Abstract
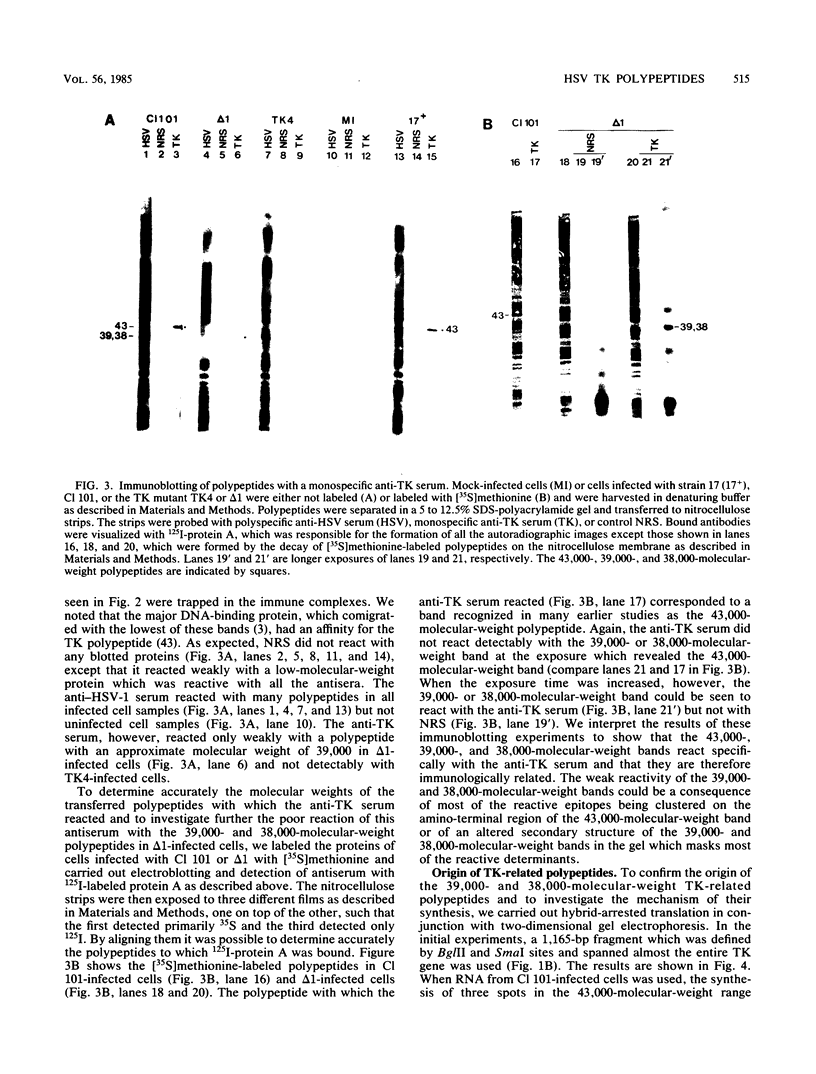
Previous studies (H.S. Marsden, L. Haarr, and C.M. Preston, J. Virol. 46:434-445, 1983) have shown that at least three polypeptides, with molecular weights of 43,000, 39,000, and 38,000, are encoded by the herpes simplex virus type 1 (HSV-1) thymidine kinase (TK) gene. It has been suggested that the 39,000- and 38,000-molecular-weight polypeptides arise from preinitiation complexes bypassing the first and second AUG codons before commencement of translation since, according to previous work (M. Kozak, Nucleic Acids Res. 9:5233-5252, 1981), these codons are not of the most efficient structure for initiation. This possibility was investigated by using specific herpes simplex virus mutants with alterations in the TK gene. Mutant TK4 has an amber mutation between the first and second AUG codons, whereas mutant delta 1 has a deletion which removes the first AUG codon but leaves other AUG codons, as well as transcriptional promoter sequences, intact. Both mutants synthesized only the 39,000- and 38,000-molecular-weight polypeptides, and the amounts produced were normal in TK4-infected cells but increased in delta 1-infected cells. Furthermore, the levels of TK produced after infection with the mutant viruses correlated with the amounts of the 39,000- and 38,000-molecular-weight polypeptides synthesized. The 43,000-, 39,000-, and 38,000-molecular-weight polypeptides were shown to be related by their positive reaction with anti-TK serum in both immunoprecipitation and immunoblotting experiments. The production of the 39,000- and 38,000-molecular-weight polypeptides through bypassing of the first AUG codon was examined by hybrid arrest experiments with a DNA fragment complementary to only 50 bases at the 5' terminus of TK mRNA. This fragment arrested the synthesis of the 30,000- and 38,000-molecular-weight polypeptides when annealed to mRNA from wild-type HSV-1- or TK4-infected cells, showing that those polypeptides arise from an mRNA initiated upstream from the first AUG codon. mRNA from cells infected with mutant delta 1, which lacks DNA sequences upstream from the first AUG, was not affected by the 50-base-pair fragment. The data therefore confirm that three polypeptides encoded by the HSV-1 TK gene arise by differential use of in-phase AUG codons for the initiation of protein synthesis. This mechanism for the production of related but distinct polypeptides has not previously been demonstrated in a eucaryotic system, and the implications for the regulation of TK enzyme activities are discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandyopadhyay P. K., Temin H. M. Expression from an internal AUG codon of herpes simplex thymidine kinase gene inserted in a retrovirus vector. Mol Cell Biol. 1984 Apr;4(4):743–748. doi: 10.1128/mcb.4.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Bayliss G. J., Marsden H. S., Hay J. Herpes simplex virus proteins: DNA-binding proteins in infected cells and in the virus structure. Virology. 1975 Nov;68(1):124–134. doi: 10.1016/0042-6822(75)90154-3. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Gould K. G., Akashi H., Clerx-van Haaster C. M. The complete sequence and coding content of snowshoe hare bunyavirus small (S) viral RNA species. Nucleic Acids Res. 1982 Jun 25;10(12):3703–3713. doi: 10.1093/nar/10.12.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Polder L. J., Bernards R., Schrier P. I., van den Elsen P. J., van der Eb A. J., van Ormondt H. The 2.2 kb E1b mRNA of human Ad12 and Ad5 codes for two tumor antigens starting at different AUG triplets. Cell. 1981 Nov;27(1 Pt 2):121–131. doi: 10.1016/0092-8674(81)90366-4. [DOI] [PubMed] [Google Scholar]
