Abstract
Previous studies using phenylethylamine psychostimulants such as amphetamine (AMPH) have demonstrated that pretreatment with a high dose of drug followed by a low-dose challenge injection (3h later) results in an exaggerated behavioral response. In order to explore the mechanism of this exaggerated or what has been suggested to be a “sensitized” response, we investigated the effects of methamphetamine (METH) in a similar treatment paradigm. The current study found that, as suggested by previous studies, a low-dose challenge with METH substantially increased the locomotor response in animals that received a high-dose pretreatment (3.5 h prior to challenge). We also observed that rats displayed an increase in the concentrations of METH and its metabolite AMPH in the striatum following the low-dose challenge of METH if they were pretreated with METH versus saline. A similar pattern for METH and AMPH levels were measured in the plasma. Taken together, these results suggest that the accumulation of drug in animals pretreated with high-dose METH contributes to the overall enhanced behavioral response following challenges with low doses of METH.
Keywords: methamphetamine, locomotor, behavior, striatum, plasma
1. Introduction
Administration of psychostimulants (e.g. amphetamine (AMPH), methamphetamine (METH)) increases locomotor and stereotypic behaviors likely modulated by elevated extracellular dopamine (DA) in the basal ganglia and limbic regions (Kuczenski et al., 1991; Robinson and Camp, 1990; Sharp et al., 1987). Such changes in DA function have been associated with not only motor control, but control of cognitive, emotional and motivational functions as well (DiChiara et al., 2004; Gulley et al., 2004; Nordahl et al., 2003; Volkow et al., 2002).
Using a high dose of AMPH (4.0 mg/kg) followed by a challenge with a low dose of AMPH (0.5 mg/kg) 3 h later, appears to alter the pattern and duration of locomotor responses (Kuczenski and Segal, 1999a, 1999b). Interestingly, this altered pattern of locomotor activity has been speculated to reflect changes in DA release (Kuczenski and Segal, 1989, 1999a, 1999b; Kuczenski et al., 1997) or brain tissue levels of AMPH (Kuczenski and Segal, 1994; Kuczenski et al., 1997). Of interest, the “typical” locomotor activity pattern following a low-dose challenge by AMPH returns if the time between the injections is increased from 3 to 5 h suggesting that DA response to the drug has returned to “normal” (Kuczenski and Segal, 1999a). Thus, Kuczenski and Segal (1999a,b) propose that the exaggerated response of DA systems to AMPH low-dose challenge is due to increased sensitivity of DA receptors. According to this hypothesis, the priming dose of the AMPH pretreatment temporarily sensitizes DA receptors such that locomotor responses to the concentrations of DA released following the low dose challenge by AMPH are exaggerated (Kuczenski and Segal 1999a,b).
Similar to the effects of AMPH, METH produces a dramatic increase in extracellular levels of DA in limbic and basal ganglia structures contributing to a constellation of locomotor behavioral effects. Using a similar dosing paradigm to Kuczenski and Segal, the current study tested whether a low-dose challenge of METH resulted in exaggerated locomotor responses in animals receiving a high-dose METH pretreatment 3.5 h earlier. Preliminary studies determined that METH doses of 4.0 mg/kg and a challenge of 0.4 mg/kg produced similar exaggerated levels of locomotor activity as the previous studies by Kuczenski and Segal using AMPH. In order to investigate a possible pharmacokinetic role in this phenomenon, striatal and plasma drug levels of METH and its metabolite AMPH were measured at multiple time points following the challenge injection. Time points were chosen to correspond with different “phases” of locomotor activation as determined by preliminary studies in our lab and others (e.g. Kuczenski and Segal 1999a,b). Our data suggest that the pharmacokinetic responses to this METH dosing paradigm may be the principal explanation for the enhanced locomotor response following a METH challenge injection administered 3.5 h after high-dose METH pretreatment.
2. Materials and methods
Animals
Male Sprague-Dawley rats (Charles River Laboratories, Raleigh, NC) weighing between 260 to 320 g were used for the experiments. They were allowed to acclimate to the colony room for at least 2 weeks prior to experiments and were maintained in a temperature-controlled room with a 12-h light/dark cycle and were given free access to food and water. All experiments were approved by the University of Utah Institutional Animal Care and Use Committee and adhered to the National Academy of Sciences Guide for the Care and Use of Laboratory Animals.
