Abstract
Thalamocortical communication is a dynamic process influenced by both presynaptic and postsynaptic mechanisms. In this study, we recorded single-unit responses from cortical neurons that received direct input from the lateral geniculate nucleus (LGN) to address the question of whether prior patterns of cortical activity affect the ability of LGN inputs to drive cortical responses. By examining the ongoing activity that preceded the arrival of electrically evoked spikes from the LGN, we identified a number of activity patterns that were predictive of suprathreshold communication. Namely, cortical neurons were more likely to respond to LGN stimulation when their activity levels increased to 30-40Hz and/or their activity displayed rhythmic patterns (30 ms intervals) with increased power in the gamma frequency band. Cortical neurons were also more likely to respond to LGN stimulation when their activity increased 30-40 ms prior to stimulation, suggesting that the phase of gamma activity also contributes to geniculocortical communication. Based on these results, we conclude that ongoing activity in the cortex is not random, but rather organized in a manner that can influence the dynamics of thalamocortical communication.
Keywords: V1, LGN, lateral geniculate nucleus, spike rate, coding
Introduction
Multiple external and internal factors contribute to the dynamics of spike production in the cerebral cortex. Between the lateral geniculate nucleus (LGN) and visual cortex, there is a significant filtering of spikes as LGN neurons typically produce many more spikes than their postsynaptic targets (Alonso et al., 2001; Usrey et al., 2000). Although previous work indicates that much of this filtering can be accounted for on the basis of preceding patterns of presynaptic activity (Levine and Cleland, 2001; Mastronarde, 1987; Rowe and Fischer, 2001; Sincich et al., 2007; Usrey et al., 1998; Usrey et al., 2000; Weyand, 2007), it is also likely that postsynaptic activity patterns play a role. For instance, correlated cortical network activity with increased power in the gamma frequency band and/or the dynamics of ongoing activity in the cortex could influence the efficacy of thalamocortical communication (Anderson et al., 2000; Arieli et al., 1996; Fiser et al., 2004; Friedman-Hill et al., 2000; Fries et al., 2001a; Fries et al., 2001b; Ramcharitar et al., 2006; Taylor et al., 2005; Tsodyks et al., 1999; Womelsdorf et al., 2006; Womelsdorf et al., 2007). Along related lines, cortical responses to thalamic input may depend on the depolarized/hyperpolarized or Up/Down state of the cortical neuron's membrane potential (Bruno and Sakmann, 2006; Haider et al., 2006; Haider et al., 2007; Hasenstaub et al., 2005; McCormick et al., 2003; Rigas and Castro-Alamancos, 2007; Rudolph et al., 2007; Sachdev et al., 2004; Shu et al., 2003a; Shu et al., 2003b).
In this study, we examine the influence of prior cortical activity on the transfer of spikes from the LGN to visual cortex in macaque monkeys. To do so, we electrically stimulate the LGN with brief shocks while recording neuronal responses from cortical neurons that receive monosynaptic LGN input. We then compare cortical activity patterns that precede shocks that successfully evoke cortical responses to those that fail to evoke responses. Our results show that electrically evoked spikes in the LGN are more likely to drive suprathreshold responses when the activity of cortical neurons rises to 30–40 Hz. Cortical neurons are also more responsive to LGN input when they are experiencing rhythmic patterns of activity with increased power in the gamma frequency band. These results demonstrate that prior patterns of cortical activity can have a deterministic influence on the transfer of spikes between the LGN and visual cortex.
Materials and Methods
Seven adult male macaque monkeys (Macaca mulatta) were used in this study. All surgical and experimental procedures conformed to NIH guidelines and were approved by the UC Davis Institutional Animal Care and Use Committee.