- Brown S. M., Ritchie D. A., Subak-Sharpe J. H. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J Gen Virol. 1973 Mar;18(3):329–346. doi: 10.1099/0022-1317-18-3-329. [DOI] [PubMed] [Google Scholar]
- Chen M. S., Prusoff W. H. Association of thymidylate kinase activity with pyrimidine deoxyribonucleoside kinase induced by herpes simplex virus. J Biol Chem. 1978 Mar 10;253(5):1325–1327. [PubMed] [Google Scholar]
- Cremer K. J., Bodemer M., Summers W. P., Summers W. C., Gesteland R. F. In vitro suppression of UAG and UGA mutants in the thymidine kinase gene of herpes simplex virus. Proc Natl Acad Sci U S A. 1979 Jan;76(1):430–434. doi: 10.1073/pnas.76.1.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty J. J., Subak-Sharpe J. H., Preston C. M. Identification of a virus-specific polypeptide associated with a transforming fragment (BglII-N) of herpes simplex virus type 2 DNA. J Virol. 1981 Oct;40(1):126–132. doi: 10.1128/jvi.40.1.126-132.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke D., Labenz J., Brauer D., Müller W. E. Adenosine diphosphate: thymidine 5'-phosphotransferase, a new enzyme activity, associated with the Herpes simplex virus-induced deoxypyrimidine kinase. Biochim Biophys Acta. 1982 Oct 20;708(1):99–103. doi: 10.1016/0167-4838(82)90207-2. [DOI] [PubMed] [Google Scholar]
- Falke D., Nehrbass W., Brauer D., Müller W. E. Adenylic acid: deoxythymidine 5'-phosphotransferase: evidence for the existence of a novel herpes simplex virus-induced enzyme. J Gen Virol. 1981 Apr;53(Pt 2):247–255. doi: 10.1099/0022-1317-53-2-247. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Haarr L., Marsden H. S. Two-dimensional gel analysis of HSV type 1-induced polypeptides and glycoprotein processing. J Gen Virol. 1981 Jan;52(Pt 1):77–92. doi: 10.1099/0022-1317-52-1-77. [DOI] [PubMed] [Google Scholar]
- Hall L. M., Draper K. G., Frink R. J., Costa R. H., Wagner E. K. Herpes simplex virus mRNA species mapping in EcoRI fragment I. J Virol. 1982 Aug;43(2):594–607. doi: 10.1128/jvi.43.2.594-607.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M. E., Smiley J. R. Effects of deletions on expression of the herpes simplex virus thymidine kinase gene from the intact viral genome: the amino terminus of the enzyme is dispensable for catalytic activity. J Virol. 1984 Jun;50(3):733–738. doi: 10.1128/jvi.50.3.733-738.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Absence of a requirement for host polypeptides in the herpes virus thymidine kinase. J Gen Virol. 1974 Jul;24(1):215–220. doi: 10.1099/0022-1317-24-1-215. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Hay J., Subak-Sharpe J. H. Herpesvirus proteins: induction of nucleoside phosphotransferase activity after herpes simplex virus infection. J Virol. 1976 Mar;17(3):1056–1059. doi: 10.1128/jvi.17.3.1056-1059.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson A. T., Subak-Sharpe J. H. Biochemical studies on the herpes simplex virus-specified deoxypyrimidine kinase activity. J Gen Virol. 1974 Sep;24(3):481–492. doi: 10.1099/0022-1317-24-3-481. [DOI] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Selection of initiation sites by eucaryotic ribosomes: effect of inserting AUG triplets upstream from the coding sequence for preproinsulin. Nucleic Acids Res. 1984 May 11;12(9):3873–3893. doi: 10.1093/nar/12.9.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labenz J., Friedrich D., Falke D. Analysis of the TK enzyme complex induced by HSV types 1 and 2 by means of isoelectric focusing and polyacrylamide gel electrophoresis. Arch Virol. 1982;71(3):235–249. doi: 10.1007/BF01314875. [DOI] [PubMed] [Google Scholar]