2.2. Behavioral Procedures
Animals were placed in plexiglass locomotor chambers (46 × 46 × 12 cm) under low-light conditions. A (4 × 4) grid was placed beneath each chamber and the 8-h session was recorded using VCRs (Sanyo) connected to video cameras (AV wireless monitors). All animals were habituated to the locomotor chambers for 2 days (days 1–2) prior to the locomotor session. During habituation, the rats had free access to the chamber for 8 h. On days 1 and 2, following the first hour of habituation, all animals were injected (s.c.) with saline: a second injection of saline was administered 3.5 h later. These injections served to acclimate the animals with the injection procedure in order to reduce stress-evoked activity that often occurs following injections as determined in preliminary studies. On the test day (day 3), the animals were divided into four groups and injected twice as described above: the first group (n = 8) was administered saline for both injections; the second group (n = 8) was administered a pretreatment of a high dose (4.0 mg/kg) of METH followed by a challenge injection of saline; the third group (n = 8) was administered a pretreatment of saline followed by a challenge injection of a low dose of METH (0.4 mg/kg); the fourth group (n = 9) was administered the high dose of METH (4.0 mg/kg), as a pretreatment, followed by a challenge with the low dose of METH (0.4 mg/kg). All activity was recorded on day 3 of the experiment. Locomotor behavior was defined by the number of quadrants the animal crossed (“crossings”) and was scored by an observer blinded to the treatment group. Each occurrence of rearing activity, where the animals stood on their hind legs, was also individually recorded and scored by an observer blinded to the treatment condition as vertical activity (“activity counts”).
2.3. Measurement of drug concentration in striatum and plasma
For experiments measuring drug concentration, a separate set of animals were housed in their home cages in the same colony room as the animals used in the behavioral experiment described above. As it was not possible to measure behavior for the full observation period and measure drug concentration at defined time points, we made the assumption that the pharmacokinetic effects of METH in these animals would be similar to those used in the behavioral experiment. On the day of the experiment animals were removed from their home cages, injected then returned to their home cages. All animals were sacrificed via rapid decapitation at defined time points, prior to measurement of drug concentration (see below). Measurements of drug concentrations were performed 3.5 h following the initial injection of saline or METH (4.0 mg/kg; n = 6) and 30, 60 or 90 min following the challenge injection (METH; 0.4 mg/kg (n = 5/group). Plasma samples were extracted while striatal tissues were homogenized in water prior to extraction. Fifty ng/µl of internal standards d5-AMPH and d8-METH were added to all samples, standards and quality control samples. Samples were extracted with 5 ml of 4:1 n-butyl chloride: acetonitrile (ACN) after adjusting the pH with 100 µl of NH4OH (29% w/w; Fisher Chemical). After agitation, samples were centrifuged. The organic layer was transferred to a fresh glass tube, evaporated at room temperature, and reconstituted in 100 µl of 95:5 ACN:0.1% formic acid. Then, 20 µl was injected onto the LC-MS-MS system. Chromatographic separation was obtained on a Varian MetaChem MetaSil Basic column (3.0 × 100 mm) (Varian Inc., Palo Alto, CA). A solvent ramp was used beginning with 90% of 0.1% formic acid and 10% MeOH. After 1 min the MeOH was increased to 50% MeOH over 7.5 min. The proportion of MeOH was then returned to 10% over 0.5 min and held at 10% for an additional 3 min in order to re-equilibrate the column. The mass spectrometric system consisted of a Surveyor HPLC autosampler and pump (ThermoFinnigan; San Jose, CA) coupled to a TSQ Quantum from ThermoFinnigan. Mass spectrometric analysis was achieved by electrospray ionization (ESI) followed by selective reaction monitoring (SRM). Mass to charge transitions were as follows: AMP: 136 → 91; METH: 150.1 → 91; d5-AMP: 141.1 → 93; d8-METH 158.1 → 92.