Surgical preparation
Animals were initially anesthetized with ketamine (10 mg/kg, IM) and maintained with sufentanil citrate (3–6 μg/kg/hour, IV) and isoflurane (0.4%) in nitrous oxide and oxygen (2:1). Animals were intubated, placed in a stereotaxic apparatus and wrapped in a thermostatically controlled heating blanket. Throughout the experiment, temperature, expired CO2, electrocardiogram (ECG), electroencephalogram (EEG), heart rate, and SPO2 were monitored continuously. Proper anesthetic depth was assessed by monitoring the EEG for changes in slow-wave/spindle activity, the ECG, and the expired CO2. If changes in any of these measures indicated a decreased level of anesthesia, additional sufentanil was given and the rate of infusion increased. A midline scalp incision was made, wound edges were infused with lidocaine, and craniotomies were made over the LGN and V1. Once the dura was reflected, craniotomies were filled with 2% agar in saline. The eyes were dilated with 1% atropine sulfate, fitted with contact lenses and focused on a tangent screen located 172 cm in front of the animal. Following the completion of all surgical procedures, animals were paralyzed with vencuronium bromide (0.2 mg/kg/hour, IV). Animals were euthanized at the end of each experiment with an overdose of sodium pentobarbital (80 mg/kg).
Data acquisition, electrical stimulation, and neuronal identification
Single-unit recordings were made from V1 neurons using tungsten-in-glass microelectrodes (Alan Ainsworth, London, UK). Neuronal responses were amplified, filtered, and recorded to a PC equipped with a Power 1401 data acquisition system and the Spike2 software package (Cambridge Electronic Design, Cambridge, England). Spike isolation was based on waveform analysis (on-line and off-line) and presence of a refractory period, as indicated by the autocorrelogram.
Neurons in the LGN were electrically stimulated via two platinum/iridium microelectrodes (Frederick Haer and Co., Bowdoinham, ME) implanted in regions of the LGN that were in retinotopic register with recording sites in V1. The exposed tips of the stimulating electrodes (500 μm) spanned multiple LGN layers. Stimulating electrodes were connected to an AM systems isolated pulse stimulator (Carlsborg, WA) that delivered brief, biphasic pulses (0.2 ms, ∼10 V). Electrical stimulation was delivered in one of two modes. In the first mode (non-collision trials), a shock was delivered every 5 seconds. In the second mode (collision trials), shocks were triggered to occur within 1 ms of a spontaneous spike from the cortical neuron. These two types of trials were used to identify and distinguish geniculocortical recipient neurons from corticogeniculate neurons in V1 (Briggs and Usrey, 2005; Briggs and Usrey, 2007). Following the identification of a geniculocortical recipient neuron, additional data were collected while electrical stimulation was delivered every 5 seconds (non-collision mode). These data were used to examine the influence of ongoing cortical activity on the efficacy of geniculocortical spike transfer.
Data analysis
To examine the influence of cortical activity on geniculocortical spike transfer, we first sorted stimulation trials according to whether or not the electrical shock in the LGN successfully evoked a cortical response (termed “successful” and “unsuccessful” trials). We then performed three sets of analyses. In the first analysis, we compared the occurrence of cortical spikes at different times before and after electrical stimulation over a range extending from 300 ms prior to stimulation to 500 ms after stimulation. For each neuron and trial type, spikes counts were binned in 10 ms intervals and normalized to the mean level of spontaneous activity. Spontaneous activity was measured over a 1-second window beginning 2 seconds after stimulation (also separated into corresponding successful and unsuccessful trials). Normalized activity profiles were then averaged across neurons and individual bins that exceeded ±2 standard deviations of mean spontaneous activity were identified. A t-test was then performed (adjusted using the Bonferroni method to correct for Type 1 errors) to compare the activity of successful and unsuccessful trials in these identified bins.
In the second analysis, we compared the preceding spike rates of cortical neurons for trials where electrical stimulation successfully and unsuccessfully evoked cortical responses. For both types of trials, preceding spike rate was calculated over a 130 ms period immediately preceding LGN stimulation. Results from the first analysis identified this window as a time period of interest. For each neuron, the proportion of successful versus unsuccessful trials was determined (using 10 Hz bins) and these proportions were averaged across the sample of neurons. In addition, the total distribution of spike rates preceding all trials, both successful and unsuccessful, was determined by calculating the relative proportion of spike rates in 10 Hz bins.
