Abstract
Coordinated proliferation and differentiation of growth plate chondrocytes is required for endochondral bone growth, but the mechanisms and pathways that control these processes are not completely understood. Recent data demonstrate important roles for nitric oxide (NO) and C-type natriuretic peptide (CNP) in the regulation of cartilage development. Both NO and CNP stimulate the synthesis of cGMP and thus the activation of common downstream pathways. One of these downstream mediators, cGMP-dependent kinase II (cGKII), has itself been shown to be essential for normal endochondral bone formation. This review summarizes our knowledge of the roles and mechanisms of NO, CNP and cGKII signaling in cartilage and endochondral bone development.
Keywords: nitric oxide, C-type natriuretic peptide, cGMP, cGMP-dependent kinase, cartilage, endochondral bone, MAPK pathways
Endochondral ossification
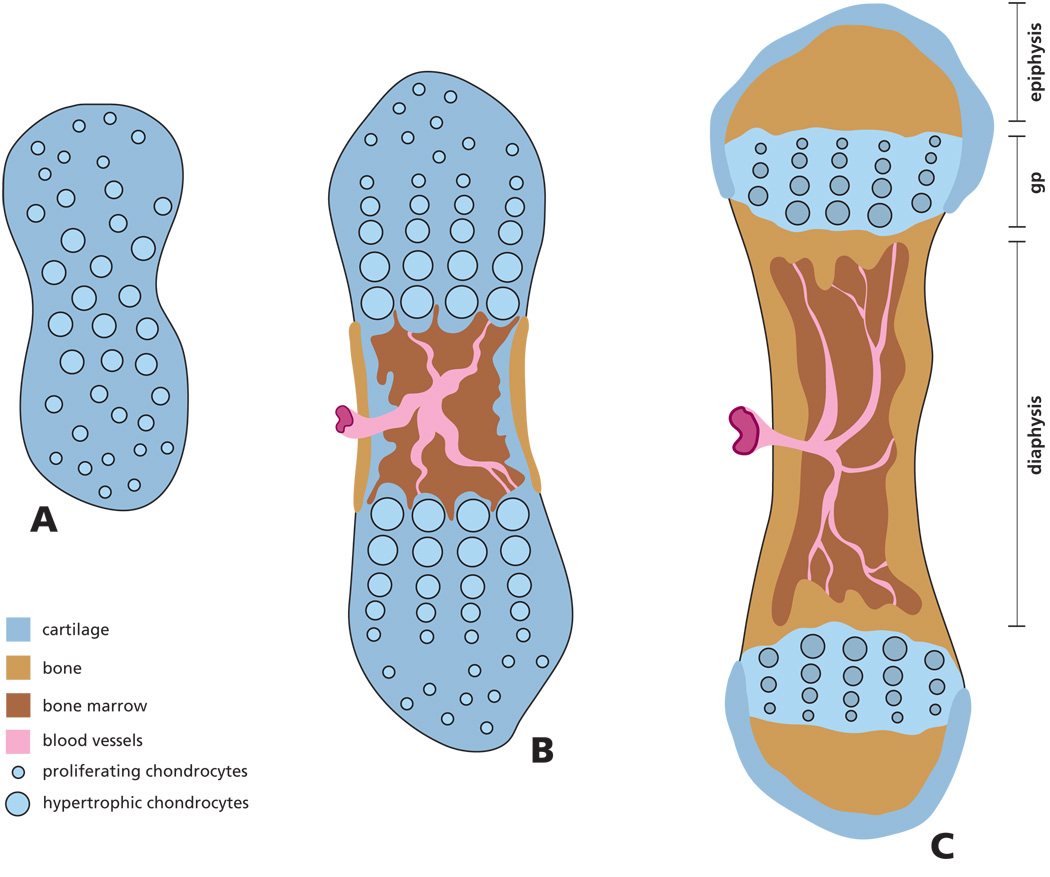
Skeletal development occurs through two different pathways: endochondral and intramembranous ossification. Intramembranous bone forms by direct differentiation of mesenchymal cells into osteoblasts (cranial vault bones). However, the process of endochondral ossification is responsible for the development of the majority of our bones. During this process, cartilage precursors are first generated and later replaced by bone (long bones, vertebral column, cranial base) (Karsenty and Wagner, 2002; Olsen et al., 2000; Provot and Schipani, 2005; Wagner and Karsenty, 2001). During this process, cartilage cells (chondrocytes) undergo a series of well-orchestrated phenotypic changes required for the development and growth of bone. Initially, mesenchymal precursor cells condense at the sites of future bone formation. Increased cell density and cell-cell interactions result in differentiation of these cells into chondrocytes, characterized by the expression of specific transcription factors (e.g. Sox9) and extracellular matrix (ECM) molecules such as collagen II and aggrecan (DeLise et al., 2000) (Figure 1). Cells in the center of these cartilage anlagen then differentiate further into large (hypertrophic) postmitotic chondrocytes. Cartilage cells on either side of the hypertrophic center start proliferating in an unidirectional manner along the longitudinal axis of the bone model, giving rise to the characteristic structure of the growth plate (Kronenberg, 2003) (Figure 1). In the growth plates, located at the end of the long bones, chondrocytes undergo a series of transitions that form distinct zones. In the resting zone, immature chondrocytes accelerate their rate of cell cycle progression and give rise to proliferative chondrocytes (Beier, 2005). After a number of divisions, these cells withdraw from the cell cycle and start terminal differentiation into hypertrophic chondrocytes. Finally, the hypertrophic zone of the growth plate becomes mineralized, is invaded by blood vessels, gradually resorbed and replaced by bone tissue.
Figure 1. Scheme of endochondral bone formation during development.
Initially the mesenchyme in the region of the future bone anlage differentiates into chondrocytes, forming a cartilage model of the future bone (A). Cells in the center of the cartilage model undergo further differentiation into hypertrophic cells inducing vascular invasion of the diaphysis and formation of a marrow cavity. As a result of osteoclast and osteoblast activity, the cartilage is resorbed and the first osteoid is deposited (B). Areas of secondary ossification develop in the epiphysis, while in the growth plate (gp), located between the epiphysis and diaphysis, columns of chondrocytes continue to proliferate, hypertrophy, secrete and mineralize the extracellular matrix (C). Over time this matrix is partially resorbed and replaced with new bone resulting in bone elongation.
Chondrocyte proliferation and hypertrophy, and ECM production, are the driving forces of longitudinal growth of endochondral bones. These processes are regulated by a plethora of hormones, growth factors and other signals, through both local and systemic mechanisms. The endocrine factors that control bone growth include growth hormone, glucocorticoids and thyroid hormones, while local factors include parathyroid hormone-related peptide and members of the transforming growth factor β, fibroblast growth factor, hedgehog and Wnt families, amongst others (Beier et al., 1999a; van der Eerden, 2003). The intracellular pathways that are triggered by many of these signals in chondrocytes are not well understood. The best characterized effectors include Sox9 and Runx2, two central transcription factors that regulate early chondrocyte differentiation and hypertrophic differentiation, respectively (Kronenberg, 2003; Kronenberg, 2006; Lefebvre and Smits, 2005). In recent years, nitric oxide and C-type natriuretic peptide have been identified as new regulators of endochondral bone growth that act through a common mediator, cGMP. The role of nitric oxide, C-type natriuretic peptide and the cGMP pathway in endochondral ossification is discussed in this review, and the pathways are outlined in Figure 2.
