Abstract
Decorin is a member of the family of small leucine-rich proteoglycans that are present in blood vessels and synthesized by vascular smooth muscle cells (VSMCs). Decorin plays complex roles in both normal vascular physiology and the pathogenesis of various types of vascular disorders. However, the mechanisms of regulation of decorin expression in vasculature are not clearly understood. Particularly little information is available about a role of nuclear receptors in the regulation of decorin expression. In the present study, we report that activation of vascular FXR by a specific ligand resulted in upregulation of decorin at the levels of both mRNA and protein. FXR appears to induce decorin expression at a transcriptional level because (1) upregulation of decorin mRNA expression was abolished by the treatment of a transcription inhibitor, actinomycin D; and (2) decorin promoter activity was significantly increased by activation of FXR. Functional analysis of human decorin promoter identified an imperfect inverted repeat DNA motif, IR8 (−2313TGGTCAtagtgtcaTGACCT−2294), as a likely FXR-responsive element that is involved in decorin regulation.
Keywords: FXR, decorin, smooth muscle cells, vascular, regulation
Introduction
Extracellular matrix (ECM) provides a number of structural and functional characteristics to tissues including cell support, mechanical integrity and molecular signaling. In cardiovascular tissues, cells produce various ECM components such as collagen, elastin, proteoglycans (PGs), matrix metalloproteinases, growth factors and signaling molecules [1]. Collagen is the most abundant protein in cardiovascular tissues; it is secreted by cells to provide tensile strength and serve as an organizational scaffold. Elastin is a major fibrillar ECM protein that provides elastic recoil and is an essential component of blood vessels. PGs consist of one or more glycosaminoglycan (GAG) chains attached to a core protein. The principal ECM chondroitin/dermatan sulfate PGs include members of two gene families--the large aggregating chondroitin sulfate proteoglycans (lecticans) and the small leucine-rich proteoglycans (SLRPs) [1]. SLRPs can be divided into class I consisting of decorin and biglycan and class II consisting of lumican and fibromodulin [2].
PGs are involved in a number of important physiological processes such as cell adhesion, proliferation, migration, and angiogenesis, and therefore play a critical role in tissue development and maintenance [3]. In addition, PGs contribute to the development of a number of vascular diseases such as atherosclerosis [4]. Vascular smooth muscle cells (VSMCs) are a major source of PGs and their expression and synthesis are regulated by a number of factors such as mechanical stress, growth factors, cytokines etc [5]. However, little is known about a role of nuclear receptors in modulation of PGs expression in VSMCs.
The farnesoid X receptor (FXR) (NR1H4) is a member of the nuclear receptor superfamily that is highly expressed in liver, kidney, adrenals, and intestine [6]. FXR is activated by bile acids (BAs), such as the primary BA chenodeoxycholic acid (CDCA) [7]. In addition to BAs, synthetic agonists that are highly specific for FXR have also been developed [8]. FXR plays an important role in the maintenance of cholesterol and BA homeostasis via regulating the expression and function of genes involved in BA synthesis, uptake and excretion. FXR ligands have recently been proposed as novel therapeutics in cardiovascular diseases based on their effectiveness in lowering circulating triglycerides and cholesterol [9], and improving hyperglycaemia [10]. These effects are largely attributed to FXR activation in the liver and intestine. Interestingly, a study by Bishop-Bailey et al. has shown that FXR is also expressed in the vasculature including EC and SMC. Activation of FXR results in inhibition of SMC proliferation, inflammation, and migration [11, 12]. We have extended their studies by showing that activation of FXR in vascular EC resulted in down-regulation of endothelin-1 (ET-1) expression and upregulation of eNOS expression [13, 14]. In addition, we have demonstrated that activation of FXR in SMCs resulted in upregulation of angiotensin type 2 receptor and inhibition of angiotensin II-mediated growth proliferation [15]. In this study we further examine if FXR plays a role in VSMCs with respect to the regulation of the expression of three widely studied PGs, versican, decorin and biglycan. Our results show that FXR ligands enhance the expression of decorin while showing minimal effect on versican and biglycan. These, together with previously published data, strongly suggest a likely direct effect of FXR on vasculature via influencing a number of molecular targets.
Material and methods
Materials
GW4064 was synthesized following a published protocol [8]. Actinomycin D was purchased from Sigma Chemical Co. (St. Louis, MO). All products for cell culture were purchased from Invitrogen (Carlsbad, CA). pCMX, pCMX-FXR, and pCMV- βgal were described previously [16].
Cell Culture
Human coronary artery smooth muscle cells (HCASMCs) were obtained from Cell Application, Inc. (San Diego, CA). Rat aortic artery smooth muscle cells (RASMCs) were kindly provided by Dr. Timothy Billiar at University of Pittsburgh. Normal African green monkey kidney fibroblast cells (CV-1 line) were obtained from American Type Culture Collection (Manassas, VA). These cells were cultured in DMEM supplemented with 10% FBS.
