movement.
ACKNOWLEDGMENTS
We thank Georg Krohne for many helpful discussions and for mAbs R27 and PKB8, Marie-Christine Dabauvalle for mAb against p62, Micheline Paulin-Levasseur for MAN antibodies, and Rosie Rudd for correction of this manuscript. This work was supported by a grant of the Deutsche Forschungsgemeinschaft to R.B. (Be 1168/4-2).
Abbreviations used:
- LAPs2
lamina-associated polypeptides 2
- NE
nuclear envelope
- SC
synaptonemal complex
REFERENCES
- Alsheimer M, Benavente R. Change of karyoskeleton during mammalian spermatogenesis: expression pattern of nuclear lamin C2 and its regulation. Exp Cell Res. 1996;228:181–188. doi: 10.1006/excr.1996.0315. [DOI] [PubMed] [Google Scholar]
- Alsheimer M, Fecher E, Benavente R. Nuclear envelope remodelling during rat spermiogenesis: distribution and expression pattern of LAP2/thymopoietins. J Cell Sci. 1998;111:2227–2234. doi: 10.1242/jcs.111.15.2227. [DOI] [PubMed] [Google Scholar]
- Bass HW, Marshall WF, Sedat JW, Agard DA, Cande WZ. Telomeres cluster de novo before the initiation of synapsis: a three-dimensional spatial analysis of telomere positions before and during meiotic prophase. J Cell Biol. 1997;137:5–18. doi: 10.1083/jcb.137.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont AS, Zhai Y, Thilenius A. Lamin B distribution and association with peripheral chromatin revealed by optical sectioning and electron microscopic tomography. J Cell Biol. 1993;123:1671–1685. doi: 10.1083/jcb.123.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavente R, Dabauvalle MC, Scheer U, Chaly N. Functional role of newly formed pore complexes in postmitotic nuclear reorganization. Chromosoma. 1989;98:233–241. doi: 10.1007/BF00327308. [DOI] [PubMed] [Google Scholar]
- Benavente R, Krohne G. Change of karyoskeleton during spermatogenesis of Xenopus: expression of lamin LIV, a nuclear lamina protein specific for the male germ line. Proc Natl Acad Sci USA. 1985;82:6176–6180. doi: 10.1073/pnas.82.18.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collas P, Courvalin JC, Poccia D. Targeting of membranes to sea urchin sperm chromatin is mediated by a lamin B receptor-like integral membrane protein. J Cell Biol. 1996;135:1715–1725. doi: 10.1083/jcb.135.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechat T, Gotzmann J, Stockinger A, Harris CA, Talle MA, Siekierka JJ, Foisner R. Detergent-resistance of LAP2α in interphase nuclei and phosphorylation-dependent association with chromosomes early in nuclear assembly implies functions in nuclear structure dynamics. EMBO J. 1998;17:4887–4902. doi: 10.1093/emboj/17.16.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esponda P, Giménez-Martín G. The attachment of the synaptonemal complex to the nuclear envelope. Chromosoma. 1972;38:405–417. doi: 10.1007/BF00320159. [DOI] [PubMed] [Google Scholar]
- Fawcett DW, Chemes H. Changes in distribution of nuclear pores during differentiation of the male germ cells. Tissue Cell. 1979;11:147–162. doi: 10.1016/0040-8166(79)90015-6. [DOI] [PubMed] [Google Scholar]
- Foisner R, Gerace L. Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell. 1993;73:1267–1279. doi: 10.1016/0092-8674(93)90355-t. [DOI] [PubMed] [Google Scholar]
- Franke WW, Schmid E, Vanderkerckhove J, Weber K. A permanently proliferating rat vascular smooth cell with maintained expression of smooth muscle characteristics, including actin of the smooth muscle type. J Cell Biol. 1980;87:594–600. doi: 10.1083/jcb.87.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K, Hotta Y. cDNA cloning of a germ cell-specific lamin B3 from mouse spermatocytes and analysis of its ectopic expression in somatic cells. EMBO J. 1993;12:97–106. doi: 10.1002/j.1460-2075.1993.tb05635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K, Inagaki H, Hotta Y. Identification and cloning of an mRNA coding for a germ cell-specific A-type lamin in mice. Exp Cell Res. 1994;212:426–430. doi: 10.1006/excr.1994.1164. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Panté N, Aebi U, Gerace L. Cloning and cDNA for lamina-associated polypeptide 2 (LAP2) and identification of regions that specify targeting to nuclear envelope. EMBO J. 1995;14:1626–1636. doi: 10.1002/j.1460-2075.1995.tb07151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos SD, Meier J, Simos G. Lamins and lamin-associated proteins. Curr Opin Cell Biol. 1994;6:347–353. doi: 10.1016/0955-0674(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Gerace L, Burke B. Functional organization of the nuclear envelope. Annu Rev Cell Biol. 1988;4:335–374. doi: 10.1146/annurev.cb.04.110188.002003. [DOI] [PubMed] [Google Scholar]
