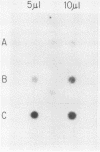
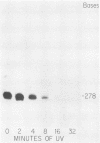
Abstract
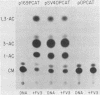
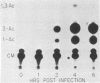
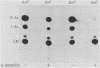
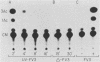
We investigated the protein and DNA sequence requirements for the expression of an immediate-early frog virus 3 (FV3) gene, infected-cell RNA (ICR) 169. We used a plasmid containing the 78 nucleotides 5' to the transcription start site of ICR-169 placed upstream from the coding sequence for the bacterial enzyme chloramphenicol acetyltransferase (CAT). This construction, when introduced by CaPO4-mediated transfection into various eucaryotic cell lines, promoted CAT synthesis only if the transfected cells were subsequently infected with FV3. Dot-blot hybridization of RNA extracted from transfected, FV3-infected cells with a radioactive CAT probe showed that the induction of CAT synthesis by FV3 was at the level of transcription. When transfected cells were infected with FV3 in the presence of cycloheximide, induction of CAT-specific RNA still occurred, demonstrating that a virion protein was responsible for the trans activation. FV3-induced CAT synthesis was inhibited by alpha-amanitin in wild-type Chinese hamster ovary (CHO) cells but not in CHO cells with an alpha-amanitin-resistant RNA polymerase II. The results suggest that a virion protein alters either the DNA template or the host polymerase to allow transcription from immediate-early FV3 promoters.
Full text
PDF
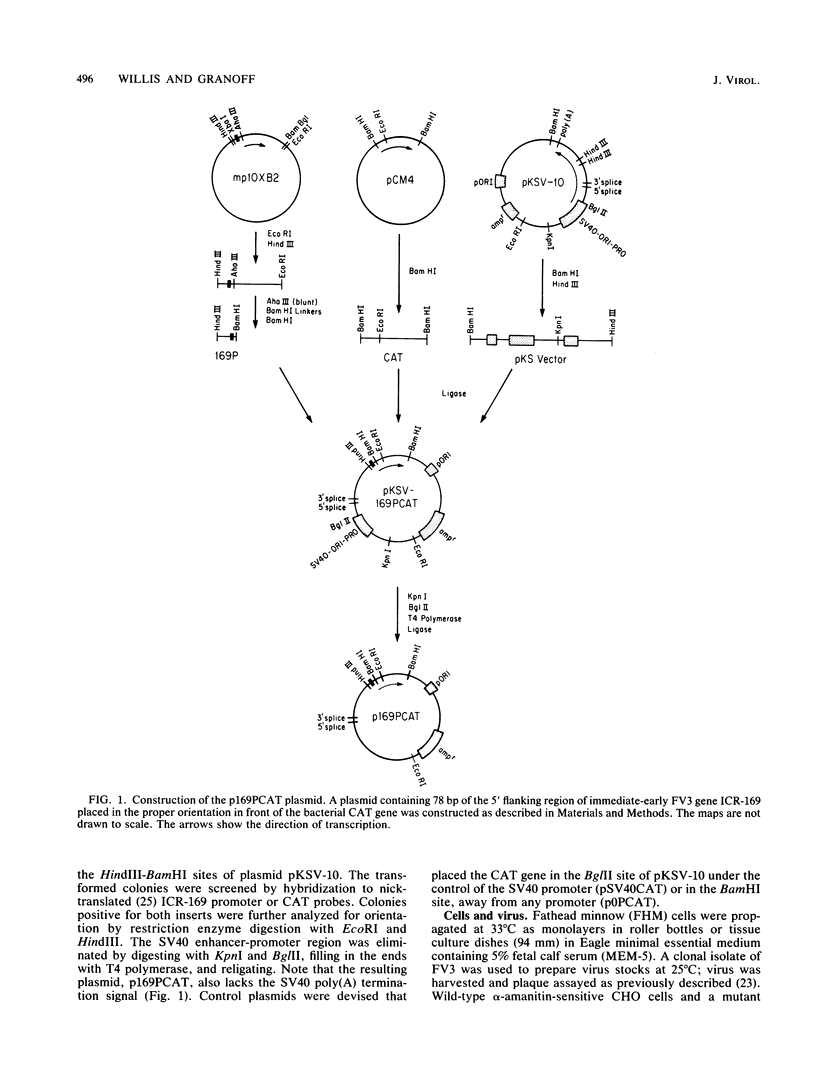

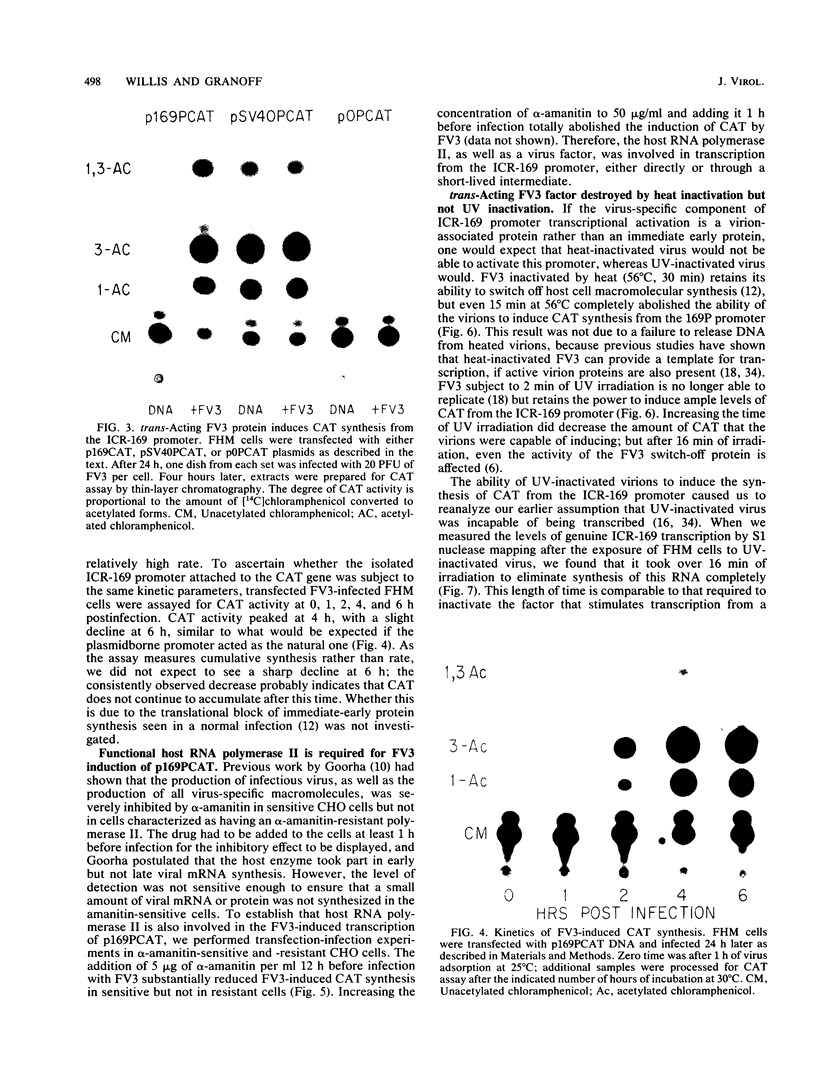


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubertin A. M., Tondre L., Lopez C., Obert G., Kirn A. Sodium dodecyl sulfate-mediated transfer of electrophoretically separated DNA-binding proteins. Anal Biochem. 1983 May;131(1):127–134. doi: 10.1016/0003-2697(83)90143-4. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bertholet C., Drillien R., Wittek R. One hundred base pairs of 5' flanking sequence of a vaccinia virus late gene are sufficient to temporally regulate late transcription. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2096–2100. doi: 10.1073/pnas.82.7.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan V. L., Whitmore G. F., Siminovitch L. Mammalian cells with altered forms of RNA polymerase II. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3119–3123. doi: 10.1073/pnas.69.11.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi A., Church G. M., Tonegawa S., Gilbert W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985 Jan 11;227(4683):134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- Everett R. D., Dunlop M. Trans activation of plasmid-borne promoters by adenovirus and several herpes group viruses. Nucleic Acids Res. 1984 Aug 10;12(15):5969–5978. doi: 10.1093/nar/12.