Abstract
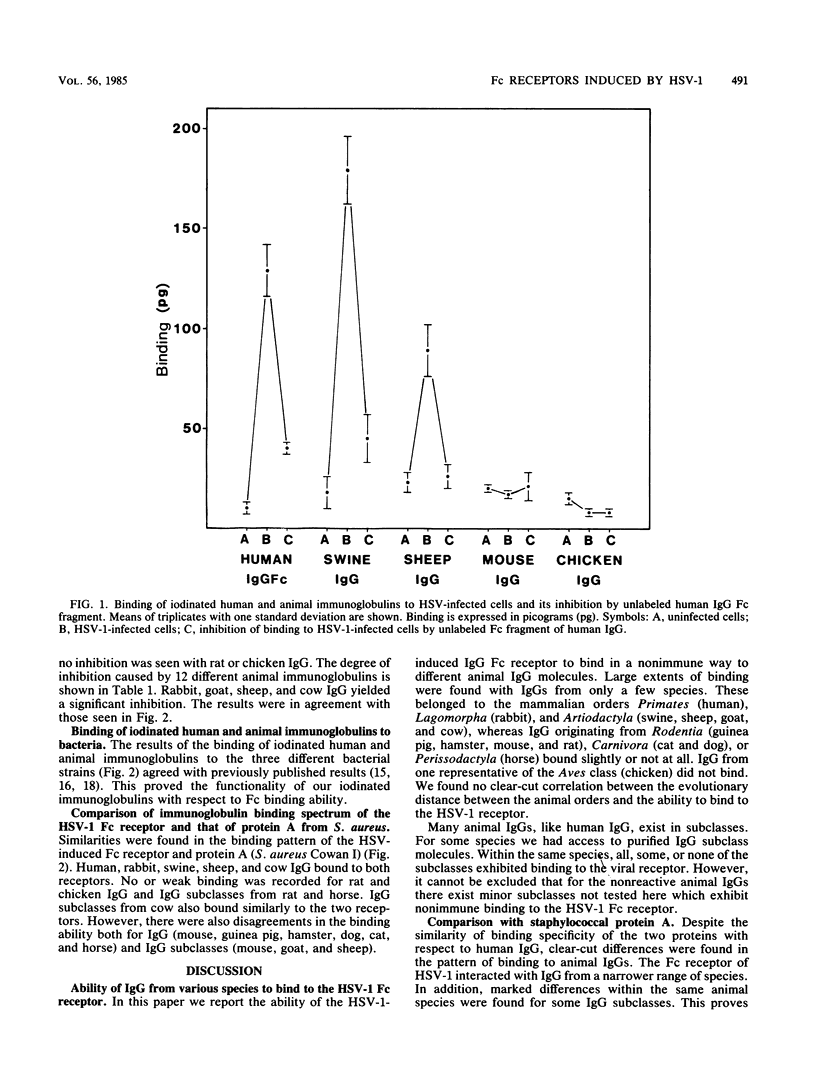
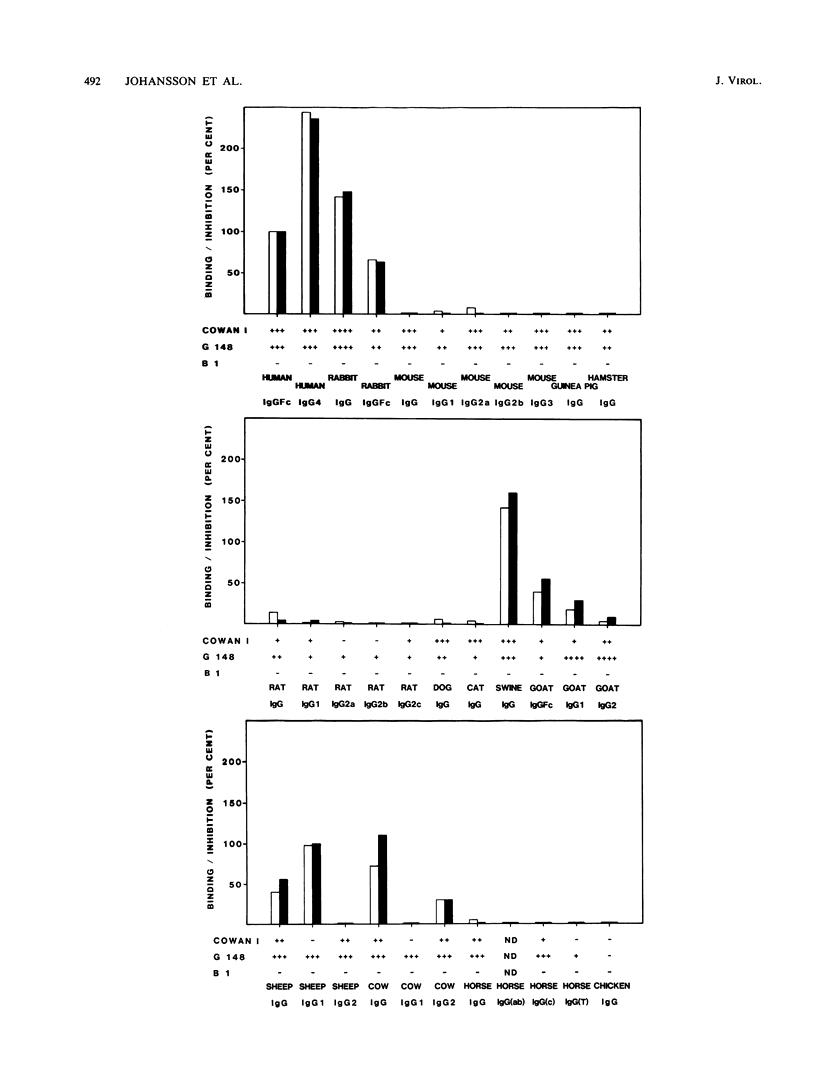
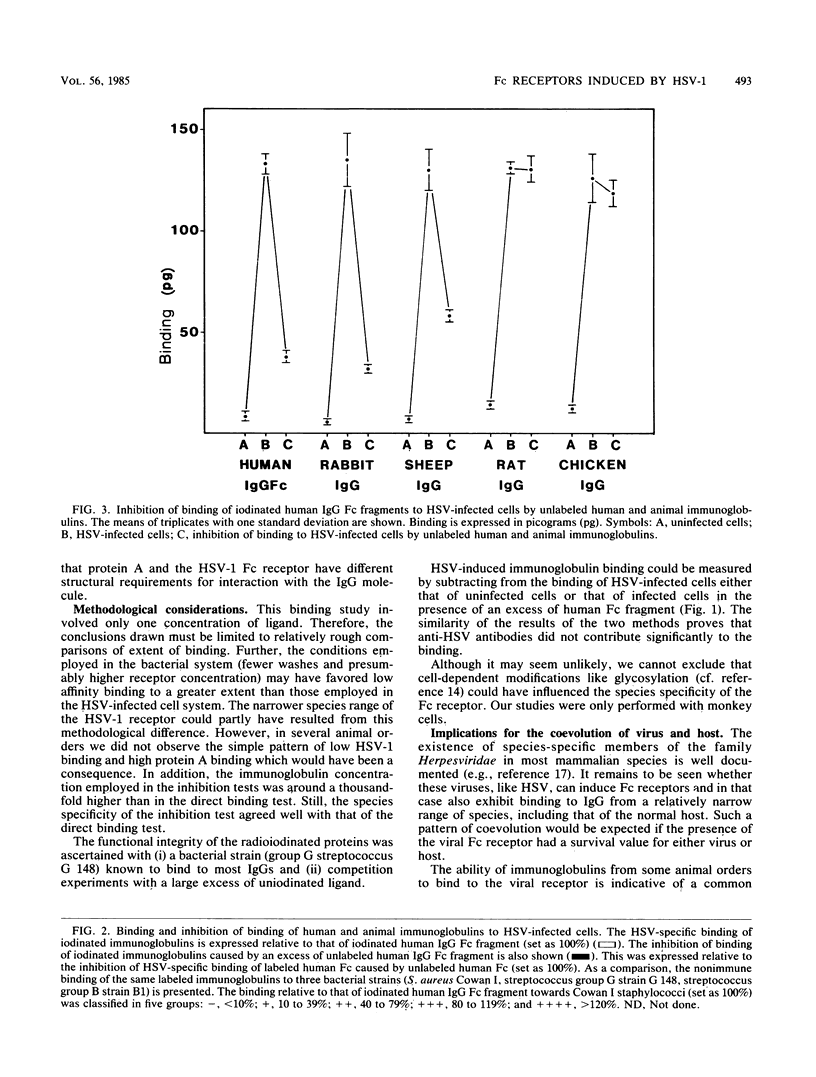
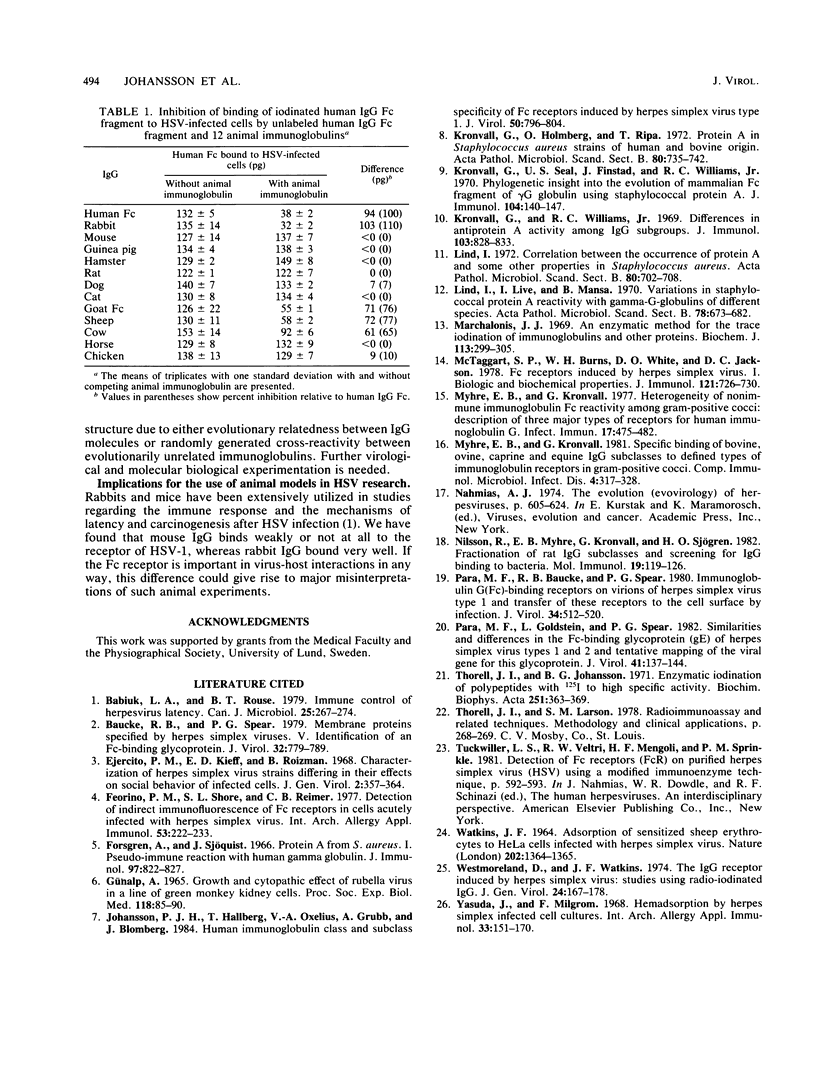
Cells infected with herpes simplex virus type 1 (HSV-1) express a cell surface receptor able to bind the Fc portion of immunoglobulin G (IgG). Of the four human IgG subclasses, the HSV-1 Fc receptor, like staphylococcal protein A, binds to all except IgG3. In this paper, we describe the binding of a number of animal IgG and IgG subclass molecules to HSV-1-infected cells and compare this binding to that of protein A. Although only few representatives from each animal order were tested, we found that IgG from Carnivora and Rodentia did not bind or bound only slightly to the HSV-1 receptor, whereas IgG from Primates, Lagomorpha, and Artiodactyla bound well. This pattern was clearly different from the species spectrum of IgG binding of protein A. Differences between the two receptors were also found when animal IgG subclasses were tested. The pronounced differences in affinity for the HSV-1 Fc receptor between immunoglobulins from, for example, mouse and rabbit may influence the interpretation of animal studies with this virus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiuk L. A., Rouse B. T. Immune control of herpesvirus latency. Can J Microbiol. 1979 Mar;25(3):267–274. doi: 10.1139/m79-043. [DOI] [PubMed] [Google Scholar]