2.3. Statistical Analysis
All data were analyzed using SAS V 9.1 software. Time course data for locomotor activity were broken down into 20 separate 10-min time bins and a three-way ANOVA was used to detect pretreatment × challenge treatment × time interactions. In the case that a significant interaction occurred, differences between treatment groups were determined at each 10-min time point using a one-way ANOVA followed by post hoc comparisons with Duncan’s multiple range test with an experimentwise error rate of α = 0.05. In addition, the total amount of locomotor activity over the 3.5 hour period following the challenge injection was also analyzed using a one-way ANOVA followed by Duncan’s multiple range test with an experimentwise error rate of α = 0.05. The data for the drug concentration experiments were analyzed using a one-way ANOVA followed by a t-test at each time point measured with an experimentwise error rate o of α = 0.5.
2.4. Drugs
Methamphetamine hydrochloride was a generous gift from the National Institute on Drug Abuse (NIDA). Methamphetamine (0.4 mg/kg or 4.0 mg/kg) was dissolved in saline (0.9%). All injections were subcutaneous (s.c.).
RESULTS
Locomotor activity
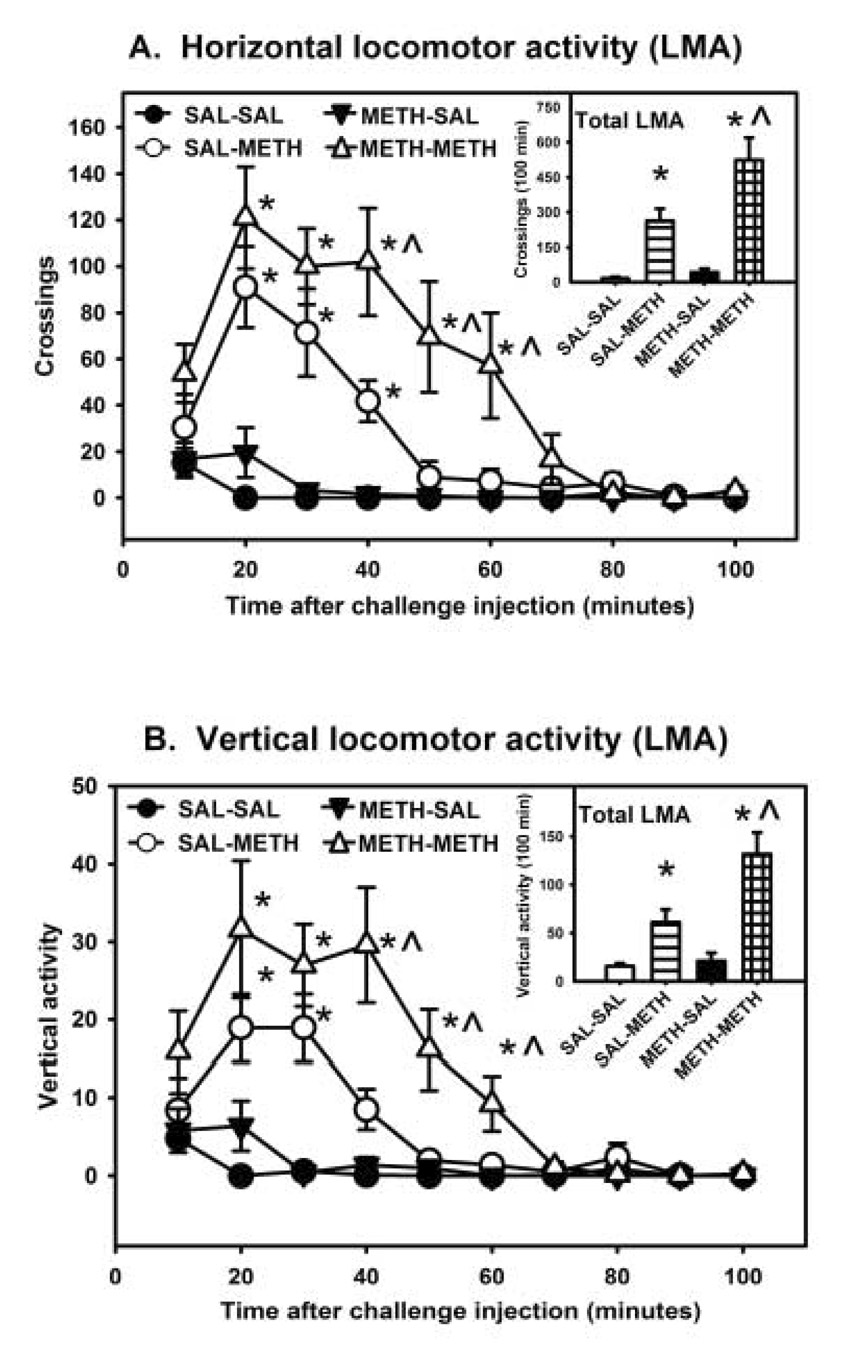
Saline injections alone had little impact on horizontal or vertical activity (Figure 1A and 1B). The three-way ANOVA yielded a significant interaction (pretreatment × challenge treatment × time) for both horizontal [F(20,669) = 2.22, p < 