Plasmid Construction
Decorin promoter was amplified by PCR using human BAC clone RP11-864B16 (BACPAC Resources) as template. Primers for plasmid construction are described in Table 1. The mutant reporter construct was generated using TaKaRa mutanBEST Kit (TaKaRa, Japan).
Table 1.
Primer sets for plasmid construction
| Vector | Promoter Region | Primer Sequence | Product Size (bp) | Enzyme site |
|---|---|---|---|---|
| −2977~ +298 | 5’-GGGGTACCAGGACACCAAACACAATGG-3’(F) | 3275 | KpnI+XhoI | |
| 5’-GCGCTCGAGTTTGCAGGTGTGGAAAGG-3’(R) | ||||
| pGL3-basic | −2428~ +192 | 5’-GGGGTACCGGGAATCCTACCCCAAACAT-3’(F) | 2620 | KpnI+XhoI |
| 5’-GGCTCGAGATGAACACAATCCGGCTGAC-3’(R) | ||||
| −1069~ +192 | 5’-GGGGTACCCATGGACTTTTGCACCAATG-3’ (F) | 1261 | KpnI+BglII | |
| 5’-GAAGATCTATGAACACAATCCGGCTGAC-3’(R) | ||||
| −2428~ −1591 | 5’- CGGTCGAC GGGAATCCTACCCCAAACAT–3’(F) | 838 | SalI+BamHI | |
| 5’- CGGGATCC TCAGGCAACTTGCTTAACCTC–3’(R) | ||||
| tk-luc | −2428~ −2057 | 5’- CCAAGCTT GGGAATCCTACCCCAAACAT–3’(F) | 372 | HindIII+BamHI |
| 5’- CGGGATCC CATGACCAAGTTGCAATGCT–3’(R) | ||||
| −2428~ −2261 | 5’- CCAAGCTT GGGAATCCTACCCCAAACAT–3’(F) | 168 | HindIII+BamHI | |
| 5’- CGGGATCC CCCAACAAAGTCATACGCAGT–3’(R) | ||||
| 5’- CATAGTGTCATGACCTCTTGG -3’(1F) | ||||
| tk-luc | −2428~ −2261 | 5’-GACAGAAACCAGGTCTTTGCA -3’(1R) | 168 (mutated) | HindIII+BamHI |
| 5’- GTCT CTTGGTGACTTAACT -3’(2F) | ||||
| 5’-CATGACACTATGGACAGAAA -3’(2R) |
F, forward primer; R, reverse primer
RT-PCR & Real-Time PCR
Total RNA was extracted from cells with TRIzol reagent and the first-strand cDNA was synthesized using SuperScript III reverse transcriptase. The primers used for rat decorin (Dcn), biglycan (Bgn), and versican (Vcan) are: Dcn (468bp) forward: 5’-ACGCATGAGACAACCATGAA-3’; reverse: 5’-TCGAAGCTCCTGGAGTGTTT-3’; Bgn (407bp) forward: 5’-GAGGCTTCAGGCTCAGACAC-3’; reverse: 5’-ACTTTGCGGATACGGTTGTC-3’; Vcan (466bp) forward: 5’-CTTGGGGTGAGAACCCTGTA-3’; reverse: 5’-TAGCATGTTGGGAGGTGACA-3’. As an internal control, β- actin (360bp) was analyzed in parallel by using the following primers: forward: 5'-GTTCCGATGCCCCGAGGCT-3', reverse: 5'-GCATTTGCGGTGCACGATGGA-3'.
Real time PCR was performed with an ABI PRISM 7000 Sequence Detection System. The probes (Applied Biosystems) used are Rn01503162 for decorin and Rn01775763_g1 for G3PDH.
Western Blotting
Western blot analysis was performed as described previously [13]. Rabbit antibody to decorin was kindly provided by Dr. Larry Fisher at National Institutes of Health (Bethesda, MD).
Transfection Assays
CV-1 cells grown to 60–70% confluence in 48-well plates were transiently transfected using Lipofectamine2000 (Invitrogen) with pGL3-DCN or TK-DCN-Luc in the presence or absence of pCMX-FXR. pCMX was added to ensure identical amounts of DNA in each well. Transfection efficiency was monitored by co-transfection of pCMV-βgal plasmid. Cell extracts were prepared after transfection, and the luciferase and β-galactosidase assays were performed as described [17]. Luciferase activity was then normalized against β-galactosidase activity. Transfection experiments were done at least three times in triplicate. Data were represented as fold induction over reporter gene alone.
Electrophoretic Mobility Shift Assay
In vitro produced FXR and RXR were performed as described [15]. The radiolabeled probes used are: IR-1: 5′-CGATCAAG AGGTCATTGACCTTTTTG-3′, wt Dcn/IR8: 5’-CGATTCTGGTCATAGTGTC-ATGACCTCTT-3’ or mt Dcn/IR8: 5’-CGATTCTGTCCATAGTGTCATGGTCTCTT-3’, where underlined nucleotides represent response element half-sites and bases in bold are mutated. For supershift experiments, the anti-FXR antibody (sc-13063; Santa Cruz Biotechnology) was preincubated for 20 minutes before the addition of FXR and/or RXR proteins. For competition experiments, the unlabeled oligonucleotides were included in the binding reaction at the 100x excess concentrations over the probe prior to the addition of labeled oligonucleotides. EMSA with SMC nuclear proteins was similarly performed.