In the final analysis, we compared preceding activity patterns for successful and unsuccessful trials by examining cumulative autocorrelograms (1 ms bin-width; Friedman-Hill et al., 2000) made from spikes occurring during the 1-second period immediately preceding LGN stimulation. Cumulative autocorrelograms for the two trial types were generated by first calculating mean autocorrelograms for each neuron and then summing these autocorrelograms across neurons. A difference autocorrelogram was calculated by subtracting the cumulative autocorrelogram made from unsuccessful trials from the cumulative autocorrelogram made from successful trials. Fast-Fourier transform and power spectrum analyses were also performed on the two cumulative autocorrelograms and integrals of the power spectra in the gamma frequency range (20–80 Hz) were calculated. The ratio of power in the gamma range was then determined for successful and unsuccessful trials. The same analysis was performed using spontaneous activity measured during a 1-second period beginning 2 seconds after stimulation for all of the successful and unsuccessful trials.
Results
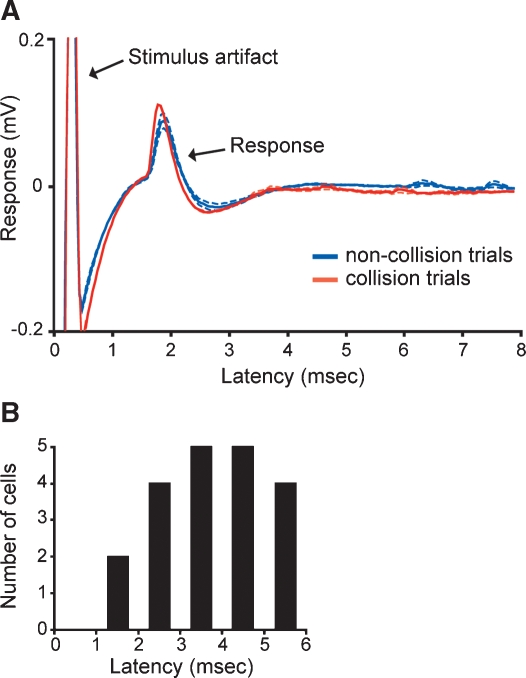
We recorded single-unit activity from V1 neurons in the macaque monkey that received monosynaptic input from the LGN to determine whether prior patterns of cortical activity affect the efficacy of geniculocortical spike transfer. Cortical neurons with monosynaptic LGN input were identified by their responses to electrical stimulation in the LGN. This was accomplished by first identifying neurons that followed a brief (0.2 ms), electrical shock with a short-latency response with little temporal jitter (Figure 1A). A collision test was then performed to determine whether the recorded neuron received feedforward input from the LGN and/or provided feedback input to the LGN. In a collision test, the electrical shock is triggered by a spontaneous spike from the recorded neuron. If the recorded neuron receives feedforward input from the LGN, then the spike resulting from the shock will propagate to the cortex and drive a postsynaptic spike (Figure 1A). If, however, the recorded neuron is a feedback neuron that projects to the LGN, then the antidromic spike resulting from the shock will collide with the spontaneous spike and the antidromic spike will not reach the cortex (Briggs and Usrey, 2005; Briggs and Usrey, 2007). Using these criteria, we identified 20 V1 neurons that received monosynaptic input from the LGN. Receptive fields of these neurons were located within the central 20 degrees. Based on the relative location of recording sites, these neurons were believed to be located in layers 4C and 6. Across the sample, the average latency for electrically evoked spikes to propagate from the LGN to the cortex was 3.8 ± 0.3 ms (Figure 1B). Similar conduction latencies have been reported previously (Briggs and Usrey, 2007; Bullier and Henry, 1980).
Figure 1.
Identifying cortical neurons that receive LGN input and measuring conduction latency. (A) Responses of a representative cortical neuron to electrical stimulation in the LGN. Solid blue and red lines represent average responses to non-collision and collision trials, respectively (average of 12 trials each; aligned to the stimulus artifact at time zero). Dashed lines represent standard errors. The response latency for this neuron was 1.6 ms. (B) Distribution of conduction latencies across the sample of identified cortical neurons with direct LGN input (n = 20).