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidoni D., Dynan W. S., Tjian R. Multiple specific contacts between a mammalian transcription factor and its cognate promoters. 1984 Nov 29-Dec 5Nature. 312(5993):409–413. doi: 10.1038/312409a0. [DOI] [PubMed] [Google Scholar]
- Goorha R. DNA-binding proteins in frog virus 3-infected cells. J Gen Virol. 1981 Sep;56(Pt 1):131–140. doi: 10.1099/0022-1317-56-1-131. [DOI] [PubMed] [Google Scholar]
- Goorha R. Frog virus 3 requires RNA polymerase II for its replication. J Virol. 1981 Jan;37(1):496–499. doi: 10.1128/jvi.37.1.496-499.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorha R., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. I. Virus-specific protein synthesis and its regulation. Virology. 1974 Jul;60(1):237–250. doi: 10.1016/0042-6822(74)90381-x. [DOI] [PubMed] [Google Scholar]
- Goorha R., Murti G., Granoff A., Tirey R. Macromolecular synthesis in cells infected by frog virus 3. VIII. The nucleus is a site of frog virus 3 DNA and RNA synthesis. Virology. 1978 Jan;84(1):32–50. doi: 10.1016/0042-6822(78)90216-7. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gravell M., Cromeans T. L. Mechanisms involved in nongenetic reactivation of frog polyhedral cytoplasmic deoxyribovirus: evidence for an RNA polymerase in the virion. Virology. 1971 Oct;46(1):39–49. doi: 10.1016/0042-6822(71)90004-3. [DOI] [PubMed] [Google Scholar]
- Gravell M., Granoff A. Viruses and renal carcinoma of Rana pipiens. IX. The influence of temperature and host cell on replication of frog polyhedral cytoplasmic deoxyribovirus (PCDV). Virology. 1970 Aug;41(4):596–602. doi: 10.1016/0042-6822(70)90425-3. [DOI] [PubMed] [Google Scholar]
- Gravell M., Naegele R. F. Nongenetic reactivation of frog polyhedral cytoplasmic deoxyribovirus (PCDV). Virology. 1970 Jan;40(1):170–174. doi: 10.1016/0042-6822(70)90390-9. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Pero J. Cascades of Sigma factors. Cell. 1981 Sep;25(3):582–584. doi: 10.1016/0092-8674(81)90164-1. [DOI] [PubMed] [Google Scholar]
- Martin J. P., Aubertin A. M., Kirn A. Expression of frog virus 3 early genes after ultraviolet irradiation. Virology. 1982 Oct 30;122(2):402–410. doi: 10.1016/0042-6822(82)90239-2. [DOI] [PubMed] [Google Scholar]
- Naegele R. F., Granoff A. Viruses and renal carcinoma of Rana pipiens. XI. Isolation of frog virus 3 temperature-sensitive mutants; complementation and genetic recombination. Virology. 1971 May;44(2):286–295. doi: 10.1016/0042-6822(71)90260-1. [DOI] [PubMed] [Google Scholar]