- Baucke R. B., Spear P. G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979 Dec;32(3):779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Feorino P. M., Shore S. L., Reimer C. B. Detection by indirect immunofluorescence of Fc receptors in cells acutely infected with Herpes simplex virus. Int Arch Allergy Appl Immunol. 1977;53(3):222–233. doi: 10.1159/000231756. [DOI] [PubMed] [Google Scholar]
- Forsgren A., Sjöquist J. "Protein A" from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. J Immunol. 1966 Dec;97(6):822–827. [PubMed] [Google Scholar]
- GUENALP A. GROWTH AND CYTOPATHIC EFFECT OF RUBELLA VIRUS IN A LINE OF GREEN MONKEY KIDNEY CELLS. Proc Soc Exp Biol Med. 1965 Jan;118:85–90. [PubMed] [Google Scholar]
- Johansson P. J., Hallberg T., Oxelius V. A., Grubb A., Blomberg J. Human immunoglobulin class and subclass specificity of Fc receptors induced by herpes simplex virus type 1. J Virol. 1984 Jun;50(3):796–804. doi: 10.1128/jvi.50.3.796-804.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G., Holmberg O., Ripa T. Protein A in Staphylococcus aureus strains of human and bovine origin. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(5):735–742. doi: 10.1111/j.1699-0463.1972.tb00201.x. [DOI] [PubMed] [Google Scholar]
- Kronvall G., Seal U. S., Finstad J., Williams R. C., Jr Phylogenetic insight into evolution of mammalian Fc fragment of gamma G globulin using staphylococcal protein A. J Immunol. 1970 Jan;104(1):140–147. [PubMed] [Google Scholar]