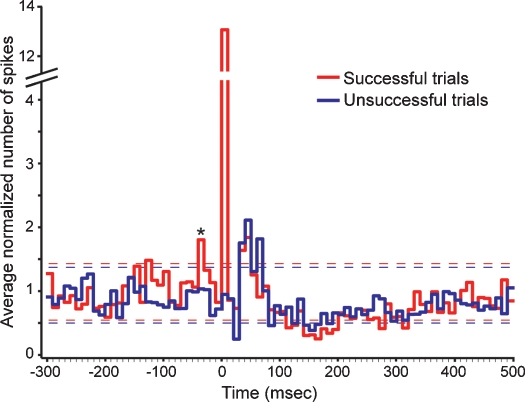
Once we identified a cortical neuron that received direct LGN input, we delivered a shock to the LGN every 5 seconds and examined whether the ability of the shock to evoke a postsynaptic spike was influenced by prior activity from the cortical neuron. For each neuron, shocks were sorted according to whether or not they were successful in evoking a postsynaptic spike. Across our sample of neurons, 51% of all shocks were successful in evoking postsynaptic spikes. More importantly, the pattern of cortical activity that preceded successful trials differed significantly from that which preceded unsuccessful trials (Figure 2). In particular, there were two time intervals prior to the shock (−120 to −130 ms and −30 to −40 ms) where activity preceding successful trials exceeded two times the standard deviation of spontaneous activity (assessed 2 seconds after each shock). For the −30 to −40 ms interval, activity preceding successful trials was significantly greater than activity preceding unsuccessful trials (p < 0.02, t-test).
Figure 2.
Cortical activity before and after electrical stimulation in the LGN. The two traces show the average occurrence of cortical spikes (normalized to spontaneous levels) before and after electrically stimulating the LGN. Red traces correspond to activity profiles before and after shocks that successfully evoked a monosynaptic response; blue traces correspond to activity profiles for shocks that failed to evoke a monosynaptic response. Dashed lines indicate two standard deviations above and below the mean spontaneous levels for the two trial types. Asterisk indicates the bin where cortical activity differed significantly between successful and unsuccessful trials (p < 0.02, t-tests). Shock timing is aligned to time = 0. Bin width is equal to 10 ms.
Cortical activity following electrical stimulation also displayed several noteworthy patterns (Figure 2). As expected, during the first 10 ms following a shock, successful shocks evoked significantly greater activity from recorded neurons than unsuccessful shocks (p ≪ 0.0001; t-test). Following this time, cortical activity dipped briefly at ∼30 ms for both successful and unsuccessful trials before showing a pronounced elevation between ∼40 and 70 ms. Activity levels for both trial types then decreased for a prolonged period from ∼100–350 ms, which included a period from 150–200 ms where activity levels decreased below spontaneous levels, before returning to baseline levels.
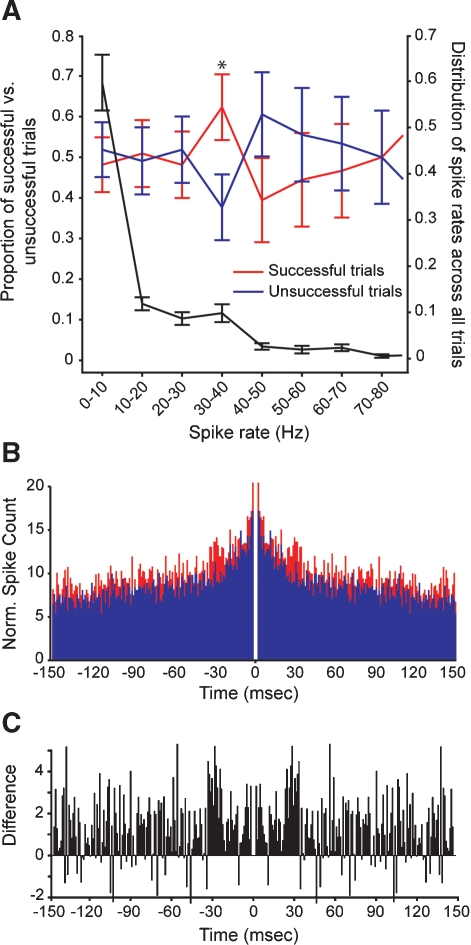
Having found that prior cortical activity can influence the transfer of electrically evoked spikes at geniculocortical synapses, we next asked whether other features of prior activity influence the efficacy of geniculocortical spike transfer. In particular, we wished to determine whether specific spike rates and/or spike correlation patterns preceded successful trials. To study the possible influence of prior spike rate, we calculated spike rates within a 130 ms window immediately preceding successful and unsuccessful shocks. Preceding spike rates between 30 and 40 Hz occurred significantly more often in successful trials compared to unsuccessful trials (Figure 3A, red and blue traces, left axis; p < 0.03; t-test). An examination of the distribution of preceding spike rates shows that 30–40 Hz rates occurred in ∼10% of all trials; rates below 10 Hz occurred most frequently (Figure 3A, black trace, right axis). These results indicate that preceding spike rate, in addition to spike timing, can influence the transfer of spikes at geniculocortical synapses.