0.05] and vertical activity [F(20,669) = 2.00, p < 0.05]. Individual one-way ANOVAs performed at each 10-min time point indicated significant differences for horizontal activity at 20 min [F(3,31) = 14.58, p < 0.01], 30 min [F(3,31) =15.78, p < 0.01], 40 min [F(3,31) = 14.84, p < 0.01], 50 min [F(3,31) = 7.05, p < 0.01] and 60 min [F(3,31) = 5.51, p < 0.01] post injection (Figure 1A). Likewise, individual one-way ANOVAs performed at each 10-min time point indicated significant differences for vertical activity at 20 min [F(3,31) =7.36, p < 0.01], 30 min [F(3,31) = 14.77, p < 0.01], 40 min [F(3,31) = 11.79, p < 0.01], 50 min [F(3,31) = 7.79, p < 0.01] and 60 min [F(3,31) = 6.10, p < 0.01] post injection (Figure 1B). Post hoc comparisons demonstrated that pretreatment with saline followed by challenge injections of METH (0.4 mg/kg) produced a significant increase interaction for both horizontal and vertical activity. Animals pretreated with the high dose of METH (4.0 mg/kg) followed by the challenge injection of METH (0.4 mg/kg) displayed a greater locomotor response as measured by both horizontal and vertical activity over a more prolonged period of time compared to METH-challenged animals that received saline pretreatment (Figures 1A and 1B).
Figure 1.