- O'Hare P., Hayward G. S. Expression of recombinant genes containing herpes simplex virus delayed-early and immediate-early regulatory regions and trans activation by herpesvirus infection. J Virol. 1984 Nov;52(2):522–531. doi: 10.1128/jvi.52.2.522-531.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Kettman R., Burny A., Haseltine W. A. Trans activation of the bovine leukemia virus long terminal repeat in BLV-infected cells. Science. 1985 Jan 18;227(4684):320–322. doi: 10.1126/science.2981432. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Corden J., Kédinger C., Chambon P. Promotion of specific in vitro transcription by excised "TATA" box sequences inserted in a foreign nucleotide environment. Nucleic Acids Res. 1981 Aug 25;9(16):3941–3958. doi: 10.1093/nar/9.16.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P., Wildeman A., Chambon P. A trans-acting factor is responsible for the simian virus 40 enhancer activity in vitro. Nature. 1985 Feb 7;313(6002):458–463. doi: 10.1038/313458a0. [DOI] [PubMed] [Google Scholar]
- Weeks D. L., Jones N. C. E1A control of gene expression is mediated by sequences 5' to the transcriptional starts of the early viral genes. Mol Cell Biol. 1983 Jul;3(7):1222–1234. doi: 10.1128/mcb.3.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. B., Goorha R., Chinchar V. G. Macromolecular synthesis in cells infected by frog virus 3. Curr Top Microbiol Immunol. 1985;116:77–106. doi: 10.1007/978-3-642-70280-8_5. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Goorha R., Granoff A. Nongenetic reactivation of frog virus 3 DNA. Virology. 1979 Oct 30;98(2):476–479. doi: 10.1016/0042-6822(79)90572-5. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Goorha R., Miles M., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. VII. Transcriptional and post-transcriptional regulation of virus gene expression. J Virol. 1977 Oct;24(1):326–342. doi: 10.1128/jvi.24.1.326-342.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. B., Granoff A. Macromolecular synthesis in cells infected by frog virus 3. IX. Two temporal classes of early viral RNA. Virology. 1978 May 15;86(2):443–453. doi: 10.1016/0042-6822(78)90084-3. [DOI] [PubMed] [Google Scholar]
- Willis D. B., Granoff A. Macromolecular synthesis in cells infected with frog virus 3. V. The absence of polyadenylic acid in the majority of frog virus 3-specific mRNA species. Virology. 1976 Sep;73(2):543–547. doi: 10.1016/0042-6822(76)90417-7. [DOI] [PubMed] [Google Scholar]
- Willis D., Foglesong D., Granoff A. Nucleotide sequence of an immediate-early frog virus 3 gene. J Virol. 1984 Dec;52(3):905–912. doi: 10.1128/jvi.52.3.905-912.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]