Figure 3.
Comparison of spike rates and spike correlations that precede successful and unsuccessful stimulation trials. (A) Relative occurrence of different spike rates that preceded electrical stimulation in the LGN. Red and blue traces (left axis) show the average proportion of spike rates that preceded shocks successful at evoking cortical responses and unsuccessful at evoking responses, respectively. Error bars indicate the SEM; asterisk indicates a significant difference in the proportion of successful versus unsuccessful trials (p < 0.03, t-test). The black trace (right axis) shows the distribution of all preceding spike rates for all trials (successful and unsuccessful). Error bars indicate the SEM. (B) Cumulative autocorrelograms made from cortical spike trains in trials where electrical stimulation was successful in evoking a cortical response (shown in red) and unsuccessful in evoking a response (shown in blue). (C) A difference autocorrelogram made from the two cumulative autocorrelograms shown in B.
To determine whether preceding spike-correlation patterns differed for successful and unsuccessful trials, we generated cumulative autocorrelograms for spikes occurring within a 1-second window prior to electrical stimulation. Across our sample of cortical neurons, autocorrelograms from successful trials contained peaks at ∼30 ms that were not evident in autocorrelograms from unsuccessful trials (Figures 3B and 3C). As a final analysis, we calculated the integral of the power spectrum within the gamma frequency range (20–80 Hz) for each autocorrelogram. Compared to autocorrelograms made from unsuccessful trials, there was 40% more power in the gamma frequency range for autocorrelograms made from successful trials. The analogous comparison made from spontaneous activity measured 2 seconds after successful and unsuccessful trials yielded a difference of less than 4%. Because cortical neurons are more likely to respond to LGN stimulation when their activity increases 30–40 ms before stimulation (Figure 2), it seems likely that the phase of gamma activity is also important for geniculocortical communication.
Discussion
We combined single-unit recordings from neurons in the primary visual cortex with electrical stimulation in the LGN to address the question of whether ongoing patterns of cortical activity affect the ability of LGN inputs to drive cortical responses. Our results show that high frequency and/or rhythmic activity can increase the efficacy of geniculocortical communication. In particular, cortical neurons are more likely to respond to LGN stimulation when their activity levels increase to 30–40 Hz and/or their activity displays rhythmic patterns (30 ms intervals) with increased power in the gamma frequency band. Our results also show that cortical neurons are more likely to respond to LGN stimulation when their activity increases 30–40 ms prior to stimulation, suggesting that the phase of gamma activity contributes to geniculocortical communication. Here, we consider the potential mechanisms that underlie these results as well as their functional implications.
Geniculocortical communication and cortical gamma band activity
Recent work demonstrates that ensembles of cortical neurons often display periodic episodes of oscillatory activity in the gamma frequency band (20–80 Hz). This type of activity has been observed across visual cortical areas and is frequently associated with neuronal and behavioral responses to visual stimuli (Friedman-Hill et al., 2000; Fries et al., 2001a; Fries et al., 2001b; Fries et al., 2007; Jensen et al., 2007; Taylor et al., 2005; Womelsdorf et al., 2006; Womelsdorf et al., 2007). This role for gamma-band oscillations is not restricted to cortex or mammals, as gamma-band oscillations have been found to directly enhance the direction selectivity of movement responsive cells in electric fish (Ramcharitar et al., 2006). While increasing evidence indicates that coordinated cortical activity can have an important influence on neuronal and behavioral responses to stimuli, the cellular mechanisms underlying these influences have yet to be elucidated. Here, we show that oscillatory activity in the gamma frequency band is associated with an increase in the efficacy of geniculocortical communication. Furthermore, our results indicate that geniculocortical transmission is enhanced during a specific epoch or phase of the gamma cycle, as predicted from recent modeling efforts and consistent with intracortical communication (Fries et al., 2007; Womelsdorf et al., 2007).