Effects of a challenge injection of methamphetamine (METH; 0.4 mg/kg) or saline (SAL) on horizontal (A) and vertical activity (B) in rats (n =8 /group) administered 3.5 h after SAL or METH (4.0 mg/kg) administration. Data points are represented as mean horizontal activity (A) or mean vertical activity (B) ± SEM. Inset graphs represent total activity during the first 100 min after challenge injection. * = p < 0.05 significantly different than SAL-SAL. ^ = p <0.05 significantly different from SAL-METH.
Drug concentration in brain
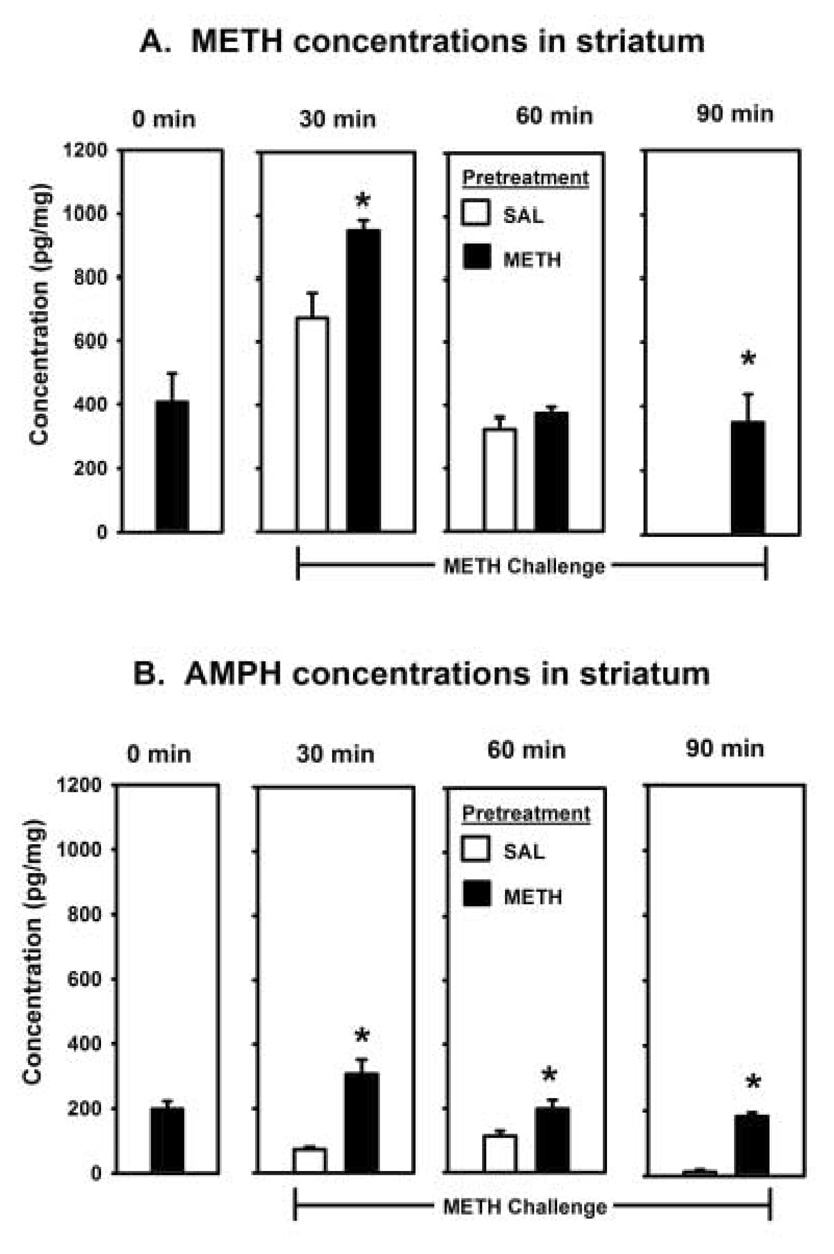
There were significant METH and AMPH levels in the striatum 3.5 h after a METH pretreatment just prior to the low-dose METH challenge (0 min, Figures 2A and 2B). Following the challenge-drug injection, METH concentrations in the striatum were significantly increased at 30 min [F(1,9) = 10.37, p < 0.05] and 90 min [F(1,8) = 22.52, p < 0.05], but not 60 min, in rats pretreated with a high-dose METH relative to rats pretreated with saline (Figure 2A). Amphetamine concentrations in the striatum of rats receiving both METH injections were also increased at all three time points [30 min: F(1,9) = 26.2, p < 0.5; 60 min: F(1,8) = 5.79, p < 0.05; 90 min: F(1,8) = 172.3, p < 0.05] relative to rats pretreated with saline (Figure 2B).
Figure 2.
Methamphetamine (A) and AMPH (B) concentrations in the striatum 0 min (n = 6) prior to or 30 min (n = 5/group), 60 min (n = 5/group) or 90 min (n = 5/group) after the challenge injection of METH (0.4 mg/kg). Rats were pretreated with either saline (SAL) or METH (4.0 mg/kg) 3.5 h prior to administration of the challenge injection. * = p < 0.05 significantly different than SAL-METH.