Statistical Analysis
All data are expressed as means ± SD unless otherwise stated. Comparisons between two groups were made with unpaired Student's t-tests. Non-parametric comparisons between three or more groups were made with ANOVA followed by Kruskal-Wallis post hoc analysis. In all cases, P < 0.05 was considered statistically significant.
Results
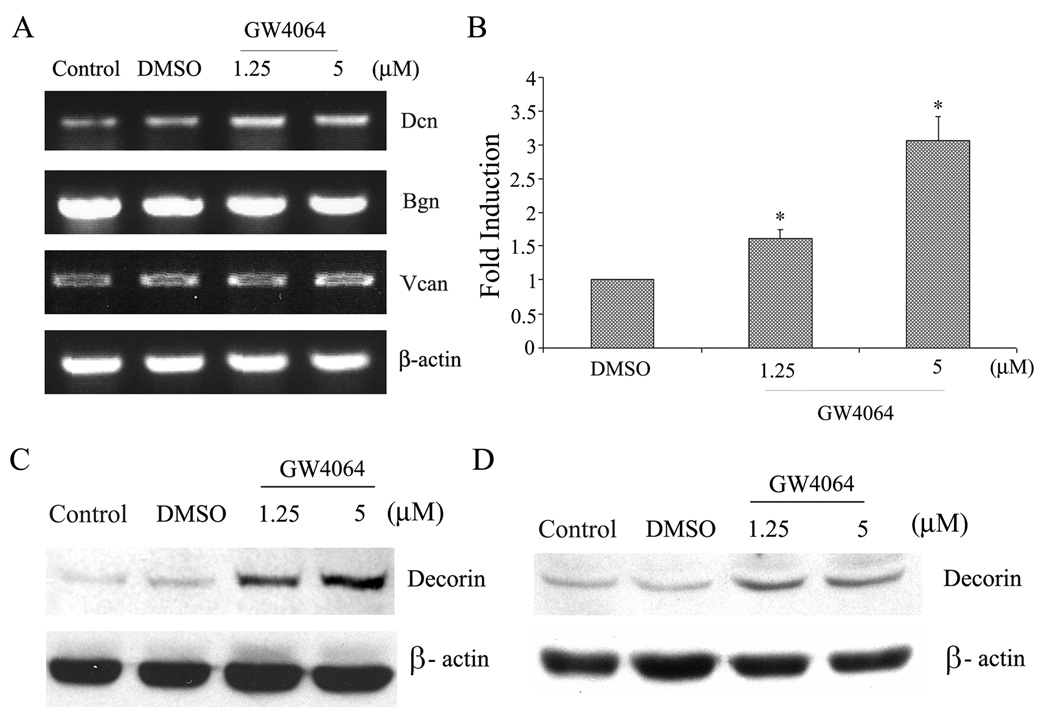
We and others have previously shown that functional FXR is expressed in vascular SMCs and activation of FXR led to growth inhibition of VSMCs [11, 12, 15]. In this study we examine whether activation of FXR affects the expression of PGs in VSMCs. In the preliminary study we examined the effect of GW4064 on the expression of three PGs (decorin, biglycan, and versican) in RASMCs via semi-quantitative RT-PCR. These PGs were chosen in our study because their roles in vascular physiology and vascular diseases have been well addressed and their regulations have been somewhat elucidated. GW4064 is a synthetic ligand that is highly specific for FXR [8]. As shown in Fig. 1A, GW4064 treatment led to increases in the mRNA levels of decorin in RASMCs. However, there were little changes in the mRNA levels of biglycan and versican. The GW4064-induced upregulation of decorin mRNA was also confirmed by the quantitative real-time RT-PCR (Fig. 1B). GW4064 similarly induced upregulation of decorin mRNA in HCASMCs (data not shown). GW4064 treatment also led to upregulation of decorin core protein in both RASMCs (Fig. 1C) and HCASMCs (Fig. 1D).
Fig. 1. GW4064-mediated upregulation of decorin expression in SMCs.
(A) RASMCs were treated with various concentrations of GW4064 and the mRNA expression of Dcn, Bgn, and Vcan was analyzed by semi-quantitative RT-PCR 24 h later. (B) Quantitative real-time RT-PCR analysis of Dcn mRNA expression in RASMCs before and after treatment with GW4064. *P < 0.05 (vs. DMSO), n = 3. (C) Western blot analysis of Dcn core protein expression in RASMCs 24 h following treatment with GW4064. (D) Western blot analysis of Dcn core protein expression in HCASMCs 24 h following treatment with GW4064.