Geniculocortical communication and cortical Up/Down states
In addition to gamma frequency band activity, ensembles of cortical neurons can also fluctuate together between Up (depolarized) and Down (hyperpolarized) states (reviewed in McCormick, 2005; Steriade, 2001). Given our finding that electrically evoked spikes in the LGN are more likely to drive cortical responses when spontaneous activity levels are high (30–40 Hz), it is tempting to speculate that these periods of increased activity occurred during neuronal Up states. For instance, recordings from brain slices show that neurons in prefrontal cortex and visual cortex are more sensitive to afferent input during Up states (McCormick et al., 2003; Shu et al., 2003b). In addition, the visual responses of cortical neurons are enhanced during Up states (Haider et al., 2007). These effects may not be ubiquitous across cortical areas, however, as results from somatosensory cortex indicate that sensory stimuli or thalamic stimulation evoke larger PSPs and have an increased likelihood of driving suprathreshold responses during cortical Down states (Bruno and Sakmann, 2006; Sachdev et al., 2004, but see Hasenstaub et al., 2007). Although previous studies indicate that Up/Down states are not an artifact of anesthesia (Destexhe et al., 2007; Steriade, 2001), we cannot rule out a possible influence of anesthesia on our results as anesthesia can certainly influence the temporal properties of spike trains.
Studies of conductance changes and subthreshold activity of cortical neurons during Up/Down states reveal their dependence upon local excitatory and inhibitory inputs (Haider et al., 2006; Hasenstaub et al., 2005; Rudolph et al., 2007; Shu et al., 2003a). Thalamocortical circuits are also likely to play a significant role in regulating Up/Down state behavior as thalamic activity has been shown to activate cortical Up states (Hirata and Castro-Alamancos, 2005; MacLean et al., 2005; Rigas and Castro-Alamancos, 2007; Shu et al., 2003a). Accordingly, we found a consistent increase in the spiking responses of cortical neurons for approximately 70 ms following brief (0.2 ms) electrical stimulation in the LGN. As activity in thalamocortical circuits ultimately affects activity in corticothalamic circuits and vice versa (Blumenfeld and McCormick, 2000; Hirata and Castro-Alamancos, 2005; Wolfart et al., 2005), it seems likely that there exists a dynamic interplay between the thalamus and cortex in the generation of Up and Down states. Similarly, the increase in cortical activity following electrical stimulation may reflect persistent activity within cortical networks with reciprocal connections (Arieli et al., 1996; Tsodyks et al., 1999).
In closing, patterns of presynaptic activity in the LGN have been shown to influence the efficacy of geniculocortical communication (Usrey, 2002a; Usrey, 2002b). Here we demonstrate geniculocortical efficacy is also influenced by postsynaptic activity in the cortex. Taken together, these results suggest that geniculocortical communication is not simply a bottom-up process, but a process that reflects the dynamics of activity at thalamic and cortical levels of visual processing.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Kelly Henning and Dan Sperka for technical assistance. This work was supported by the NIH, NSF, the McKnight Foundation, and the Esther A. and Joseph Klingenstein Fund.