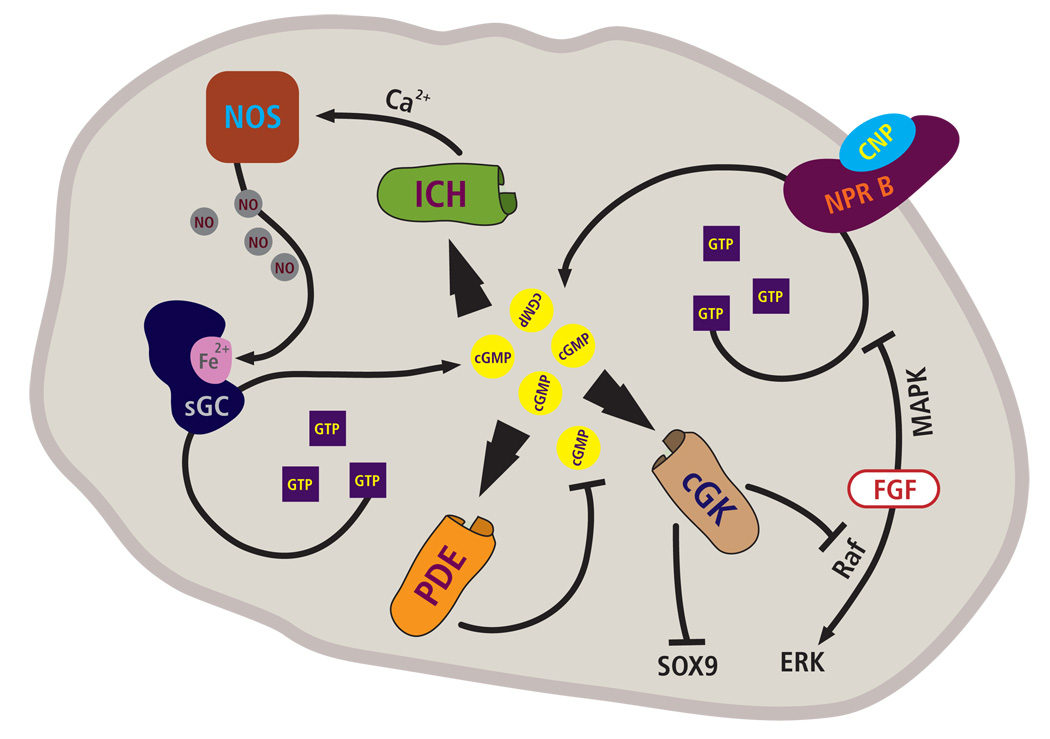
Figure 2. Scheme of cGMP pathways active in growth plate cartilage.
Nitric oxide (NO) generation by nitric oxide synthases (NOS) present in chondrocytes leads to activation of the soluble guanylate cyclase (sGC) and an increase in the intracellular levels of cGMP. Binding of the C-type natriuretic peptide (CNP) to the natriuretic peptide receptor B (NPR2) also increases cGMP levels in chondrocytes. The increase in cGMP concentration may lead to activation of phosphodiesterases (PDE), cGMP-regulated ion channels (ICH) and cGMP-dependent kinases (cGK). In particular, cGK II has been shown to play an important role in endochondral bone formation. Possible downstream pathways include the inhibition of nuclear translocation of the transcription factor Sox 9 (SOX9), and the repression of FGF signaling and ERK activation through Raf 1.
Nitric oxide in cartilage development
Nitric oxide (NO) was identified as the endothelial-derived relaxation factor (Ignarro et al., 1987; Palmer et al., 1987), and the importance of that pioneering work was recognized with the 1998 Nobel Prize in Physiology/Medicine. NO is an uncharged molecule with an unpaired electron, which makes it an ideal messenger molecule. Uncharged, NO can diffuse freely across membranes without need for a receptor and the unpaired electron makes it a highly reactive molecule (Lowenstein et al., 1994; Stamler et al., 1992). NO is synthesized enzymatically from L-arginine in the presence of oxygen by at least three different nitric oxide synthases. The neuronal (nNOS or NOS1) and the endothelial (eNOS or NOS3) isoforms are constitutively expressed, and their activity is regulated by intracellular calcium levels and the calcium binding protein calmodulin. The inducible isoform (iNOS or NOS2) is stimulated by factors that include lipopolysacharide and cytokines such as IL-1, TNFα and IFNα, and its activation leads to a sustained generation of NO (Stadler et al., 1991).
A well studied model of NO action involves its binding to the heme-containing soluble protein guanylate cyclase, causing increased enzymatic activity and formation of cGMP. Depending on the cell type, the increase in cGMP concentration may lead to activation of phosphodiesterases, cGMP-regulated ion channels and cGMP-dependent kinases (cGKs) (Figure 2) (Knowles and Moncada, 1998). NO may also change the enzymatic function of other heme-containing proteins, cyclooxygenase 1 and 2, and in this way modulate inflammatory responses (Stamler, 1994; Stamler et al., 1992). NO can influence the activity of a number of biochemical pathways by reacting with thiol-containing domains in a number of proteins, Enzymes that contain cysteines in their active sites, such as glyceraldehyde-3-phosphate, alcohol and aldehyde dehydrogenases, are most amenable to this form of control (Stamler, 1994), implicating NO in the regulation of cellular metabolism. Aside from its role in modulating energy status, NO can also serve to control cell proliferation (in Drosophila imaginal discs) and cell death in different systems (chondrocytes, hepatocytes, macrophages, neurons, and others) (Blanco and Lotz, 1995; Blanco et al., 1995; Kim et al., 2005; Kuzin et al., 1996; Marriott et al., 2004; Peunova and Enikolopov, 1998).
Research on the role of NO in the regulation of chondrocyte function has focused on the articular chondrocyte and the osteoarthritic joint cartilage. NO plays a role in almost every aspect of osteoarthritis pathophysiology. The effects of NO on articular chondrocytes include inhibition of cartilage matrix synthesis, acceleration of chondrocyte-mediated matrix degradation, promotion of chondrocyte inflammatory responses, and chondrocyte apoptosis (Abramson et al., 2001; Amin and Abramson, 1998; Lotz, 1999). Interestingly, our recent studies have shown that the three NOS isoforms are also expressed by growth plate chondrocytes, and NO adducts (nitroso cysteine, nitrotyrosine) accumulate in the hypertrophic and calcified regions of the chick cartilage (Teixeira et al., 2005). In culture, growth plate chondrocytes can generate levels of NO metabolites comparable to articular chondrocytes (Hauselmann et al., 1998; Palmer et al., 1993; Taskiran et al., 1994) as well as other cells (Chang et al., 1996; MacPherson et al., 1999). Furthermore, both cGMP-dependent and -independent pathways mediate the effect of NO in growth plate chondrocytes. Using an in vitro model for chondrocyte maturation we have found that NO donors (S-nitrosoglutathione, S-Nitroso-N-acetyl-DL-penicillamine, spermine–nitric oxide) or transfection with eNOS enhanced the expression of markers of hypertrophy, such as type X collagen and alkaline phosphatase activity. Conversely, inhibition of NOS (NG-nitro-L-arginnine methyl ester, NG-Monomethyl-L-arginnine), guanylate cyclase (1H-[1,2,4] Oxadiazolo[4,3-a]quinoxalin-1-one) or cGKs (KT 5823) blocked retinoic acid-induced chondrocyte maturation (Teixeira et al., 2003). Once chondrocytes became hypertrophic, the same NO donors were capable of inducing chondrocyte apoptosis in a cGMP-independent manner. In cultured hypertrophic chondrocytes, blocking NO generation with NOS inhibitors prevented loss of reduced thiols, mitochondria depolarization, and ultimately phosphate-induced apoptosis (Teixeira et al., 2001). These in vitro data suggest that distinct NO pathways may be active at different stages of chondrocyte differentiation and maturation during endochondral bone formation.
However, the in vivo role of NO and the different NOS genes in endochondral ossification still awaits detailed analyses. The eNOS knockout mice present limb deficiencies, with terminal and transverse defects of both hind limbs and forelimbs. The weight and crown-rump length were significantly reduced (10%) in the knockout animals, with continued growth delays from E17 to 21 days postnatal. Other abnormalities ranged from meromelia to syndactyly, and hypoplasia of carpals, metacarpals, radius and ulna (Table 1). It was proposed that these abnormalities were caused by insufficient blood flow to the limbs and hemorrhage. Microscopic findings were consistent with vascular etiology, with constricted arterioles, hemorrhage and necrosis observed in the area of the cartilage anlagen. While no growth abnormalities have been described for the iNOS and nNOS knockout animals, suggesting compensatory interactions among the different isoforms, the administration of NOS inhibitors to the drinking water of pregnant rats induced fetal growth retardation and hind limb disruptions in the pups (Diket et al., 1994). These alterations were also attributed to disturbed blood flow to the developing limbs, but direct effects on skeletal elements cannot be excluded and await further investigation.