- Gerace L, Comeau C, Benson M. Organization and modulation of nuclear lamina structure. J Cell Sci Suppl. 1984;1:137–160. doi: 10.1242/jcs.1984.supplement_1.10. [DOI] [PubMed] [Google Scholar]
- Gerace L, Foisner R. Integral membrane proteins and dynamic organization of the nuclear envelope. Trends Cell Biol. 1994;4:127–131. doi: 10.1016/0962-8924(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Graham RC, Karnovsky MJ. The early stage of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966;14:291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Harris CA, Andryuk PJ, Cline SC, Natarajan A, Siekierka JJ, Goldstein G. Three distinct human thymopoietins are derived from alternatively spliced mRNAs. Proc Natl Acad Sci USA. 1994;91:6283–6287. doi: 10.1073/pnas.91.14.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R, McKeon F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell. 1990;61:579–589. doi: 10.1016/0092-8674(90)90470-y. [DOI] [PubMed] [Google Scholar]
- Heyting C, Dietrich AJJ. Meiotic chromosome preparation and protein labeling. Methods Cell Biol. 1991;35:177–202. doi: 10.1016/s0091-679x(08)60573-7. [DOI] [PubMed] [Google Scholar]
- Hiraoka T. Observational and experimental studies of meiosis with special reference to the bouquet stage. XIV. Some considerations on a probable mechanism of the bouquet formation. Cytologia. 1952;17:292–299. [Google Scholar]
- Hock R, Wilde F, Scheer U, Bustin M. Dynamic relocation of chromosomal protein HMG-17 in the nucleus is dependent on transcriptional activity. EMBO J. 1998;17:6992–7001. doi: 10.1093/emboj/17.23.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höger T, Grund C, Franke WW, Krohne G. Immunolocalization of lamins in the thick nuclear lamina of human synovial cells. Eur J Cell Biol. 1991;54:150–156. [PubMed] [Google Scholar]
- Kralewski M, Benavente R. XY body formation during rat spermatogenesis: an immunocytochemical study using antibodies against XY body-associated proteins. Chromosoma. 1997;106:304–307. doi: 10.1007/s004120050251. [DOI] [PubMed] [Google Scholar]
- Krohne G. Lamin assembly in vivo. In: Herrmann H, Harris JR, editors. Subcellular Biochemistry: Intermediate Filaments. London: Plenum Publishing; 1998. pp. 563–586. [PubMed] [Google Scholar]
- Krohne G, Debus E, Osborn M, Weber K, Franke WW. A monoclonal antibody against nuclear lamina proteins reveals cell type-specificity in Xenopus laevis. Exp Cell Res. 1984;150:47–59. doi: 10.1016/0014-4827(84)90700-6. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lammers JHM, Offenberg HH, van Aalderen M, Vink ACG, Dietrich AJJ, Heyting C. The gene encoding a major component of the lateral elements of synaptonemal complexes of the rat is related to X-linked lymphocyte-regulated genes. Mol Cell Biol. 1994;14:1137–1146. doi: 10.1128/mcb.14.2.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C, Paulin-Levasseur M, Gajewski A, Alsheimer M, Benavente R, Krohne G. Molecular characterization and developmentally regulated expression of Xenopus lamina-associated polypeptide 2 (XLAP2) J Cell Sci. 1999;112:749–759. doi: 10.1242/jcs.112.5.749. [DOI] [PubMed] [Google Scholar]
- Loidl J. The initiation of meiotic chromosome pairing: the cytological view. Genome. 1990;33:759–778. doi: 10.1139/g90-115. [DOI] [PubMed] [Google Scholar]
- Martin L, Crimaudo C, Gerace L. cDNA cloning and characterization of lamina-associated polypeptide 1C (LAP1C), an integral protein of the inner nuclear membrane. J Biol Chem. 1995;270:8822–8828. doi: 10.1074/jbc.270.15.8822. [DOI] [PubMed] [Google Scholar]
- Matsudaira PT. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- Meistrich ML. Separation of spermatogenic cells and nuclei from rodent testes. Methods Cell Biol. 1977;15:15–54. doi: 10.1016/s0091-679x(08)60207-1. [DOI] [PubMed] [Google Scholar]
- Moens PB. Quantitative electron microscopy of chromosome organization at meiotic prophase. Cold Spring Harbor Symp Quant Biol. 1973;38:99–107. doi: 10.1101/sqb.1974.038.01.013. [DOI] [PubMed] [Google Scholar]
- Moens PB, Pearlman RE, Heng HHQ, Traut W. Chromosome cores and chromatin at meiotic prophase. Curr Top Dev Biol. 1998;37:241–262. doi: 10.1016/s0070-2153(08)60176-3. [DOI] [PubMed] [Google Scholar]