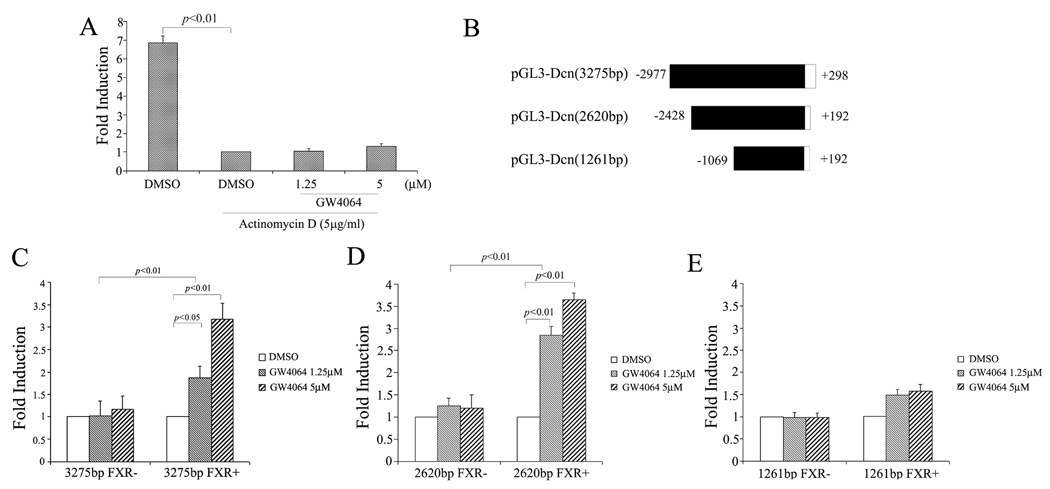
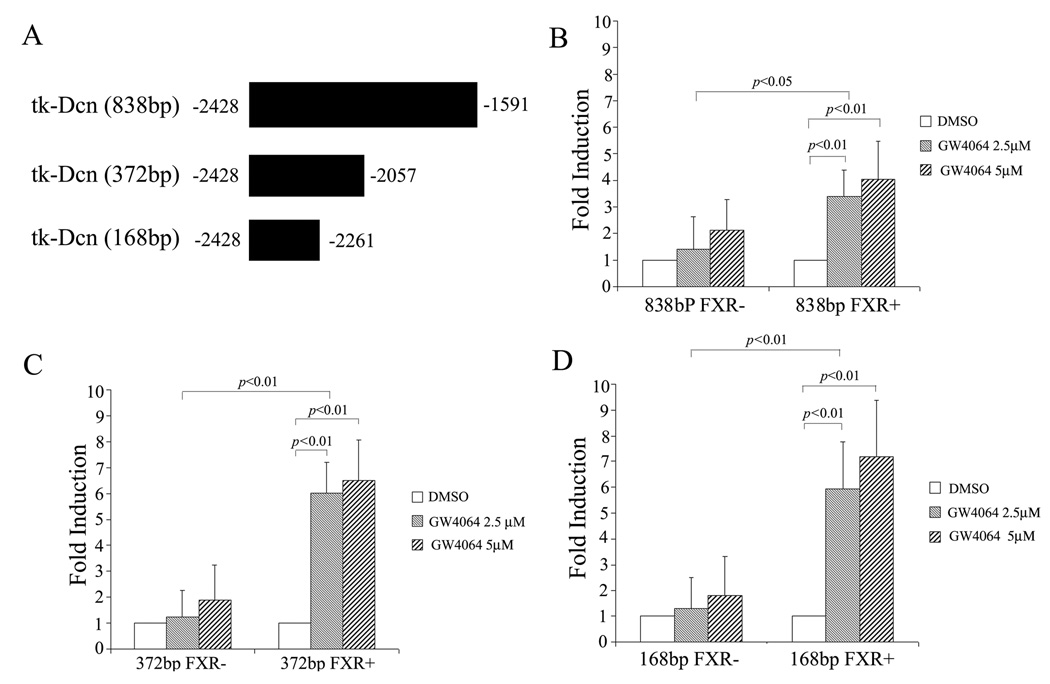
Fig. 2A shows that pretreatment with actinomycin D substantially inhibited GW4064-mediated upregulation of decorin mRNA, suggesting that GW4064 enhanced decorin mRNA expression at the transcriptional level. To further examine whether decorin promoter is a transcriptional target of FXR, we constructed a luciferase reporter expression plasmid (pGL3-DCN) that is driven by a human decorin promoter (−2977/+298) (Fig. 2B). CV-1 cells were transfected with pGL3-DCN in the absence or presence of a FXR expression vector. As shown in Fig. 2C, GW4064 alone showed no effect on decorin promoter activity. This might be due to the fact that CV-1 cells lack the expression of endogenous FXR. Indeed, GW4064 significantly enhanced the decorin promoter activity in CV-1 cells that were co-transfected with a FXR expression plasmid. A similar result was obtained with a reporter that is driven by a −2428/+192 human decorin promoter (Fig. 2D). However, deletion of the sequence of −2428/−1070 from the decorin promoter drastically reduced the GW4064/FXR-mediated transactivation of the decorin promoter (Fig. 2E), suggesting the presence of a FXR response element (FXRE) in this sequence. To further define the region that contains a potential FXRE, the −2428/−1070 sequence was then subjected to serial deletions and subcloned to a luciferase expression plasmid that was driven by a minimal TK promoter (Fig. 3A). As shown in Fig. 3B–C, decreasing the size of the sequence from 838 (−2428/−1591) to 168 (−2428/−2261) bp was associated with no significant changes in the promoter activity.