Table 1.
Mutants in the natriuretic peptide and nitric oxide pathways with skeletal phenotypes
| Common Name | Gene Name | Mutation | skeletal phenotype | Reference |
|---|---|---|---|---|
| CNP | Nppc (Human) | translocation, overexpression | tall stature, bone abnormalities | Bocciardi et al. 2007 |
| Nppc (Mouse) | KO | dwarfism, smaller, proliferative and hypertrophic zones in growth plate | Chusho et al. 2001 | |
| cartilage overexpression | rescues effects of Nppc KO rescues model of achondrodysplasia | Chusho et al. 2001 | ||
| BNP | Nppb (Mouse) | ubiquitous overexpression | skeletal overgrowth | Suda et al. 1998 |
| NPR2 | NPR2 (Human) | loss-of-function | acromesomelic dysplasia, type Maroteaux short stature | Bartels et al. 2004 |
| Npr2 (Mouse) | natural loss-of-function | cn mice, dwarfism, short limbs | Tamura et al. 2004 | |
| KO | dwarfism, reduced endochondral bone growth | Tsuji et al. 2005 | ||
| NPR3 | Npr3 (Mouse) | natural loss-of-function | skeletal overgrowth | Jaubert et al. 1999 |
| KO | overgrowth of long bones increased skeletal turnover | Matsukawa et al. 1999 | ||
| cGK II | Prkg2 (Mouse) | KO | dwarfism, disorganized growth plate | Pfeifer et al. 1996 |
| Prkg2 (Rat) | natural loss-of-function | Komeda miniature rat Ishikawa dwarfism, abnormal growth plates | Chikuda et al. 2004 | |
| eNOS | NOS3 (Mouse) | KO | limb abnormalities (low penetrance) short digits | Gregg et al. 1998 Hefler et al. 2001 |
Interestingly, the double knockouts for two NOS isofoms (including eNOS) do not present significant growth defects, suggesting that the presence of a single NOS isoform allows compensatory NO production (Tranguch and Huet-Hudson, 2003). However, increased embryonic lethality in double knockouts suggests the occurrence of major developmental abnormalities (that were not investigated further) and underlines the importance of these pathways during development. Mice lacking all three NOS isoforms also have a low number of offspring as well as a very low survival rate (Morishita et al., 2005; Tsutsui et al., 2006). Postnatal death occurred mostly due to cardiovascular disease. Surprisingly, no gross growth defects were described for the surviving triple knockout suggesting that the phenotype is variable, ranging from very mild phenotypes to lethality. A detailed study of their skeletal development has not been completed, neither during embryonic development nor postnatally. Therefore, while our in vitro studies clearly show a role for NO in growth plate cartilage, no definite conclusion can be drawn from current in vivo observations.
NO is also an important signaling molecule in bone in response to different stimuli including inflammatory cytokines and mechanical stress (Klein-Nulend et al., 1998; MacPherson et al., 1999; van't Hof and Ralston, 2001). Major defects in osteoblast activity (reduced proliferation, differentiation and mineralization) have been reported both in vivo and in vitro, in eNOS knockout animals (Aguirre et al., 2001; Armour et al., 2001), and NO produced by eNOS mediates the effect of sex hormones in bone (Grassi et al., 2006; Wimalawansa et al., 1996). Furthermore, NOS inhibitors or high concentrations of NO reduce the formation, activity and motility of osteoclasts (Brandi et al., 1995). The nNOS knockout mice have reduced osteoclast numbers and activity both in vivo and in vitro (Jung et al., 2003). However, the study of the eNOs and iNOS deficient mice showed no major defect in bone resorption, suggesting some NO effects on osteoclasts are indirect, either mediated by osteoblasts and/or inflammatory pathways (van't Hof and Ralston, 2001). While these NO effects on bone function could help explain some of the growth abnormalities in the knockout animals, our previous work suggests that the process of endochondral bone formation is affected by the altered activity of growth plate chondrocytes themselves. Due to indirect effects of NO in different cell types involved in endochondral ossification (osteoblast, chondrocytes, osteoclasts, endothelial cells), clarification of the role of NO in this process will require generation and analyses of tissue specific knockout mice.
C-type natriuretic peptide in endochondral ossification
The second main class of cGMP inducers are the natriuretic peptides, ANP (atrial natriuretic peptide), BNP (brain natriuretic peptide) and CNP (C-type natriuretic peptide). These are secreted peptides that control cell behavior through activation of two transmembrane receptors, NPR1 and NPR2 (natriuretic peptide receptor 1 and 2) (Anand-Srivastava, 2005; Baxter, 2004; Cea, 2005; Leist et al., 1997; Potter et al., 2005). Importantly, these receptors possess guanylyl cyclase activity, synthesize cGMP in response to ligand binding and are thus also known as GC-A and GC-B (guanylyl cyclase A and B). ANP and BNP signal mainly through NPR1/GC-A, while CNP predominantly activates NPR2/GC-B. All three ligands also bind to a third receptor, NPR3, that is thought to act mainly as a clearance receptor that limits ligand availability and natriuretic peptide signaling.
While several in vitro studies had suggested a role of natriuretic peptide signaling in chondrocytes (Fujishige et al., 1999; Hagiwara et al., 1996; Hagiwara et al., 1994; Mericq et al., 2000; Yasoda et al., 1998), the importance of this pathway for endochondral ossification was only fully recognized after disruption of the CNP gene in mice (Chusho et al., 2001). The most striking phenotype of these mice is severe dwarfism caused by direct effects on growth plate chondrocytes. At birth, these mice_display a 10 % reduction in length, but the growth retardation becomes more severe postnatally, and about 70 % of null mice die in the first 100 days after birth. Notably, all endochondral bones examined (e.g. long bones, vertebrae) show growth reduction, whereas intramembraneous bones were of normal size. Furthermore, cartilage-specific overexpression of CNP rescued the phenotype of CNP-deficient mice, strongly suggesting that CNP stimulates bone growth through direct effects on chondrocytes (rather than systemically). In agreement with this phenotype, organ culture studies of endochondral bones showed that exogenous CNP is a potent stimulator of longitudinal bone growth (Mericq et al., 2000; Yasoda et al., 1998). Subsequent to the description of CNP-deficient mice, it was shown that targeted inactivation as well as naturally occurring mutations of the Npr2 gene (encoding the CNP receptor) result in a similar phenotype (Tsuji and Kunieda, 2005; Yasoda et al., 2004). In particular, Npr2-null mice display significantly reduced postnatal growth of endochondral, but not intramembranous, bones and increased mortality (Yasoda et al., 2004). In contrast, both natural and engineered loss-of-function mutants of the Npr3 gene show the opposite phenotype of skeletal overgrowth (Jaubert et al., 1999; Matsukawa et al., 1999). These findings support a model where CNP promotes endochondral bone growth through NPR2, whereas the decoy receptor NPR3 antagonizes this activity. In contrast, mice deficient for ANP, BNP or NPR1 genes do not display skeletal phenotypes (Enomoto et al., 2000; Jimenez et al., 1999; John et al., 1995; John et al., 1996), suggesting that these genes play minor role, if any, in endochondral ossification. Overexpression of BNP in transgenic mice has been shown to cause skeletal overgrowth, but this is most likely due to stimulation of NPR2 by unphysiologically high levels of BNP (Suda et al., 1998).