- Moses MJ. Synaptinemal complex. Annu Rev Genet. 1968;2:363–412. [Google Scholar]
- Moss SB, Donovan MJ, Bellvé AR. The occurrence and distribution of lamin proteins during mammalian spermatogenesis and early embryonic development. Ann NY Acad Sci. 1987;513:74–89. doi: 10.1111/j.1749-6632.1987.tb24999.x. [DOI] [PubMed] [Google Scholar]
- O’Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Parvinen M, Söderström KO. Chromosome rotation and formation of synapsis. Nature. 1976;260:534–535. doi: 10.1038/260534a0. [DOI] [PubMed] [Google Scholar]
- Paulin-Levasseur M, Blake DL, Julien M, Rouleau L. The MAN antigens are nonlamin constituents of the nuclear lamina in vertebrate cells. Chromosoma. 1996;104:367–379. doi: 10.1007/BF00337226. [DOI] [PubMed] [Google Scholar]
- Rattner JB, Goldsmith M, Hamkalo BA. Chromatin organization during meiotic prophase of Bombyx mori. Chromosoma. 1980;79:215–224. doi: 10.1007/BF01175187. [DOI] [PubMed] [Google Scholar]
- Scherthan H, Weich S, Schwegler H, Heyting C, Härle M, Cremer T. Centromere and telomere movements during early meiotic prophase of mouse and man are associated with the onset of chromosome pairing. J Cell Biol. 1996;134:1109–1125. doi: 10.1083/jcb.134.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A, Gerace L. Integral membrane proteins specific to the inner nuclear membrane and associated with the nuclear lamina. J Cell Biol. 1988;107:2029–2036. doi: 10.1083/jcb.107.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Benavente R. Identification of a short lamin protein selectively expressed during meiotic stages of rat spermatogenesis. Differentiation. 1992;52:55–60. doi: 10.1111/j.1432-0436.1992.tb00499.x. [DOI] [PubMed] [Google Scholar]
- Solari AJ. The behavior of chromosomal axes during diplotene in mouse spermatocytes. Chromosoma. 1970;31:217–230. doi: 10.1007/BF00285149. [DOI] [PubMed] [Google Scholar]
- Solari AJ. The behavior of the XY pair in mammals. Int Rev Cytol. 1974;38:273–317. doi: 10.1016/s0074-7696(08)60928-6. [DOI] [PubMed] [Google Scholar]
- Solari AJ. Sex Chromosomes and Sex Determination in Vertebrates. Boca Raton, FL: CRC Press; 1994. [Google Scholar]
- Stick R, Schwarz H. The disappearance of the nuclear lamina during spermatogenesis: an electron microscopic and immunofluorescence study. Cell Differ. 1982;11:235–243. doi: 10.1016/0045-6039(82)90071-9. [DOI] [PubMed] [Google Scholar]
- Stuurman N, Hein S, Aebi U. The nuclear lamins: their structure, assembly, and interactions. J Struct Biol. 1998;122:42–66. doi: 10.1006/jsbi.1998.3987. [DOI] [PubMed] [Google Scholar]
- Vázquez-Nin GH, Flores E, Echeverría OM, Merkert H, Wettstein R, Benavente R. Immunocytochemical localization of DNA in synaptonemal complexes of rat and mouse spermatocytes, and of chick oocytes. Chromosoma. 1993;102:457–463. doi: 10.1007/BF00357100. [DOI] [PubMed] [Google Scholar]
- Vester B, Smith A, Krohne G, Benavente R. Presence of a nuclear lamina in pachytene spermatocytes of the rat. J Cell Sci. 1993;104:557–563. doi: 10.1242/jcs.104.2.557. [DOI] [PubMed] [Google Scholar]
- Wettstein R, Sotelo JR. The molecular architecture of synaptonemal complexes. Adv Cell Mol Biol. 1971;1:109–152. [Google Scholar]
- Wilson EB. The Cell in Development and Heredity. New York: Macmillan; 1925. [Google Scholar]
- Wiltshire T, Park C, Caldwell KA, Handel MA. Induced premature G2/M-phase transition in pachytene spermatocytes includes events unique to meiosis. Dev Biol. 1995;169:557–567. doi: 10.1006/dbio.1995.1169. [DOI] [PubMed] [Google Scholar]
- Worman HJ, Evans CD, Blobel G. The lamin B receptor of the nuclear envelope inner membrane: a polytopic protein with eight potential transmembrane domains. J Cell Biol. 1990;117:245–258. doi: 10.1083/jcb.111.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Barton RM, Worman HJ. Nuclear lamin binding proteins. In: Herrmann H, Harris JR, editors. Subcellular Biochemistry: Intermediate Filaments. London: Plenum Publishing; 1998. pp. 586–610. [Google Scholar]
- Yuan L, Pelttari J, Brundell E, Björkroth B, Zhao J, Liu JG, Brismar H, Daneholt B, Höög C. The synaptonemal complex protein SCP3 can form multistranded, cross-striated fibers in vivo. J Cell Biol. 1998;142:331–339. doi: 10.1083/jcb.142.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]