References
- Alonso J.-M., Usrey W. M., Reid R. C. (2001). Rules of connectivity between geniculate cells and simple cells in cat primary visual cortex. J. Neurosci. 21, 4002–4015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J., Lampl I., Reichova I., Carandini M., Ferster D. (2000). Stimulus dependence of two-state fluctuations of membrane potential in cat visual cortex. Nat. Neurosci. 3, 617–621 [DOI] [PubMed] [Google Scholar]
- Arieli A., Sterkin A., Grinvald A., Aertsen A. (1996). Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 273, 1868–1871 [DOI] [PubMed] [Google Scholar]
- Blumenfeld H., McCormick D. A. (2000). Corticothalamic inputs control the pattern of activity generated in thalamocortical networks. J. Neurosci. 20, 5153–5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F., Usrey W. M. (2005). Temporal properties of feedforward and feedback pathways between thalamus and visual cortex in the ferret. Thalamus Relat. Syst. 3, 133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F., Usrey W. M. (2007). A fast, reciprocal pathway between the lateral geniculate nucleus and visual cortex in the macaque monkey. J. Neurosci. 27, 5431–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno R. M., Sakmann B. (2006). Cortex is driven by weak but synchronously active thalamocortical synapses. Science 312, 1622–1627 [DOI] [PubMed] [Google Scholar]
- Bullier J., Henry G. H. (1980). Ordinal position and afferent input of neurons in monkey striate cortex. J. Comp. Neurology 193, 913–935 [DOI] [PubMed] [Google Scholar]
- Destexhe A., Hughes S. W., Rudolph M., Crunelli V. (2007). Are corticothalamic “up” states fragments of wakefulness? Trends Neurosci. 30, 334–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiser J., Chiu C., Weliky M. (2004). Small modulation of ongoing cortical dynamics by sensory input during natural vision. Nature 431, 573–578 [DOI] [PubMed] [Google Scholar]
- Friedman-Hill S., Maldonado P. E., Gray C. M. (2000). Dynamics of striate cortical activity in the alert macaque: I. Incidence and stimulus-dependence of gamma-band neuronal oscillations. Cereb. Cortex 10, 1105–1116 [DOI] [PubMed] [Google Scholar]
- Fries P., Neuenschwander S., Engel A. K., Goebel R., Singer W. (2001a). Rapid feature selective neuronal synchronization through correlated latency shifting. Nat. Neurosci. 4, 194–200 [DOI] [PubMed] [Google Scholar]
- Fries P., Reynolds J. H., Rorie A. E., Desimone R. (2001b). Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291, 1560–1563 [DOI] [PubMed] [Google Scholar]
- Fries P., Nikolić D., Singer W. (2007). The gamma cycle. Trends Neurosci. 30, 310–316 [DOI] [PubMed] [Google Scholar]
- Haider B., Duque A., Hasenstaub A. R., McCormick D. A. (2006). Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J. Neurosci. 26, 4535–4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider B., Duque A., Hasenstaub A. R., Yu Y., McCormick D. A. (2007). Enhancement of visual responsiveness by spontaneous local network activity in vivo. J. Neurophysiol. 97, 4186–4202 [DOI] [PubMed] [Google Scholar]
- Hasenstaub A. R., Shu Y., Haider B., Kraushaar U., Duque A., McCormick D. A. (2005). Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron 47, 423–435 [DOI] [PubMed] [Google Scholar]
- Hasenstaub A. R., Sachdev R. N. S., McCormick D. A. (2007). State changes rapidly modulate cortical neuroanl responsiveness. J Neurosci. 27, 9607–9622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A., Castro-Alamancos M. A. (2005). Enhancement of synaptic depression produces long-term enhancement in thalamocortical networks. J. Neurophysiol. 95, 2479–2491 [DOI] [PubMed] [Google Scholar]
- Jensen O., Kaiser J., Lachaux J.-P. (2007). Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 30, 317–324 [DOI] [PubMed] [Google Scholar]
- Levine M. W., Cleland B. G. (2001). An analysis of the effect of retinal ganglion cell impulses upon the firing probability of neurons in the dorsal lateral geniculate nucleus of the cat. Brain Res. 902, 244–254 [DOI] [PubMed] [Google Scholar]
- MacLean J. N., Watson B. O., Aaron G. B., Yuste R. (2005). Internal dynamics determine the cortical response to thalamic stimulation. Neuron 48, 811–823 [DOI] [PubMed] [Google Scholar]