The cellular mechanisms of CNP action on chondrocytes are complex. The proliferative zone of the growth plate is shortened in mice deficient for CNP or NPR2, but while CNP-null mice show lower proliferation rates (Chusho et al., 2001), these rates appear to be unchanged in the absence of NPR2 (Yasoda et al., 2004). One possible explanation for these contradictory results is that CNP might not affect the rate of cell cycle progression while cells proliferate (e.g. no differences in the proportion of cells in the S-phase within the proliferative zone), but possibly controls the timing of cell cycle exit, thus affecting the length of the proliferating zone and the proportion of S phase cells within the context of the entire growth plate. While the effects of CNP on chondrocyte proliferation are still not completely clear, the more dramatic phenotype in both CNP- and NPR2-null mice was the shortening of the hypertrophic zone. Similarly, CNP treatment of endochondral bones in organ cultures resulted in longer hypertrophic zones (Agoston et al., 2007; Mericq et al., 2000), due to increased number and size of hypertrophic chondrocytes. No differences in chondrocyte apoptosis were observed in CNP-deficient mice (Chusho et al., 2001). Finally, CNP has been shown to increase the production of extracellular matrix (ECM) and the expression of genes involved in proteoglycan synthesis (Krejci et al., 2005; Mericq et al., 2000; Woods et al., 2007) and to suppress the expression of enzymes involved in ECM breakdown (Krejci et al., 2005). Together, these data suggest a model where CNP promotes endochondral bone growth through several mechanisms, including the stimulation of chondrocyte proliferation, the promotion of chondrocyte hypertrophy and the enhancement of extracellular matrix synthesis by chondrocytes.
Another landmark discovery was the identification of mutations in the human NPR2 gene as the cause of acromesomelic dysplasia, type Maroteaux, a rare human chondrodysplasia characterized by dwarfism (Bartels et al., 2004). Heterozygosity for this mutation is associated with short stature (Olney et al., 2006). The fact that loss of one functional allele of NPR2 is sufficient to affect height confirms the clinical importance of the CNP signaling system in controlling endochondral bone growth. Very recently, skeletal overgrowth in one patient was correlated with increased CNP expression due to a chromosomal translocation involving the NCCP gene that encodes CNP (Bocciardi et al., 2007).
The importance of the CNP signaling system in mammalian endochondral ossification is now clearly established, and focus has recently shifted towards the analyses of both the upstream regulators and the downstream mechanisms of CNP signaling in skeletal development. CNP is thought to act in an auto-/paracrine manner, but is expressed in and acts on multiple tissues, some of which have the potential to indirectly control bone growth. For example, CNP controls pituitary growth hormone secretion (McArdle et al., 1994; Shimekake et al., 1994) which is known to regulate endochondral bone growth. However, chondrocytes express both CNP and NPR2 (Chusho et al., 2001), suggesting that local effects constitute the majority of the anabolic effects of this pathway. This model is further supported by the observation that CNP overexpression in cartilage rescues the effects of the CNP knockout (Chusho et al., 2001) and that CNP application in organ cultures mimics these effects. However, the question arises how CNP signaling is coordinated with the many other signaling systems controlling endochondral ossification. Whether the CNP axis is regulated by systemic/endocrine factors is of particular interest. Our recent in vitro studies have shown that the glucocorticoid Dexamethasone increases CNP mRNA expression in chondrocytes (Agoston et al., 2006) however, since glucocorticoids (at least in the applied pharmacological doses) suppress bone growth (Baron et al., 1992; Robson et al., 2002; Siebler et al., 2001), the physiological significance of these data needs to be established.
Using microarray analyses of microdissected mouse tibiae, we determined that CNP, NPR2 and NPR3 are expressed in all zones of the growth plate (Agoston et al., 2007). Interestingly, transcript levels for both cGKI and II are strongly upregulated during chondrocyte differentiation, in agreement with earlier immunohistochemistry data (Pfeifer et al., 1996). However, these data also identified a feedback loop where endogenous CNP induces the expression of its decoy receptor NPR3. A different feedback loop has recently been described in smooth muscle cells where CNP suppresses expression of NPR2 (Rahmutula and Gardner, 2005). Thus, it appears that the CNP signaling system exerts a high level of self-control to fine tune its activity. However, very little is known about regulation of CNP signaling components by other local or systemic factors involved in skeletal growth control, with exception of the crosstalk with fibroblast growth factor signaling described below.
CNP effectors
In contrast to the upstream regulators, more insight has been obtained into the downstream mechanisms of CNP signaling in cartilage. CNP induces synthesis of cGMP and thus activation of several downstream pathways (Figure 2), namely cGMP-dependent kinases, phosphodiesterases and specific ion channels. Since NO also induces cGMP synthesis, it is likely that it shares many of the biological activities of CNP described below; however, this has not been formally demonstrated (to our knowledge).
To date, analyses of cGMP effectors in cartilage have been largely restricted to cGKs. The description of growth plate deficiencies in cGKII null mice (Pfeifer et al., 1996) predated the recognition of CNP’s importance in cartilage. The cartilage phenotype of cGKII null mice shows both similarities with and differences from that of CNP null mice. CNP-deficient mice display greater and earlier growth reduction and increased postnatal lethality than cGKII null mice, although disorganization of the growth plate appears to be more severe in cGKII mutant mice (Pfeifer et al., 1996). Notably, cGKII deficiency blocks the anabolic effects of CNP overexpression in the cartilage of transgenic mice, demonstrating that cGKII is required for CNP effects on bone growth (Miyazawa et al., 2002). However, these data do not exclude a) additional (although potentially minor) roles of cGKI or other effectors of cGMP in CNP signaling, and b) contributions from additional upstream activators of cGKII (such as nitric oxide) in cartilage. A loss-of-function mutation in the cGKII gene has also recently been identified as the cause of dwarfism in the naturally occurring Komeda miniature rat Ishikawa (KMI) strain (Chikuda et al., 2004).
Identification of downstream mediators of cGKs is currently under way in several laboratories. It was noticed early on that CNP-deficient mice displayed features of achondroplasia, the most common form of human dwarfism. Achondroplasia is caused by activating mutations in the fibroblast growth factor receptor 3 (FGFR3) gene (Bellus et al., 1995; Bonaventure et al., 1996; Rousseau et al., 1994; Shiang et al., 1994), resulting in constitutive activation of the receptor and downstream pathways, including STAT1 and ERK1/2 signaling (Ornitz, 2005). Yasoda and colleagues (Yasoda et al., 2004) demonstrated that overexpression of CNP in cartilage rescues dwarfism and other phenotypes in a mouse model of achondroplasia. Not only does this study suggest a potential for CNP in the treatment of some forms of human dwarfism, it also demonstrates that the FGF and CNP signaling pathways are intimately linked in the control of endochondral bone growth. Yasoda et al. showed that CNP signaling blocks activation of the ERK (but not the STAT1) pathway by FGF signaling. These findings were further substantiated and extended by subsequent studies demonstrating that CNP repression of ERK activation requires cGK activity and occurs at the level of the MAP kinase kinase kinase Raf1 (Krejci et al., 2005), a major mediator of FGFR3 signaling that has been implicated in control of chondrocyte differentiation (Beier et al., 1999b; Beier et al., 1999c). In addition, it was shown that interaction between the two pathways is reciprocal in that FGF signaling blocks CNP-induced cGMP production in a MAPK-dependent manner (Yasoda et al., 2004).
p38 kinases constitute a second MAPK pathway that controls chondrocyte differentiation in vitro and in vivo (Agoston et al., 2007; Zhang et al., 2006; Zhen et al., 2001). Our recent data show that CNP activates the p38 pathway in primary chondrocytes and in tibia organ cultures (Agoston et al., 2007). Moreover, pharmacological inhibition of p38 blocks the anabolic effects of CNP in mouse tibia organ cultures. However, not all effects of CNP in organ cultures required p38 activity; for example, CNP induction of several downstream genes was not affected by p38 inhibitors. In addition to these two MAP kinase pathways, our results also suggest roles for the PI3K/Akt pathway in CNP-induced bone growth (Ulici et al., 2008) , suggesting a complex network of signaling interactions in response to CNP.