- Kronvall G., Williams R. C., Jr Differences in anti-protein A activity among IgG subgroups. J Immunol. 1969 Oct;103(4):828–833. [PubMed] [Google Scholar]
- Lind I. Correlation between the occurrence of protein A and some other properties in Staphylococcus aureus. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(5):702–708. doi: 10.1111/j.1699-0463.1972.tb00197.x. [DOI] [PubMed] [Google Scholar]
- Lind I., Live I., Mansa B. Variation in staphylococcal protein A reactivity with gamma G-globulins of different species. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(6):673–682. doi: 10.1111/j.1699-0463.1970.tb04357.x. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTaggart S. P., Burns W. H., White D. O., Jackson D. C. Fc receptors induced by herpes simplex virus. I. Biologic and biochemical properties. J Immunol. 1978 Aug;121(2):726–730. [PubMed] [Google Scholar]
- Myhre E. B., Kronvall G. Heterogeneity of nonimmune immunoglobulin Fc reactivity among gram-positive cocci: description of three major types of receptors for human immunoglobulin G. Infect Immun. 1977 Sep;17(3):475–482. doi: 10.1128/iai.17.3.475-482.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhre E. B., Kronvall G. Specific binding of bovine, ovine, caprine and equine IgG subclasses to defined types of immunoglobulin receptors in Gram-positive cocci. Comp Immunol Microbiol Infect Dis. 1981;4(3-4):317–328. doi: 10.1016/0147-9571(81)90018-7. [DOI] [PubMed] [Google Scholar]