Fig. 2. GW4064 enhanced decorin mRNA expression at the transcriptional level.
(A) Actinomycin D substantially inhibited GW4064-mediated upregulation of decorin mRNA. RASMCs were treated with actinomycin D (5µg/mL) for 30 min prior to GW4064 treatment. Twenty-four hours later, Dcn mRNA expression in RASMCs was similarly examined by real-time RT–PCR. In a separate experiment, a series of human Dcn promoter luciferase reporter constructs (shown in panel B) were transiently transfected in CV-1 cells. After 12h, the transfected cells were treated for 24h with different concentrations of GW4064 or vehicle DMSO. The luciferase activity was normalized against the β-gal activity, and the data are reported as fold induction observed with GW treatment relative to DMSO control. Shown in panels C, D, and E are data from transfection with a reporter driven by a Dcn promoter of 3275bp, 2620 bp, and 1261 bp, respectively.
Fig. 3. FXR-dependent regulation of the (−2428~−2261) DNA fragment.
The −2428/−1591 sequence was subjected to serial deletions and subcloned to a luciferase expression plasmid that was driven by a minimal TK promoter (A). Transfection and luciferase assay were similarly performed as described in the legend to Fig. 2. Shown in panels B, C, and D are data from transfection with a reporter driven by a regulatory sequence of 838 bp, 372 bp, and and168 bp, respectively.
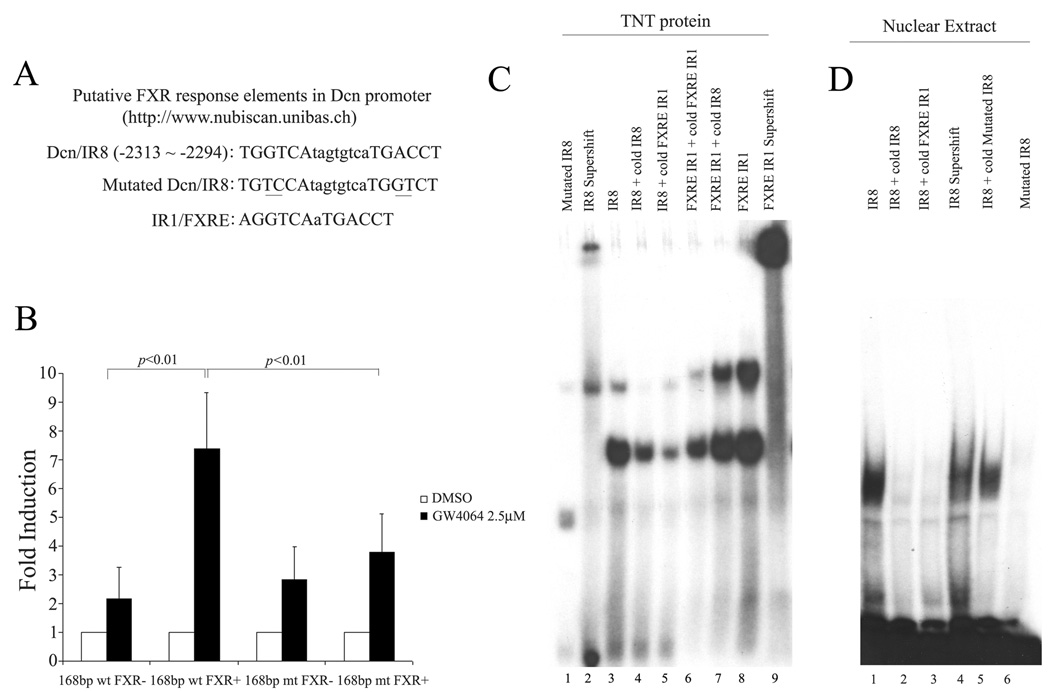
To search for FXREs that may mediate decorin induction by GW4064, the 168 bp sequence in the decorin promoter region was subjected to in silico analysis with a Web-based algorithm (NUBIScan) [18]. One such potential FXRE was identified and its sequence and location are shown in Fig. 4A. There is a hexameric core sequence TGGTCA between −2313 and −2318. Eight base pairs downstream from this half-site is an imperfect inverted repeat sequence TGACCT (−2299 to −2294). This forms an imperfect inverted repeat motif IR8 (−2313TGGTCAtagtgtcaTGACCT−2294). This IR8 motif is likely to be involved in the GW4064-mediated transactivation of decorin promoter as mutation of this motif resulted in a significant decrease in the promoter activity (Fig. 4B).