Another mechanism of action for cGK signaling in cartilage was elucidated in studies of the KMI rat (Chikuda et al., 2004). The authors showed that cGKII signaling is required to prevent entry of the chondrogenic transcription factor Sox9 into the cell nucleus. Downregulation of Sox9 activity is required for hypertrophic differentiation, but loss of cGKII leads to prolonged activity of Sox9, resulting in loss of coordination between chondrocyte proliferation and differentiation and thus in dwarfism. While cGKII was shown to induce Sox9 phosphorylation in this study, it is not clear whether this occurs through direct interaction or involves intermediary kinases. In addition, phosphorylation does not appear to be required for inactivation of Sox9 by cGKII.
Open Questions
Over the last few years, significant progress has been made in our understanding of NO, CNP and cGMP signaling during cartilage development. Findings from human diseases, spontaneous mutations in rodents and genetically engineered mice have demonstrated unequivocally that the CNP-cGMP pathway is a central regulator of endochondral ossification. However, numerous questions and challenges remain and need to be solved in order to contemplate therapeutic applications. For example, the role of the nitric oxide pathway in endochondral ossification in vivo is still poorly understood and will require detailed analyses of knockout mice for single and multiple NOS genes. The contribution of cGMP effectors other than cGKs to the activities of NO and CNP needs to be investigated. Interaction of the different downstream effectors of cGMP/cGKs – such as the various MAP kinases and other kinases – has not been addressed. Substrates of these kinases in chondrocytes (e.g. transcription factors) and direct target genes of the cGMP pathway need to be identified. While we have recently performed microarray studies on CNP-treated tibiae (Agoston et al., 2007), these were done at a late time point (six days of stimulation) and not designed to identify direct target genes.
Interaction of the CNP pathway with other signals (in addition to FGFs) in endochondral ossification needs to be examined in detail. Are components of the NO/CNP/cGMP pathways regulated by other systemic or paracrine regulators of endochondral bone growth, such as growth hormone, Indian hedgehog or parathyroid hormone-related peptide? Conversely, do CNP and/or NO regulate these pathways? Our microarray analyses indeed identified factors such as the BMP family member GDF15 as target genes of CNP in chondrocytes (Agoston et al., 2007), but detailed analyses of effects on spatial and temporal expression patterns of key regulators of cartilage development (e.g. FGFR3, IHH etc.) still needs to be performed. Another question is whether and where NO/CNP and other pathways intersect in the intracellular signaling cascades or at specific target genes. Mouse models with deficiencies in NO and CNP pathways show clear differences as discussed above. In general, interruption of CNP signaling appears to have more drastic consequences than disturbance of NO signaling. Potential explanations for these discrepancies include greater redundancy among NO-producing enzymes, differential coupling to downstream mediators, additional roles in other cell types (e.g. endothelial cells) and potential effects of genetic background.
In addition, it will be of great interest to further examine the roles of NO and CNP in articular chondrocytes and osteoarthritis. While NO is an established player in this context (Abramson et al., 2001; Finger et al., 2003; Scher et al., 2007), CNP has, to our knowledge, never been implicated in osteoarthritis. However, a recent study shows that CNP can induce hypertrophy in articular chondrocytes (Johnson et al., 2003), which is thought to contribute to disease progression. Thus, while current evidence suggests that CNP/cGMP signaling might be of use in the treatment of growth retardation, potential detrimental effects on articular cartilage need to be considered.
Another unexplored area is the relationship between CNP and NO signaling. While both pathways converge on a common mediator (cGMP), it is not clear whether they act in an additive or synergistic manner, or whether both have unique roles based on developmental stage and/or location within the body. Compound mutants will be very useful to address these questions (e.g. do double knockout mice for CNP and a NOS gene have a more severe phenotype? Can overexpression of a NOS gene in cartilage rescue the effects of CNP deficiency?). Similarly, we don’t know whether these two pathways directly regulate each other; for example, our data show upregulation of Npr3 expression in response to CNP – can increased NO levels perform the same function? Does CNP affect NO levels and/or NOS expression and function?
While numerous questions remain to be answered, the combination of high throughput technologies in genomics and proteomics with sophisticated in vivo models and real-time imaging of signaling processes will solve many of these questions over the next years.
Acknowledgements
Cristina Teixeira’s work was supported by NIDCR grant 5K08DE017426 and the Portuguese Foundation for Science and Technology. Hanga Agoston was supported by Ontario Graduate Scholarships. Frank Beier is a Canada Research Chair and holds operating grants from the Canadian Institutes of Health Research, The Arthritis Society and the Canadian Arthritis Network. Illustrations were created by Nuno Pontes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramson SB, Attur M, Amin AR, Clancy R. Nitric oxide and inflammatory mediators in the perpetuation of osteoarthritis. Curr Rheumatol Rep. 2001;3:535–541. doi: 10.1007/s11926-001-0069-3. [DOI] [PubMed] [Google Scholar]
- Agoston H, Baybayan L, Beier F. Dexamethasone stimulates expression of C-type Natriuretic Peptide in chondrocytes. BMC Musculoskelet Disord. 2006;7:87. doi: 10.1186/1471-2474-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agoston H, Khan S, James CG, Gillespie JR, Serra R, Stanton LA, Beier F. C-type natriuretic peptide regulates endochondral bone growth through p38 MAP kinase-dependent and -independent pathways. BMC Dev Biol. 2007;7:18. doi: 10.1186/1471-213X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre J, Buttery L, O'Shaughnessy M, Afzal F, Fernandez, d. M. I, Hukkanen M, Huang P, MacIntyre I, Polak J. Endothelial nitric oxide synthase gene-deficient mice demonstrate marked retardation in postnatal bone formation, reduced bone volume, and defects in osteoblast maturation and activity. Am.J.Pathol. 2001;158:247–257. doi: 10.1016/S0002-9440(10)63963-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin AR, Abramson SB. The role of nitric oxide in articular cartilage breakdown in osteoarthritis. Curr.Opin.Rheumatol. 1998;10:263–268. doi: 10.1097/00002281-199805000-00018. [DOI] [PubMed] [Google Scholar]