Drug concentration in plasma
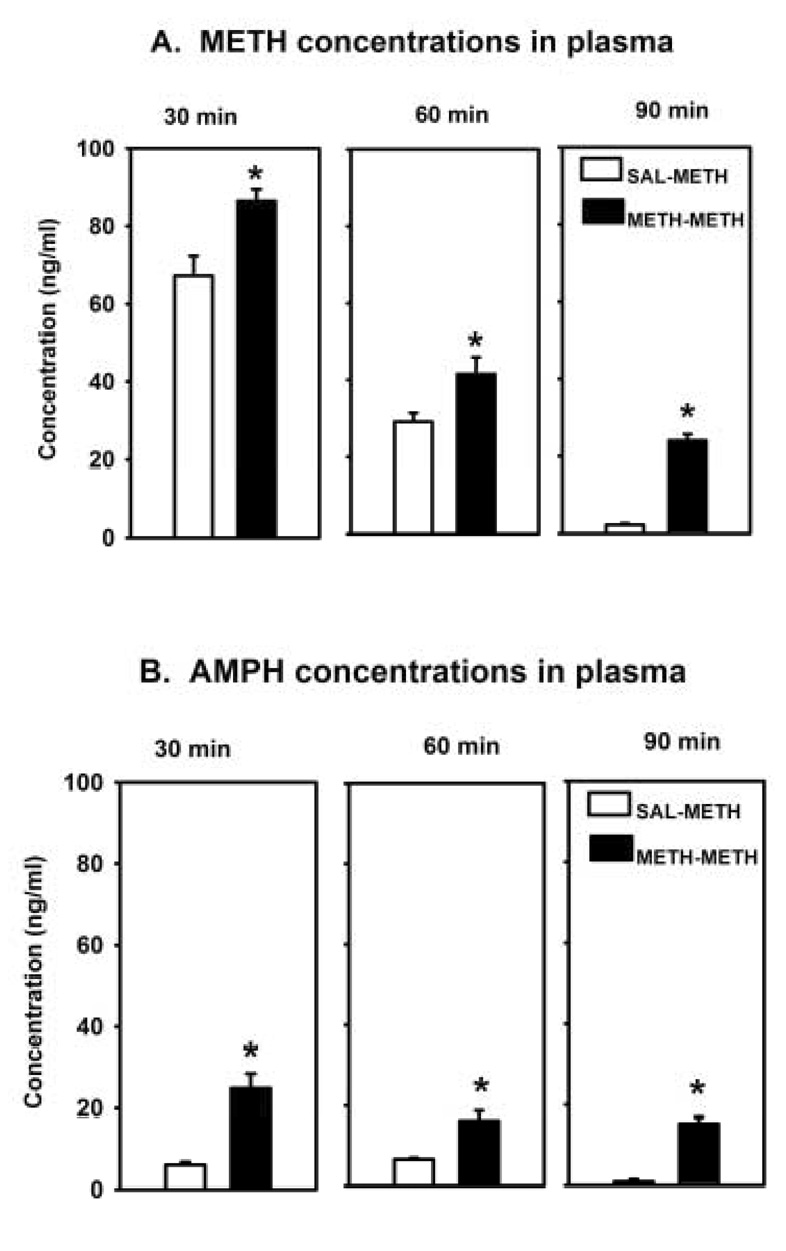
Following the challenge-drug injection, METH and AMPH plasma concentrations were significantly increased in rats receiving both injections of METH at all time points observed (Figure 3). Although both METH and AMPH plasma concentrations seemed to decrease over time, they remained elevated 90 minutes following the challenge injection in rats pretreated with the high dose of METH.
Figure 3.
Methamphetamine (A) and AMPH (B) concentrations in the plasma (n = 5/group) 30 min, 60 min or 90 min after the challenge injection of METH (0.4 mg/kg). Rats were pretreated with either saline (SAL) or METH (4.0 mg/kg) 3.5 h prior to administration of the challenge injection. * = p < 0.05 significantly different than SAL-METH.
DISCUSSION
Unlike the earlier work with AMPH (Kuczenski and Segal, 1999a,b), the current study investigated the contribution of pharmacokinetics to the locomotor response following repeated METH treatment. Our results demonstrated an amplified locomotor response in rats pretreated by a high-dose and then challenged with a low dose of METH such that these rats expressed a greater amount of motor activity for a longer duration. Thus, following the challenge injection of METH (0.4 mg/kg) we observed increased horizontal (Figure 1A) and vertical activity (Figure 1B) in animals pretreated with METH (4.0 mg/kg) relative to animals pretreated with saline.
Drug measurements also demonstrated increased striatal concentrations of METH and its metabolite AMPH in rats pretreated 3.5 h prior with a high-dose of METH (Figure 2). Relevant to this finding was the observation that residual striatal concentrations of METH and AMPH were still quantifiable 3.5 h after the pretreatment with 4.0 mg/kg of METH, suggesting that following the challenge injection, the total amount of drug present in the striatum at 30 min post-challenge, was accumulative with the residual drug from the METH pretreatment. It follows that the increased amount of drug and active metabolite in the brain from 30 – 90 min post METH-challenge in METH pretreated animals may account for the amplified locomotor response. This observation is supported by a similar pattern of elevated plasma METH and AMPH in METH pretreated animals also found in the plasma (Figure 3).