- Nilsson R., Myhre E., Kronvall G., Sjögren H. O. Fractionation of rat IgG subclasses and screening for IgG Fc-binding to bacteria. Mol Immunol. 1982 Jan;19(1):119–126. doi: 10.1016/0161-5890(82)90253-x. [DOI] [PubMed] [Google Scholar]
- Para M. F., Baucke R. B., Spear P. G. Immunoglobulin G(Fc)-binding receptors on virions of herpes simplex virus type 1 and transfer of these receptors to the cell surface by infection. J Virol. 1980 May;34(2):512–520. doi: 10.1128/jvi.34.2.512-520.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Goldstein L., Spear P. G. Similarities and differences in the Fc-binding glycoprotein (gE) of herpes simplex virus types 1 and 2 and tentative mapping of the viral gene for this glycoprotein. J Virol. 1982 Jan;41(1):137–144. doi: 10.1128/jvi.41.1.137-144.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- WATKINS J. F. ADSORPTION OF SENSITIZED SHEEP ERYTHROCYTES TO HELA CELLS INFECTED WITH HERPES SIMPLEX VIRUS. Nature. 1964 Jun 27;202:1364–1365. doi: 10.1038/2021364a0. [DOI] [PubMed] [Google Scholar]
- Westmoreland D., Watkins J. F. The IgG receptor induced by herpes simplex virus: studies using radioiodinated IgG. J Gen Virol. 1974 Jul;24(1):167–178. doi: 10.1099/0022-1317-24-1-167. [DOI] [PubMed] [Google Scholar]
- Yasuda J., Milgrom F. Hemadsorption by herpes simplex-infected cell cultures. Int Arch Allergy Appl Immunol. 1968;33(2):151–170. doi: 10.1159/000229985. [DOI] [PubMed] [Google Scholar]