- Anand-Srivastava MB. Natriuretic peptide receptor-C signaling and regulation. Peptides. 2005;26:1044–1059. doi: 10.1016/j.peptides.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Armour KE, Armour KJ, Gallagher ME, Godecke A, Helfrich MH, Reid DM, Ralston SH. Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase. Endocrinology. 2001;142:760–766. doi: 10.1210/endo.142.2.7977. [DOI] [PubMed] [Google Scholar]
- Baron J, Huang Z, Oerter KE, Bacher JD, Cutler GB., Jr Dexamethasone acts locally to inhibit longitudinal bone growth in rabbits. Am J Physiol. 1992;263:E489–E492. doi: 10.1152/ajpendo.1992.263.3.E489. [DOI] [PubMed] [Google Scholar]
- Bartels CF, Bukulmez H, Padayatti P, Rhee DK, van Ravenswaaij-Arts C, Pauli RM, Mundlos S, Chitayat D, Shih LY, Al-Gazali LI, Kant S, Cole T, Morton J, Cormier-Daire V, Faivre L, Lees M, Kirk J, Mortier GR, Leroy J, Zabel B, Kim CA, Crow Y, Braverman NE, van den Akker F, Warman ML. Mutations in the transmembrane natriuretic peptide receptor NPR-B impair skeletal growth and cause acromesomelic dysplasia, type Maroteaux. Am J Hum Genet. 2004;75:27–34. doi: 10.1086/422013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter GF. The natriuretic peptidesAn introduction. Basic Res Cardiol. 2004;99:71–75. doi: 10.1007/s00395-004-0457-8. [DOI] [PubMed] [Google Scholar]
- Beier F. Cell-cycle control and the cartilage growth plate. J Cell Physiol. 2005;202:1–8. doi: 10.1002/jcp.20111. [DOI] [PubMed] [Google Scholar]
- Beier F, Leask TA, Haque S, Chow C, Taylor AC, Lee RJ, Pestell RG, Ballock RT, LuValle P. Cell cycle genes in chondrocyte proliferation and differentiation. Matrix Biology. 1999a;18:109–120. doi: 10.1016/s0945-053x(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Beier F, Taylor AC, LuValle P. The Raf-1/MEK/ERK pathway regulates the expression of the p21(Cip1/Waf1) gene in chondrocytes. J Biol Chem. 1999b;274:30273–30279. doi: 10.1074/jbc.274.42.30273. [DOI] [PubMed] [Google Scholar]
- Beier F, Taylor AC, LuValle P. Raf signaling stimulates and represses the human collagen X promoter through distinguishable elements. J Cell Biochem. 1999c;72:549–557. doi: 10.1002/(sici)1097-4644(19990315)72:4<549::aid-jcb10>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Bellus GA, Hefferon TW, Ortiz de Luna RI, Hecht JT, Horton WA, Machado M, Kaitila I, McIntosh I, Francomano CA. Achondroplasia is defined by recurrent G380R mutations of FGFR3. Am J Hum Genet. 1995;56:368–373. [PMC free article] [PubMed] [Google Scholar]
- Blanco FJ, Lotz M. IL-1-induced nitric oxide inhibits chondrocyte proliferation via PGE2. Experimental Cell Research. 1995;218:319–325. doi: 10.1006/excr.1995.1161. [DOI] [PubMed] [Google Scholar]
- Blanco FJ, Ochs RL, Schwarz H, Lotz M. Chondrocyte apoptosis induced by nitric oxide. American Journal of Pathology. 1995;146:75–85. [PMC free article] [PubMed] [Google Scholar]
- Bocciardi R, Giorda R, Buttgereit J, Gimelli S, Divizia MT, Beri S, Garofalo S, Tavella S, Lerone M, Zuffardi O, Bader M, Ravazzolo R, Gimelli G. Overexpression of the C-type natriuretic peptide (CNP) is associated with overgrowth and bone anomalies in an individual with balanced t(2;7) translocation. Hum Mutat. 2007 doi: 10.1002/humu.20511. [DOI] [PubMed] [Google Scholar]
- Bonaventure J, Rousseau F, Legeai-Mallet L, Le Merrer M, Munnich A, Maroteaux P. Common mutations in the gene encoding fibroblast growth factor receptor 3 account for achondroplasia, hypochondroplasia and thanatophoric dysplasia. Acta Paediatr Suppl. 1996;417:33–38. doi: 10.1111/j.1651-2227.1996.tb14291.x. [DOI] [PubMed] [Google Scholar]
- Brandi ML, Hukkanen M, Umeda T, Moradi-Bidhendi N, Bianchi S, Gross SS, Polak JM, MacIntyre I. Bidirectional regulation of osteoclast function by nitric oxide synthase isoforms. Proc Natl Acad Sci U S A. 1995;92:2954–2958. doi: 10.1073/pnas.92.7.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cea LB. Natriuretic peptide family: new aspects. Curr Med Chem Cardiovasc Hematol Agents. 2005;3:87–98. doi: 10.2174/1568016053544309. [DOI] [PubMed] [Google Scholar]
- Chang CC, McCormick CC, Lin AW, Dietert RR, Sung YJ. Inhibition of nitric oxide synthase gene expression in vivo and in vitro by repeated doses of endotoxin. American Journal of Physiology. 1996;271:G539–G548. doi: 10.1152/ajpgi.1996.271.4.G539. [DOI] [PubMed] [Google Scholar]
- Chikuda H, Kugimiya F, Hoshi K, Ikeda T, Ogasawara T, Shimoaka T, Kawano H, Kamekura S, Tsuchida A, Yokoi N, Nakamura K, Komeda K, Chung UI, Kawaguchi H. Cyclic GMP-dependent protein kinase II is a molecular switch from proliferation to hypertrophic differentiation of chondrocytes. Genes Dev. 2004;18:2418–2429. doi: 10.1101/gad.1224204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chusho H, Tamura N, Ogawa Y, Yasoda A, Suda M, Miyazawa T, Nakamura K, Nakao K, Kurihara T, Komatsu Y, Itoh H, Tanaka K, Saito Y, Katsuki M, Nakao K. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci U S A. 2001;98:4016–4021. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage. 2000;8:309–334. doi: 10.1053/joca.1999.0306. [DOI] [PubMed] [Google Scholar]
- Diket AL, Pierce MR, Munshi UK, Voelker CA, Eloby-Childress S, Greenberg SS, Zhang XJ, Clark DA, Miller MJ. Nitric oxide inhibition causes intrauterine growth retardation and hind-limb disruptions in rats [see comments] American Journal of Obstetrics & Gynecology. 1994;171:1243–1250. doi: 10.1016/0002-9378(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Enomoto-Iwamoto M, Iwamoto M, Nomura S, Himeno M, Kitamura Y, Kishimoto T, Komori T. Cbfa1 is a positive regulatory factor in chondrocyte maturation. J Biol Chem. 2000;275:8695–8702. doi: 10.1074/jbc.275.12.8695. [DOI] [PubMed] [Google Scholar]
- Finger F, Schorle C, Zien A, Gebhard P, Goldring MB, Aigner T. Molecular phenotyping of human chondrocyte cell lines T/C-28a2, T/C-28a4, and C-28/I2. Arthritis Rheum. 2003;48:3395–3403. doi: 10.1002/art.11341. [DOI] [PubMed] [Google Scholar]
- Fujishige K, Kotera J, Yanaka N, Akatsuka H, Omori K. Alteration of cGMP metabolism during chondrogenic differentiation of chondroprogenitor-like EC cells, ATDC5. Biochim Biophys Acta. 1999;1452:219–227. doi: 10.1016/s0167-4889(99)00141-x. [DOI] [PubMed] [Google Scholar]
- Grassi F, Fan X, Rahnert J, Weitzmann MN, Pacifici R, Nanes MS, Rubin J. Bone re/modeling is more dynamic in the endothelial nitric oxide synthase(−/−) mouse. Endocrinology. 2006;147:4392–4399. doi: 10.1210/en.2006-0334. [DOI] [PubMed] [Google Scholar]
- Hagiwara H, Inoue A, Furuya M, Tanaka S, Hirose S. Change in the expression of C-type natriuretic peptide and its receptor, B-type natriuretic peptide receptor, during dedifferentiation of chondrocytes into fibroblast-like cells. J Biochem (Tokyo) 1996;119:264–267. doi: 10.1093/oxfordjournals.jbchem.a021233. [DOI] [PubMed] [Google Scholar]
- Hagiwara H, Sakaguchi H, Itakura M, Yoshimoto T, Furuya M, Tanaka S, Hirose S. Autocrine regulation of rat chondrocyte proliferation by natriuretic peptide C and its receptor, natriuretic peptide receptor-B. J Biol Chem. 1994;269:10729–10733. [PubMed] [Google Scholar]