Fig. 4. Analysis of putative FXR-responsive elements (FXREs) in human Dcn promoter.
(A) Identification of a putative FXRE in human Dcn promoter via an in silico analysis with a Web-based algorithm (NUBIScan). (B) Transfection with pTK-Dcn-Luc vector containing a wt 168 bp or mt 168 bp sequence derived from the decorin promoter region. (C) EMSA analysis of the binding of FXR/RXR to the putative FXRE in human Dcn promoter. (D) EMSA analysis of the binding of nuclear proteins of GW4064-treated HCASMCs to Dcn/IR8. Results shown in this panel are the representative data from 3 experiments.
To determine whether FXR directly binds to this element, EMSA assays were performed with a 29bp oligonucleotide that contains the decorin/IR8. A typical IR1/FXRE oligonucleotide was also included as a positive control. Interaction of decorin/IR8 oligonucleotide with in vitro translated FXR/RXR yielded a DNA/protein band of expected mobility (Fig. 4C). This binding was specific, as it was inhibited by addition of excess unlabelled (cold) decorin/IR8 or IR1/FXRE (Fig. 4C). Addition of antibody against FXR to the reaction mixture resulted in further retarded migration of the radio labeled band (Fig. 4C). This supershifting confirms the identity of the protein that interacts with the DNA as being FXR.
We also examined DNA-binding activity to decorin/IR8 in nuclear extracts generated from GW4064-treated HCASMCs (Fig. 4D). Incubation of the decorin/IR8 probe with GW4064/HCASMCs nuclear extracts produced a shifted band (lane 1). The addition of cold decorin/IR8 (lane 2) or IR1/FXRE (lane 3) probe or antibody against FXR protein (lane 4) substantially inhibited this binding, indicating that FXR was a principal DNA-binding component of this protein-DNA complex. No obvious interaction was noticed between the nuclear extracts and a mutated decorin/IR8 (lane 6). The above results suggest that decorin/IR8 is likely to mediate the transactivation of decorin promoter by FXR.
Discussion
We and others have previously shown that FXR agonists inhibit the growth [11, 12, 15] and migration of SMC [12]. In this study we show that activation of FXR in VSMCs also leads to upregulation of decorin expression. Furthermore we have identified an imperfect IR8 as a FXRE that appears to be involved in GW4064-mediated upregulation of decorin in VSMCs. Interestingly, GW4064 selectively enhanced the expression of decorin while showing minimal effect on biglycan and versican. Differential regulation of decorin and other ECM proteins in VSMCs have also been shown with growth factors and cytokines [19, 20].
Decorin is a one of the class I SLRPs that also include biglycan. It consists of a single glycosaminoglycan side chain linked to a core protein containing leucine-rich repeats of about 24 amino acids [21]. It is found in the ECM of a variety of tissues and cell types. Decorin plays a number of important functions including cell adhesion, migration, proliferation, and signaling [22]. The therapeutic implication of decorin has been largely suggested by the observations that decorin binds to TGF̣-β and other growth factors and inhibits their biological activity in a number of cell types. For example, overexpression of decorin in mouse lungs via adenoviral gene transfer significantly reduced fibrotic responses induced by either TGF̣-β adenoviral gene transfer [23] or bleomycin treatment [24]. Local expression of decorin by cell-mediated gene transfer also reduced neointimal formation in a rat model of balloon-induced vascular injury [25]. In a study by Al Haj Zen and colleagues, it was shown that overexpression of decorin in mouse liver via adenoviral gene transfer attenuated atherosclerosis development in Apoe−/− mice [26]. Despite the demonstrated protective roles of decorin in a number of disease models, several studies have suggested that decorin as well as several other PGs may be involved in the pathogenesis of atherosclerosis. It has been shown that decorin interacts with the amino-terminal part of apoB, which could lead to entrapment of lipoproteins in the circulation [27]. Thus while ectopic (e.g., in liver) expression of PGs may help to lower the circulating levels of lipoproteins, PGs expressed in situ in vasculature may contribute to the lipid accumulation in the deep intimal layer which represents the earliest stage of lesion in the development of atherosclerosis. This hypothesis appears to be supported by the observations that lipid accumulation correlates with expression of PGs such as decorin and biglycan in the very early lesion of atherosclerosis [28]. Thus the roles of decorin are rather complex and might be dependent on the type of vascular disorders and may also vary with the stages for a particular disease.
A role of FXR-mediated regulation of decorin in the vascular physiology as well as vascular diseases is unknown at present. FXR ligands have recently been proposed as a novel therapy for the treatment of cardiovascular diseases. This is largely based on their effects in lowering circulating triglycerides and cholesterol [9], and improving hyperglycemia [10] via the FXR-mediated effects in liver and intestine. However, activation of FXR has also been shown to result in some unfavorable biological effects such as decreases in the blood levels of high-density lipoprotein (HDL). This is further complicated by the conflicting results of the roles of FXR in atherosclerosis [29–31]. For example, Hanniman et al. reported that administration of a high-fat/high-cholesterol diet to male Fxr−/−Apoe−/− mice resulted in increased plasma lipids and atherosclerosis [29]. This is in contrast to the studies in female Fxr−/−Apoe−/− mice and male Fxr−/−Ldlr−/− mice which showed decreased atherosclerosis [30, 31]. It remains to be determined whether the FXR/decorin pathway contributes to the prevention or initiation/progression of atherosclerosis. The answers to these questions might be found in studies in mice in which components of the FXR/decorin pathway are selectively modulated in vasculature.