- Lee G. T., Para M. F., Spear P. G. Location of the structural genes for glycoproteins gD and gE and for other polypeptides in the S component of herpes simplex virus type 1 DNA. J Virol. 1982 Jul;43(1):41–49. doi: 10.1128/jvi.43.1.41-49.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. C., Simonsen C. C., Levinson A. D. Initiation of translation at internal AUG codons in mammalian cells. Nature. 1984 May 3;309(5963):82–85. doi: 10.1038/309082a0. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Haarr L., Preston C. M. Processing of herpes simplex virus proteins and evidence that translation of thymidine kinase mRNA is initiated at three separate AUG codons. J Virol. 1983 May;46(2):434–445. doi: 10.1128/jvi.46.2.434-445.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden H. S., Stow N. D., Preston V. G., Timbury M. C., Wilkie N. M. Physical mapping of herpes simplex virus-induced polypeptides. J Virol. 1978 Nov;28(2):624–642. doi: 10.1128/jvi.28.2.624-642.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- McKnight S. L. The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene. Nucleic Acids Res. 1980 Dec 20;8(24):5949–5964. doi: 10.1093/nar/8.24.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Ogino T., Shiman R., Rapp F. Deoxythymidine kinase from rabbit kidney cells infected with herpes simplex virus types 1 and 2. Intervirology. 1973;1(2):80–95. doi: 10.1159/000148835. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Piatak M., Subramanian K. N., Roy P., Weissman S. M. Late messenger RNA production by viable simian virus 40 mutants with deletions in the leader region. J Mol Biol. 1981 Dec 15;153(3):589–618. doi: 10.1016/0022-2836(81)90409-5. [DOI] [PubMed] [Google Scholar]
- Preston C. M. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J Virol. 1979 Jan;29(1):275–284. doi: 10.1128/jvi.29.1.275-284.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M., McGeoch D. J. Identification and mapping of two polypeptides encoded within the herpes simplex virus type 1 thymidine kinase gene sequences. J Virol. 1981 May;38(2):593–605. doi: 10.1128/jvi.38.2.593-605.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M. W., Choppin P. W., Lamb R. A. A previously unrecognized influenza B virus glycoprotein from a bicistronic mRNA that also encodes the viral neuraminidase. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4879–4883. doi: 10.1073/pnas.80.16.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley J. R., Wagner M. J., Summers W. P., Summers W. C. Genetic and physical evidence for the polarity of transcription of the thymidine kinase gene of herpes simplex virus. Virology. 1980 Apr 15;102(1):83–93. doi: 10.1016/0042-6822(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Summers W. P., Summers W. C., Laski F. A., RajBhandary U. L., Sharp P. A. Functional suppression in mammalian cells of nonsense mutations in the herpes simplex virus thymidine kinase gene by suppressor tRNA genes. J Virol. 1983 Aug;47(2):376–379. doi: 10.1128/jvi.47.2.376-379.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. P., Wagner M., Summers W. C. Possible peptide chain termination mutants in thymide kinase gene of a mammalian virus, herpes simplex virus. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4081–4084. doi: 10.1073/pnas.72.10.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan P. J., Banks L. M., Purifoy D. J., Powell K. L. Interactions between herpes simplex virus DNA-binding proteins. J Gen Virol. 1984 Nov;65(Pt 11):2033–2041. doi: 10.1099/0022-1317-65-11-2033. [DOI] [PubMed] [Google Scholar]
- Wagner M. J., Sharp J. A., Summers W. C. Nucleotide sequence of the thymidine kinase gene of herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1441–1445. doi: 10.1073/pnas.78.3.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]