- Hauselmann HJ, Stefanovic-Racic M, Michel BA, Evans CH. Differences in nitric oxide production by superficial and deep human articular chondrocytes: implications for proteoglycan turnover in inflammatory joint diseases. Journal of Immunology. 1998;160:1444–1448. [PubMed] [Google Scholar]
- Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc.Natl.Acad.Sci.U.S.A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaubert J, Jaubert F, Martin N, Washburn LL, Lee BK, Eicher EM, Guenet JL. Three new allelic mouse mutations that cause skeletal overgrowth involve the natriuretic peptide receptor C gene (Npr3) Proc Natl Acad Sci U S A. 1999;96:10278–10283. doi: 10.1073/pnas.96.18.10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez MJ, Balbin M, Lopez JM, Alvarez J, Komori T, Lopez-Otin C. Collagenase 3 is a target of Cbfa1, a transcription factor of the runt gene family involved in bone formation. Mol Cell Biol. 1999;19:4431–4442. doi: 10.1128/mcb.19.6.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John SW, Krege JH, Oliver PM, Hagaman JR, Hodgin JB, Pang SC, Flynn TG, Smithies O. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995;267:679–681. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- John SW, Veress AT, Honrath U, Chong CK, Peng L, Smithies O, Sonnenberg H. Blood pressure and fluid-electrolyte balance in mice with reduced or absent ANP. Am J Physiol. 1996;271:R109–R114. doi: 10.1152/ajpregu.1996.271.1.R109. [DOI] [PubMed] [Google Scholar]
- Johnson KA, van Etten D, Nanda N, Graham RM, Terkeltaub RA. Distinct transglutaminase 2-independent and transglutaminase 2-dependent pathways mediate articular chondrocyte hypertrophy. J Biol Chem. 2003;278:18824–18832. doi: 10.1074/jbc.M301055200. [DOI] [PubMed] [Google Scholar]
- Jung JY, Lin AC, Ramos LM, Faddis BT, Chole RA. Nitric oxide synthase I mediates osteoclast activity in vitro and in vivo. J Cell Biochem. 2003;89:613–621. doi: 10.1002/jcb.10527. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Kim PK, Vallabhaneni R, Zuckerbraun BS, McCloskey C, Vodovotz Y, Billiar TR. Hypoxia renders hepatocytes susceptible to cell death by nitric oxide. Cell Mol Biol (Noisy-le-grand) 2005;51:329–335. [PubMed] [Google Scholar]
- Klein-Nulend J, Helfrich MH, Sterck JG, MacPherson H, Joldersma M, Ralston SH, Semeins CM, Burger EH. Nitric oxide response to shear stress by human bone cell cultures is endothelial nitric oxide synthase dependent. Biochem Biophys Res Commun. 1998;250:108–114. doi: 10.1006/bbrc.1998.9270. [DOI] [PubMed] [Google Scholar]
- Knowles RG, Moncada S. Nitric Oxide, mitochondria and cytotoxicity. In: Moncada GNS, Bagetta G, Higgs EA, editors. Nitric Oxide and the Cell. Princeton, New Jersey: Princeton University Press; 1998. pp. 1–9. [Google Scholar]
- Krejci P, Masri B, Fontaine V, Mekikian PB, Weis M, Prats H, Wilcox WR. Interaction of fibroblast growth factor and C-natriuretic peptide signaling in regulation of chondrocyte proliferation and extracellular matrix homeostasis. J Cell Sci. 2005;118:5089–5100. doi: 10.1242/jcs.02618. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM. PTHrP and skeletal development. Ann N Y Acad Sci. 2006;1068:1–13. doi: 10.1196/annals.1346.002. [DOI] [PubMed] [Google Scholar]
- Kuzin B, Roberts I, Peunova N, Enikolopov G. Nitric oxide regulates cell proliferation during Drosophila development. Cell. 1996;87:639–649. doi: 10.1016/s0092-8674(00)81384-7. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res C Embryo Today. 2005;75:200–212. doi: 10.1002/bdrc.20048. [DOI] [PubMed] [Google Scholar]
- Leist M, Volbracht C, Kuhnle S, Fava E, Ferrando-May E, Nicotera P. Caspase-mediated apoptosis in neuronal excitotoxicity triggered by nitric oxide. Molecular Medicine. 1997;3:750–764. [PMC free article] [PubMed] [Google Scholar]
- Lotz M. The role of nitric oxide in articular cartilage damage. Rheumatic Diseases Clinics of North America. 1999;25:269–282. doi: 10.1016/s0889-857x(05)70067-3. [DOI] [PubMed] [Google Scholar]
- Lowenstein CJ, Dinerman JL, Snyder SH. Nitric oxide: a physiologic messenger. Annals of Internal Medicine. 1994;120:227–237. doi: 10.7326/0003-4819-120-3-199402010-00009. [DOI] [PubMed] [Google Scholar]
- MacPherson H, Noble BS, Ralston SH. Expression and functional role of nitric oxide synthase isoforms in human osteoblast-like cells. Bone. 1999;24:179–185. doi: 10.1016/s8756-3282(98)00173-2. [DOI] [PubMed] [Google Scholar]
- Marriott HM, Ali F, Read RC, Mitchell TJ, Whyte MK, Dockrell DH. Nitric oxide levels regulate macrophage commitment to apoptosis or necrosis during pneumococcal infection. Faseb J. 2004;18:1126–1128. doi: 10.1096/fj.03-1450fje. [DOI] [PubMed] [Google Scholar]
- Matsukawa N, Grzesik WJ, Takahashi N, Pandey KN, Pang S, Yamauchi M, Smithies O. The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc Natl Acad Sci U S A. 1999;96:7403–7408. doi: 10.1073/pnas.96.13.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle CA, Olcese J, Schmidt C, Poch A, Kratzmeier M, Middendorff R. C-type natriuretic peptide (CNP) in the pituitary: is CNP an autocrine regulator of gonadotropes? Endocrinology. 1994;135:2794–2801. doi: 10.1210/endo.135.6.7988473. [DOI] [PubMed] [Google Scholar]
- Mericq V, Uyeda JA, Barnes KM, De Luca F, Baron J. Regulation of fetal rat bone growth by C-type natriuretic peptide and cGMP. Pediatr Res. 2000;47:189–193. doi: 10.1203/00006450-200002000-00007. [DOI] [PubMed] [Google Scholar]
- Miyazawa T, Ogawa Y, Chusho H, Yasoda A, Tamura N, Komatsu Y, Pfeifer A, Hofmann F, Nakao K. Cyclic GMP-dependent protein kinase II plays a critical role in C-type natriuretic peptide-mediated endochondral ossification. Endocrinology. 2002;143:3604–3610. doi: 10.1210/en.2002-220307. [DOI] [PubMed] [Google Scholar]
- Morishita T, Tsutsui M, Shimokawa H, Sabanai K, Tasaki H, Suda O, Nakata S, Tanimoto A, Wang KY, Ueta Y, Sasaguri Y, Nakashima Y, Yanagihara N. Nephrogenic diabetes insipidus in mice lacking all nitric oxide synthase isoforms. Proc Natl Acad Sci U S A. 2005;102:10616–10621. doi: 10.1073/pnas.0502236102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney RC, Bukulmez H, Bartels CF, Prickett TC, Espiner EA, Potter LR, Warman ML. Heterozygous mutations in natriuretic peptide receptor-B (NPR2) are associated with short stature. J Clin Endocrinol Metab. 2006;91:1229–1232. doi: 10.1210/jc.2005-1949. [DOI] [PubMed] [Google Scholar]
- Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- Ornitz DM. FGF signaling in the developing endochondral skeleton. Cytokine and Growth Factor Reviews. 2005;16:205–213. doi: 10.1016/j.cytogfr.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Hickery MS, Charles IG, Moncada S, Bayliss MT. Induction of nitric oxide synthase in human chondrocytes. Biochemical & Biophysical Research Communications. 1993;193:398–405. doi: 10.1006/bbrc.1993.1637. [DOI] [PubMed] [Google Scholar]
- Peunova N, Enikolopov G. Nitric oxide mediates differentiation and survival of neuronal cells. In: Moncada GNS, Bagetta G, Higgs EA, editors. Nitric Oxide and the Cell. Princeton, New Jersey: Princeton University Press; 1998. pp. 171–192. [Google Scholar]