Acknowledgements
This work was supported by a NIH grant HL68688 (to S.L.), an AHA grant 0555456U (to S.L.) and a grant from National Natural Science Foundation of China (30771121) (to F.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gupta V, Grande-Allen KJ. Effects of static and cyclic loading in regulating extracellular matrix synthesis by cardiovascular cells. Cardiovasc. Res. 2006;72:375–383. doi: 10.1016/j.cardiores.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Hultgårdh-Nilsson A, Durbeej M. Role of the extracellular matrix and its receptors in smooth muscle cell function: implications in vascular development and disease. Curr. Opin. Lipidol. 2007;18:540–545. doi: 10.1097/MOL.0b013e3282ef77e9. [DOI] [PubMed] [Google Scholar]
- 3.Rahmani M, Wong BW, Ang L, Cheung CC, Carthy JM, Walinski H, McManus BM. Versican: signaling to transcriptional control pathways. Can. J. Physiol. Pharmacol. 2006;16:77–92. doi: 10.1139/y05-154. [DOI] [PubMed] [Google Scholar]
- 4.Theocharis AD, Tsolakis I, Tzanakakis GN, Karamanos NK. Chondroitin sulfate as a key molecule in the development of atherosclerosis and cancer progression. Adv. Pharmacol. 2006;53:281–295. doi: 10.1016/S1054-3589(05)53013-8. [DOI] [PubMed] [Google Scholar]
- 5.Kinsella MGA, Bressler SL, Wight TN. The regulated synthesis of versican, decorin, and biglycan: extracellular matrix proteoglycans that influence cellular phenotype. Crit. Rev. Eukaryot. Gene. Expr. 2004;14:203–234. doi: 10.1615/critreveukaryotgeneexpr.v14.i3.40. [DOI] [PubMed] [Google Scholar]
- 6.Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 7.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 8.Maloney PR, Parks DJ, Haffner CD, Fivush AM, Chandra G, Plunket KD, Creech KL, Moore LB, Wilson JG, Lewis MC, et al. Identification of a chemical tool for the orphan nuclear receptor FXR. J. Med. Chem. 2000;43:2971–2974. doi: 10.1021/jm0002127. [DOI] [PubMed] [Google Scholar]
- 9.Eloranta JJ, Kullak-Ublick GA. Coordinate transcriptional regulation of bile acid homeostasis and drug metabolism. Arch. Biochem. Biophys. 2005;433:397–412. doi: 10.1016/j.abb.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM, Edwards PA. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc. Natl. Acad. Sci. USA. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bishop-Bailey D, Walsh DT, Warner TD. Expression and activation of the farnesoid X receptor in the vasculature. Proc. Natl. Acad. Sci. USA. 2004;101:3668–3673. doi: 10.1073/pnas.0400046101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li YT, Swales KE, Thomas GJ, Warner TD, Bishop-Bailey D. Farnesoid x receptor ligands inhibit vascular smooth muscle cell inflammation and migration. Arterioscler. Thromb. Vasc. Biol. 2007;27:2606–2611. doi: 10.1161/ATVBAHA.107.152694. [DOI] [PubMed] [Google Scholar]
- 13.He F, Li J, Mu Y, Kuruba R, Ma Z, Wilson A, Alber S, Jiang Y, Stevens T, Watkins S, et al. Downregulation of endothelin-1 by farnesoid X receptor in vascular endothelial cells. Circ. Res. 2006;98:192–199. doi: 10.1161/01.RES.0000200400.55539.85. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Wilson A, Kuruba R, Zhang Q, Gao X, He F, Zhang LM, Pitt BR, Xie W, Li S. FXR-mediated regulation of eNOS expression in vascular endothelial cells. Cardiovasc. Res. 2008;77:169–177. doi: 10.1093/cvr/cvm016. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q, He F, Kuruba R, Gao X, Wilson A, Li J, Billiar TR, Pitt BR, Xie W, Li S. FXR-mediated regulation of angiotensin type 2 receptor expression in vascular smooth muscle cells. Cardiovasc. Res. 2008;77:560–569. doi: 10.1093/cvr/cvm068. [DOI] [PubMed] [Google Scholar]
- 16.Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, Waxman DJ, Evans RM. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc. Natl. Acad. Sci. USA. 2001;98:3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saini SP, Sonoda J, Xu L, Toma D, Uppal H, Mu Y, Ren S, Morre DD, Evans RM, Xie W. A novel constitutive androstane receptor-mediated and CYP3A-independent pathway of bile acid detoxification. Mol. Pharmacol. 2004;65:292–300. doi: 10.1124/mol.65.2.292. [DOI] [PubMed] [Google Scholar]