Kuczenski and Segal (1999a,b) have argued that following repeated AMPH, DA receptors may be sensitized following the initial AMPH injection and play a strong role in mediating the exaggerated behavioral response following subsequent challenges with AMPH. However, in these studies, Kuczenski and Segal did not measure AMPH levels in the striatum following repeated injections but rather cited their earlier work that demonstrated a dissociation between amphetamine concentration in the striatum and expression of stereotypy following a single administration of 8 mg/kg of AMPH (Kuczenski and Segal, 1997) or 1.75 mg/kg of AMPH (Segal and Kuczenski, 1987). In contrast, there dose not appear to be such a dissociation between drug brain concentration and locomotor activity in our experiments with METH suggesting a significant role for drug accumulation in our findings of enhanced motor responses caused by low-dose METH challenges of a high-dose METH pretreatment. A possible explanation for the discrepancy between the present findings and Segal and Kuczenski’s work may be that the effects and pharmacokinetics of METH and AMPH when given as a high-dose pretreatment followed by a low dose challenge are different.
In summary, our study suggests that rats administered METH (4.0 mg/kg) followed by a challenge injection of METH (0.4 mg/kg) experience accumulation of METH (and AMPH) in their brains, such that the challenge injection produces an increased concentration of METH (and AMPH), relative to the animals only receiving the METH challenge, that remains in the brain for a longer period of time. This increased concentration of drug in the brain and plasma likely significantly contributes to the enhanced locomotor response observed following a low-dose METH challenge.
Acknowledgements
This work was supported by PHS grants from NIDA, DA09407, DA00378.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- DiChiara G, Bassareo V, Fenu S, DeLuca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47 Suppl 1:227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Reed JL, Kuwajima M, Rebec GV. Amphetamine-induced behavioral activation is associated with variable changes in basal ganglia output neurons recorded from awake. behaving rats Brain Res. 2004;1012:108–118. doi: 10.1016/j.brainres.2004.03.044. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Melega WP, Cho AK, Segal DS. Extracellular dopamine and amphetamine after systemic amphetamine administration: comparison to the behavioral response. J Pharmacol Exp Ther. 1997;282:591–596. [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Concomitant characterization of behavioral and striatal neurotransmitter response to amphetamine using in vivo microdialysis. J Neurosci. 1989;9:2051–2065. doi: 10.1523/JNEUROSCI.09-06-02051.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Neurochemistry of amphetamine. In: Cho AK, Segal DS, editors. Amphetamine and Its Analogs: Psychopharmacology, Toxicology and Abuse. San Diego, CA: Academic Press, Inc.; 1994. pp. 81–113. [Google Scholar]
- Kuczenski R, Segal DS. Sensitization of amphetamine-induced stereotyped behaviors during the acute response. J Pharmacol Exp Ther. 1999a;288:699–709. [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Sensitization of amphetamine-induced stereotyped behaviors during the acute response: role of D1 and D2 dopamine receptors. Brain Res. 1999b;822:164–174. doi: 10.1016/s0006-8993(99)01149-x. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Aizenstein ML. Amphetamine, fencamfamine, and cocaine: Relationships between locomotor activity and stereotypy response profiles and caudate and accumbens dopamine dynamics. J Neurosci. 1991;11:2703–2712. doi: 10.1523/JNEUROSCI.11-09-02703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: a review. J Neuropsychiatry Clin Neurosci. 2003;15:317–325. doi: 10.1176/jnp.15.3.317. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Camp DM. Does amphetamine preferentially increase the extracellular concentration of dopamine in the mesolimbic system of freely moving rats? Neuropsychopharmacology. 1990;3:163–173. [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristics and neurochemical correlates. J Pharmacol Exp Ther. 1987;242:917–926. [PubMed] [Google Scholar]
- Sharp T, Zetterstrom T, Ljungberg T, Ungerstedt U. A direct comparison of amphetamine-induced behaviours and regional; brain dopamine release in the rat using intracerebral dialysis. Brain Res. 1987;401:322–330. doi: 10.1016/0006-8993(87)91416-8. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav Pharmacol. 2002;13:355–366. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]