- Pfeifer A, Aszodi A, Seidler U, Ruth P, Hofmann F, Fassler R. Intestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinase II. Science. 1996;274:2082–2086. doi: 10.1126/science.274.5295.2082. [DOI] [PubMed] [Google Scholar]
- Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic Peptides, Their Receptors and cGMP-dependent Signaling Functions. Endocr Rev. 2005 doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- Provot S, Schipani E. Molecular mechanisms of endochondral bone development. Biochem Biophys Res Commun. 2005;328:658–665. doi: 10.1016/j.bbrc.2004.11.068. [DOI] [PubMed] [Google Scholar]
- Rahmutula D, Gardner DG. C-Type Natriuretic Peptide Down-Regulates Expression of Its Cognate Receptor in Rat Aortic Smooth Muscle Cells. Endocrinology. 2005;146:4968–4974. doi: 10.1210/en.2005-0262. [DOI] [PubMed] [Google Scholar]
- Robson H, Siebler T, Shalet SM, Williams GR. Interactions between GH, IGF-I, glucocorticoids, and thyroid hormones during skeletal growth. Pediatr Res. 2002;52:137–147. doi: 10.1203/00006450-200208000-00003. [DOI] [PubMed] [Google Scholar]
- Rousseau F, Bonaventure J, Legeai-Mallet L, Pelet A, Rozet JM, Maroteaux P, Le Merrer M, Munnich A. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature. 1994;371:252–254. doi: 10.1038/371252a0. [DOI] [PubMed] [Google Scholar]
- Scher JU, Pillinger MH, Abramson SB. Nitric oxide synthases and osteoarthritis. Curr Rheumatol Rep. 2007;9:9–15. doi: 10.1007/s11926-007-0016-z. [DOI] [PubMed] [Google Scholar]
- Shiang R, Thompson LM, Zhu YZ, Church DM, Fielder TJ, Bocian M, Winokur ST, Wasmuth JJ. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994;78:335–342. doi: 10.1016/0092-8674(94)90302-6. [DOI] [PubMed] [Google Scholar]
- Shimekake Y, Ohta S, Nagata K. C-type natriuretic peptide stimulates secretion of growth hormone from rat-pituitary-derived GH3 cells via a cyclic-GMP-mediated pathway. Eur J Biochem. 1994;222:645–650. doi: 10.1111/j.1432-1033.1994.tb18908.x. [DOI] [PubMed] [Google Scholar]
- Siebler T, Robson H, Shalet SM, Williams GR. Glucocorticoids, thyroid hormone and growth hormone interactions: implications for the growth plate. Horm Res. 2001;56:7–12. doi: 10.1159/000048127. [DOI] [PubMed] [Google Scholar]
- Stadler J, Stefanovic-Racic M, Billiar TR, Curran RD, McIntyre LA, Georgescu HI, Simmons RL, Evans CH. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. Journal of Immunology. 1991;147:3915–3920. [PubMed] [Google Scholar]
- Stamler JS. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Suda M, Ogawa Y, Tanaka K, Tamura N, Yasoda A, Takigawa T, Uehira M, Nishimoto H, Itoh H, Saito Y, Shiota K, Nakao K. Skeletal overgrowth in transgenic mice that overexpress brain natriuretic peptide. Proc Natl Acad Sci U S A. 1998;95:2337–2342. doi: 10.1073/pnas.95.5.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskiran D, Stefanovic-Racic M, Georgescu H, Evans C. Nitric oxide mediates suppression of cartilage proteoglycan synthesis by interleukin-1. Biochemical & Biophysical Research Communications. 1994;200:142–148. doi: 10.1006/bbrc.1994.1426. [DOI] [PubMed] [Google Scholar]
- Teixeira CC, Ischiropoulos H, Leboy PS, Adams SL, Shapiro IM. Nitric oxide-nitric oxide synthase regulates key maturational events during chondrocyte terminal differentiation. Bone. 2005;37:37–45. doi: 10.1016/j.bone.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Teixeira CC, Mansfield K, Hertkorn C, Ischiropoulos H, Shapiro IM. Phosphate-induced chondrocyte apoptosis is linked to nitric oxide generation. Am.J.Physiol Cell Physiol. 2001;281:C833–C839. doi: 10.1152/ajpcell.2001.281.3.C833. [DOI] [PubMed] [Google Scholar]
- Teixeira CC, Rajpurohit R, Mansfield K, Nemelivsky YV, Shapiro IM. Maturation-dependent thiol loss increases chondrocyte susceptibility to apoptosis. J Bone Miner Res. 2003;18:662–668. doi: 10.1359/jbmr.2003.18.4.662. [DOI] [PubMed] [Google Scholar]
- Tranguch S, Huet-Hudson Y. Decreased viability of nitric oxide synthase double knockout mice. Mol Reprod Dev. 2003;65:175–179. doi: 10.1002/mrd.10274. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Kunieda T. A Loss-of-Function Mutation in Natriuretic Peptide Receptor 2 (Npr2) Gene Is Responsible for Disproportionate Dwarfism in cn/cn Mouse. J. Biol. Chem. 2005;280:14288–14292. doi: 10.1074/jbc.C500024200. [DOI] [PubMed] [Google Scholar]
- Tsutsui M, Shimokawa H, Morishita T, Nakashima Y, Yanagihara N. Development of genetically engineered mice lacking all three nitric oxide synthases. J Pharmacol Sci. 2006;102:147–154. doi: 10.1254/jphs.cpj06015x. [DOI] [PubMed] [Google Scholar]
- Ulici V, Hoenselaar KD, Gillespie JR, Beier F. The PI3K pathway regulates endochondral bone growth through control of hypertrophic chondrocyte differentiation. BMC Dev Biol. 2008;8:40. doi: 10.1186/1471-213X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van't Hof RJ, Ralston SH. Nitric oxide and bone. Immunology. 2001;103:255–261. doi: 10.1046/j.1365-2567.2001.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Eerden BCJ, Karperien M, Wit JM. Systemic and local regulation of the growth plate. Endocrine Reviews. 2003;24:782–801. doi: 10.1210/er.2002-0033. [DOI] [PubMed] [Google Scholar]
- Wagner EF, Karsenty G. Genetic control of skeletal development. Curr Opin Genet Dev. 2001;11:527–532. doi: 10.1016/s0959-437x(00)00228-8. [DOI] [PubMed] [Google Scholar]
- Wimalawansa SJ, De Marco G, Gangula P, Yallampalli C. Nitric oxide donor alleviates ovariectomy-induced bone loss. Bone. 1996;18:301–304. doi: 10.1016/8756-3282(96)00005-1. [DOI] [PubMed] [Google Scholar]
- Woods A, Khan S, Beier F. C-Type Natriuretic Peptide Regulates Cellular Condensation and Glycosaminoglycan Synthesis during Chondrogenesis. Endocrinology. 2007;148:5030–5041. doi: 10.1210/en.2007-0695. [DOI] [PubMed] [Google Scholar]
- Yasoda A, Komatsu Y, Chusho H, Miyazawa T, Ozasa A, Miura M, Kurihara T, Rogi T, Tanaka S, Suda M, Tamura N, Ogawa Y, Nakao K. Overexpression of CNP in chondrocytes rescues achondroplasia through a MAPK-dependent pathway. Nat Med. 2004;10:80–86. doi: 10.1038/nm971. [DOI] [PubMed] [Google Scholar]
- Yasoda A, Ogawa Y, Suda M, Tamura N, Mori K, Sakuma Y, Chusho H, Shiota K, Tanaka K, Nakao K. Natriuretic peptide regulation of endochondral ossification. Evidence for possible roles of the C-type natriuretic peptide/guanylyl cyclase-B pathway. J Biol Chem. 1998;273:11695–11700. doi: 10.1074/jbc.273.19.11695. [DOI] [PubMed] [Google Scholar]
- Zhang R, Murakami S, Coustry F, Wang Y, de Crombrugghe B. Constitutive activation of MKK6 in chondrocytes of transgenic mice inhibits proliferation and delays endochondral bone formation. PNAS. 2006;103:365–370. doi: 10.1073/pnas.0507979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen X, Wei L, Wu Q, Zhang Y, Chen Q. Mitogen-activated protein kinase p38 mediates regulation of chondrocyte differentiation by parathyroid hormone. J Biol Chem. 2001;276:4879–4885. doi: 10.1074/jbc.M004990200. [DOI] [PubMed] [Google Scholar]