- 18.Podvinec M, Kaufmann MR, Handschin C, Meyer UA. NUBIScan, an in silico approach for prediction of nuclear receptor response elements. Mol. Endocrinol. 2002;16:1269–1279. doi: 10.1210/mend.16.6.0851. [DOI] [PubMed] [Google Scholar]
- 19.Potter-Perigo S, Baker C, Tsoi C, Braun KR, Isenhath S, Altman GM, Altman LC, Wight TN. Regulation of proteoglycan synthesis by leukotriene d4 and epidermal growth factor in bronchial smooth muscle cells. Am. J. Respir. Cell. Mol. Biol. 2004;30:101–108. doi: 10.1165/rcmb.2003-0050OC. [DOI] [PubMed] [Google Scholar]
- 20.Schönherr E, Järveläinen HT, Kinsella MG, Sandell LJ, Wight TN. Platelet-derived growth factor and transforming growth factor-beta 1 differentially affect the synthesis of biglycan and decorin by monkey arterial smooth muscle cells. Arterioscler. Thromb. 1993;13:1026–1036. doi: 10.1161/01.atv.13.7.1026. [DOI] [PubMed] [Google Scholar]
- 21.McEwan PA, Scott PG, Bishop PN, Bella J. Structural correlations in the family of small leucine-rich repeat proteins and proteoglycans. J. Struct. Biol. 2006;155:294–305. doi: 10.1016/j.jsb.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Xu G, Guimond MJ, Chakraborty C, Lala PK. Control of proliferation, migration, and invasiveness of human extravillous trophoblast by decorin, a decidual product. Biol. Reprod. 2002;67:681–689. doi: 10.1095/biolreprod67.2.681. [DOI] [PubMed] [Google Scholar]
- 23.Kolb M, Margetts PJ, Sime PJ, Gauldie J. Proteoglycans decorin and biglycan differentially modulate TGF-beta-mediated fibrotic responses in the lung. Am. J. Physiol, Lung. Cell. Mol. Physiol. 2001;280:L1327–L1334. doi: 10.1152/ajplung.2001.280.6.L1327. [DOI] [PubMed] [Google Scholar]
- 24.Kolb M, Margetts PJ, Galt T, Sime PJ, Xing Z, Schmidt M, Gauldie J. Transient transgene expression of decorin in the lung reduces the fibrotic response to bleomycin. Am. J. Respir. Crit. Care Med. 2001;163:770–777. doi: 10.1164/ajrccm.163.3.2006084. [DOI] [PubMed] [Google Scholar]
- 25.Fischer JW, Kinsella MG, Clowes MM, Lara S, Clowes AW, Wight TN. Local expression of bovine decorin by cell-mediated gene transfer reduces neointimal formation after balloon injury in rats. Circ. Res. 2000;86:676–683. doi: 10.1161/01.res.86.6.676. [DOI] [PubMed] [Google Scholar]
- 26.Al Haj Zen A, Caligiuri G, Sainz J, Lemitre M, Demerens C, Lafont A. Decorin overexpression reduces atherosclerosis development in apolipoprotein E-deficient mice. Atherosclerosis. 2006;187:31–39. doi: 10.1016/j.atherosclerosis.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg IJ, Wagner WD, Pang L, Paka L, Curtiss LK, DeLozier JA, Shelness GS, Young CS, Pillarisetti S. The NH2-terminal region of apolipoprotein B is sufficient for lipoprotein association with glycosaminoglycans. J. Biol. Chem. 1998;273:35355–35361. doi: 10.1074/jbc.273.52.35355. [DOI] [PubMed] [Google Scholar]
- 28.Nakashima Y, Fujii H, Sumiyoshi S, Wight TN, Sueishi K. Early human atherosclerosis: accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler. Thromb. Vasc. Biol. 2007;27:1159–1165. doi: 10.1161/ATVBAHA.106.134080. [DOI] [PubMed] [Google Scholar]
- 29.Hanniman EA, Lambert G, McCarthy TC, Sinal CJ. Loss of functional farnesoid X receptor increases atherosclerotic lesions in apolipoprotein E-deficient mice. J. Lipid. Res. 2005;46:2595–2604. doi: 10.1194/jlr.M500390-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Wang X, Vales C, Lee FY, Lee H, Lusis AJ, Edwards PA. FXR deficiency causes reduced atherosclerosis in Ldlr−/− mice. Arterioscler. Thromb. Vasc. Biol. 2006;26:2316–2321. doi: 10.1161/01.ATV.0000235697.35431.05. [DOI] [PubMed] [Google Scholar]
- 31.Guo GL, Santamarina-Fojo S, Akiyama TE, Amar MJ, Paigen BJ, Brewer B, Jr, Gonzalez FJ. Effects of FXR in foam-cell formation and atherosclerosis development. Biochim. Biophys. Acta. 2006;1761:1401–1409. doi: 10.1016/j.bbalip.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]