- Mastronarde D. N. (1987). Two classes of single-input X-cells in cat lateral geniculate nucleus. II. Retinal inputs and the generation of receptive-field properties. J. Neurophysiol. 57, 381–413 [DOI] [PubMed] [Google Scholar]
- McCormick D. A. (2005). Neuronal networks: flip-flops in the brain. Curr. Biol. 15, R294–R296 [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Shu Y., Hasenstaub A. R., Sanchez-Vives M., Badoual M., Bal T. (2003). Persistent cortical activity: mechanisms of generation and effects on neuronal excitability. Cereb. Cortex 11, 1219–1231 [DOI] [PubMed] [Google Scholar]
- Ramcharitar J. U., Tan E. W., Fortune E. S. (2006). Global electrosensory oscillations enhance directional responses of midbrain neurons in eigenmannia. J. Neurophysiol. 96, 2319–2326 [DOI] [PubMed] [Google Scholar]
- Rigas P., Castro-Alamancos M. A. (2007). Thalamocortical Up states: differential effects of intrinsic and extrinsic cortical inputs on persistent activity. J. Neurosci. 27, 4261–4272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M. H., Fischer Q. (2001). Dynamic properties of retino-geniculate synapses in the cat. Vis. Neurosci. 18, 219–231 [DOI] [PubMed] [Google Scholar]
- Rudolph M., Pospischil M., Timofeev I., Destexhe A. (2007). Inhibition determines membrane potential dynamics and controls action potential generation in awake and sleeping cat cortex. J. Neurosci. 27, 5280–5290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev R. N. S., Ebner F. F., Wilson C. J. (2004). Effect of subthreshold Up and Down states on the whisker-evoked response in somatosensory cortex. J. Neurophysiol. 92, 3511–3521 [DOI] [PubMed] [Google Scholar]
- Shu Y., Hasenstaub A. R., McCormick D. A. (2003a). Turning on and off recurrent balanced cortical activity. Nature 423, 288–293 [DOI] [PubMed] [Google Scholar]
- Shu Y., Hasenstaub A. R., Badoual M., Bal T., McCormick D. A. (2003b). Barrages of synaptic activity control the gain and sensitivity of cortical neurons. J. Neurosci. 23, 10388–10401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sincich L. C., Adams D. L., Economides J. R., Horton J. C. (2007). Transmission of spike trains at the retinogeniculate synapse. J. Neurosci. 27, 2683–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. (2001). Impact of network activities on neuronal properties in corticothalamic systems. J. Neurophysiol. 86, 1–39 [DOI] [PubMed] [Google Scholar]
- Taylor K., Mandon S., Freiwald W. A., Kreiter A. K. (2005). Coherent oscillatory activity in monkey area V4 predicts successful allocation of attention. Cereb. Cortex 15, 1424–1437 [DOI] [PubMed] [Google Scholar]
- Tsodyks M., Kenet T., Grinvald A., Arieli A. (1999). Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science 286, 1943–1946 [DOI] [PubMed] [Google Scholar]
- Usrey W. M. (2002a). Spike timing and visual processing in the retinogeniculocortical pathway. Philos. Trans. R. Soc.: Biol. Sci. 357, 1729–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey W. M. (2002b). The role of spike timing for thalamocortical processing. Curr. Opin. Neurobiol. 12, 411–417 [DOI] [PubMed] [Google Scholar]
- Usrey W. M., Reppas J., Reid R. C. (1998). Paired-spike interactions and synaptic efficacy of retinal inputs to the thalamus. Nature 395, 384–387 [DOI] [PubMed] [Google Scholar]
- Usrey W. M., Alonso J.-M., Reid R. C. (2000). Synaptic interactions between thalamic inputs to simple cells in cat visual cortex. J. Neurosci. 20, 5461–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand T. G. (2007). Retinogeniculate transmission in wakefulness. J. Neurophysiol. 98, 769–785 [DOI] [PubMed] [Google Scholar]
- Wolfart J., Debay D., Le Masson G., Destexhe A., Bal T. (2005). Synaptic background activity controls spike transfer from thalamus to cortex. Nat. Neurosci. 8, 1760–1767 [DOI] [PubMed] [Google Scholar]
- Womelsdorf T., Fries P., Mitra P. P., Desimone R. (2006). Gamma-band synchronization in visual cortex predicts speed of change detection. Nature 439, 733–736 [DOI] [PubMed] [Google Scholar]
- Womelsdorf T., Schoffelen J.-M., Oostenveld R., Singer W., Desimone R., Engel A. K., Fries P. (2007). Modulation of neuronal interactions through neuronal synchronization. Science 316, 1609–1612 [DOI] [PubMed] [Google Scholar]