Abstract
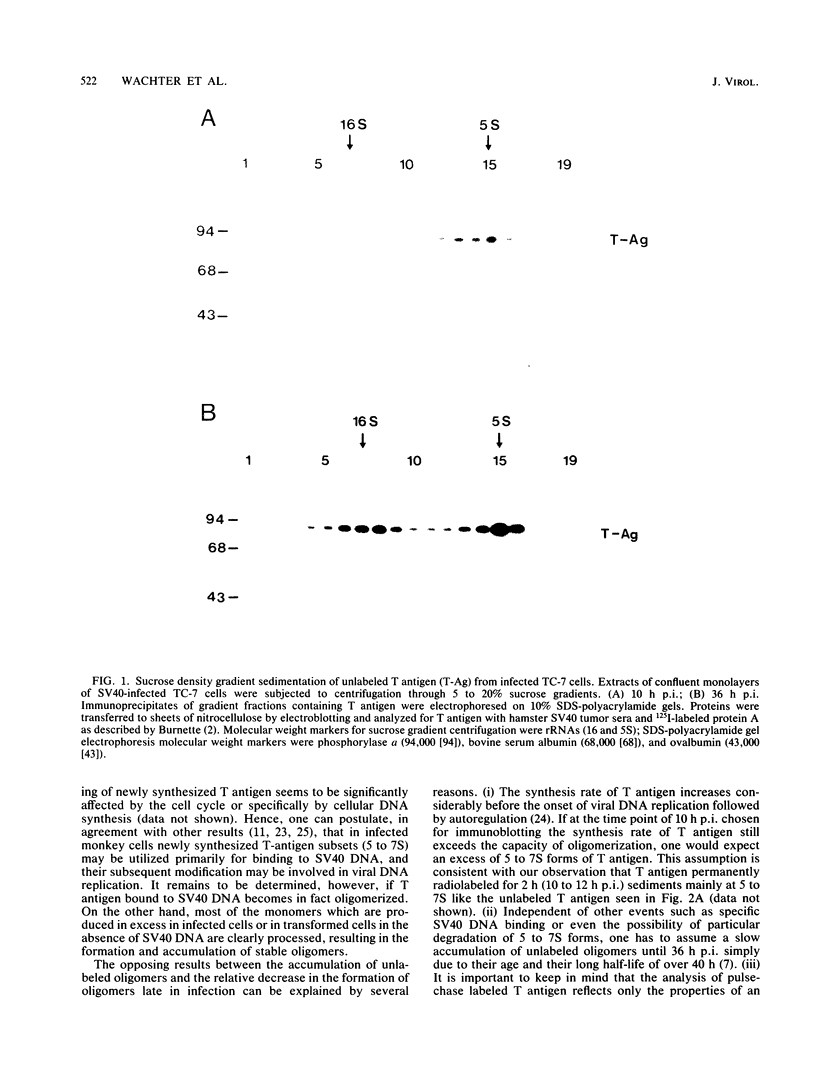
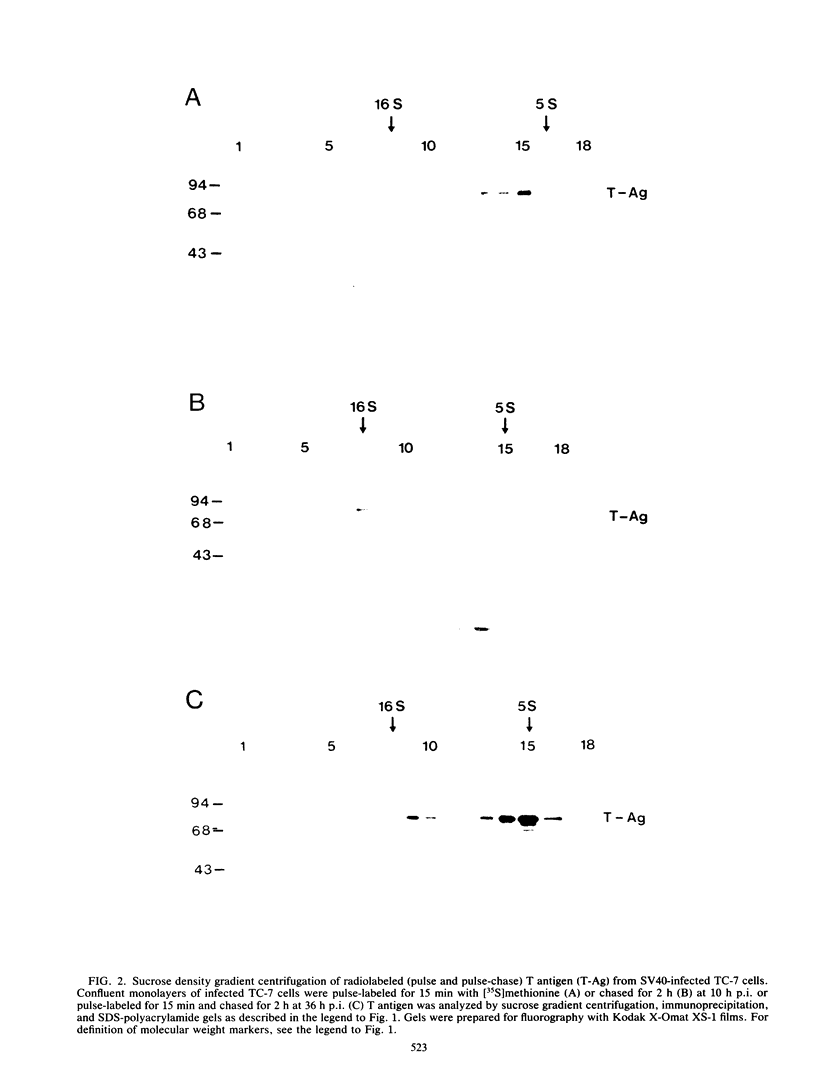
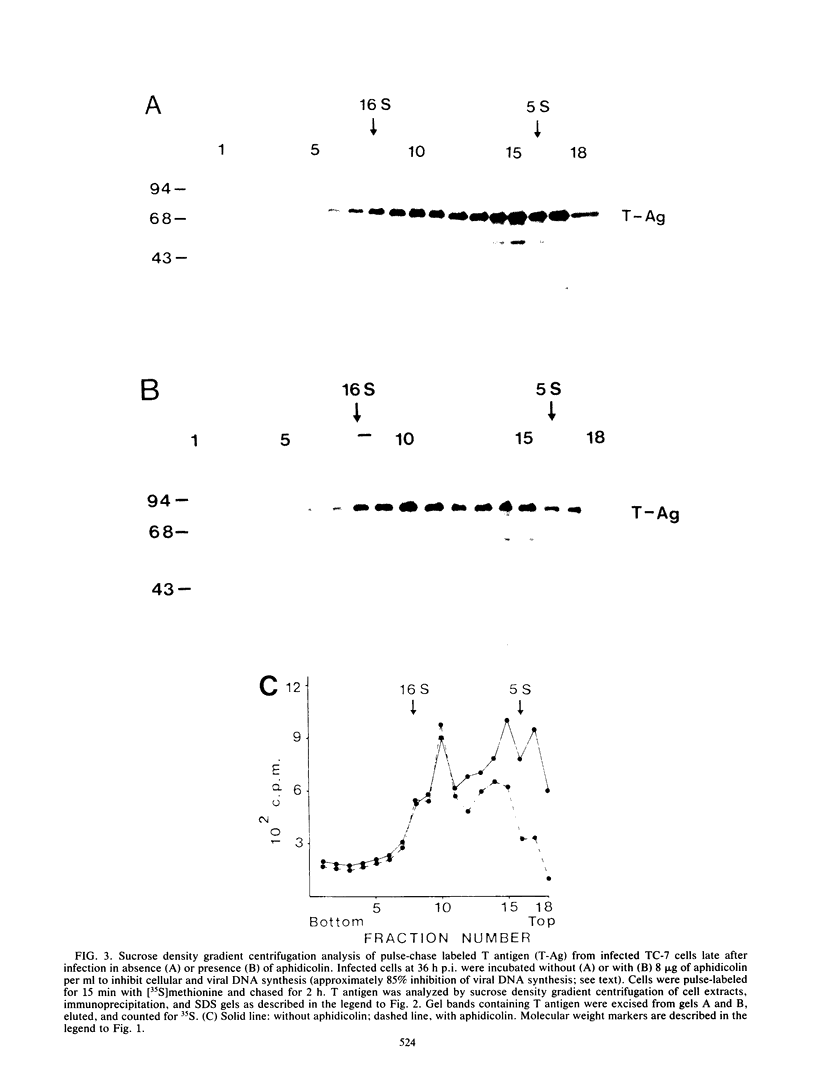
The formation of oligomers of simian virus 40 (SV40) large T antigen in SV40-infected and -transformed monkey cells was analyzed by sucrose density gradient centrifugation. The overall distribution of total T antigen during lytic infection showed mainly low-molecular-weight forms (monomers and dimers) in the early phase (10 h postinfection) and an increase in the number of oligomers in the late phase of the lytic cycle (36 h postinfection), indicating an accumulation of these final products. In contrast, studying the conversion of newly synthesized T antigen into oligomers by appropriate pulse-chase radiolabeling of infected cells revealed that this processing decelerates considerably during the late phase of infection. This mechanism can be reaccelerated by blocking DNA replication with aphidicolin. Since none of these results could be obtained by using synchronized SV40-transformed monkey cells (COS-1), these observations are compatible with the idea that the process of T antigen oligomerization may be involved in viral, but not in cellular, DNA synthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley M. K., Griffin J. D., Livingston D. M. Relationship of oligomerization to enzymatic and DNA-binding properties of the SV40 large T antigen. Cell. 1982 Jan;28(1):125–134. doi: 10.1016/0092-8674(82)90382-8. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carroll R. B., Gurney E. G. Time-dependent maturation of the simian virus 40 large T antigen-p53 complex studied by using monoclonal antibodies. J Virol. 1982 Nov;44(2):565–573. doi: 10.1128/jvi.44.2.565-573.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R. B., Hager L., Dulbecco R. Simian virus 40 T antigen binds to DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3754–3757. doi: 10.1073/pnas.71.9.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R., Lane D. P., Tjian R. Use of monoclonal antibodies as probes of simian virus 40 T antigen ATPase activity. J Biol Chem. 1981 Nov 25;256(22):11854–11858. [PubMed] [Google Scholar]
- Crawford L. The 53,000-dalton cellular protein and its role in transformation. Int Rev Exp Pathol. 1983;25:1–50. [PubMed] [Google Scholar]
- Edwards C. A., Khoury G., Martin R. G. Phosphorylation of T-antigen and control T-antigen expression in cells transformed by wild-type and tsA mutants of simian virus 40. J Virol. 1979 Feb;29(2):753–762. doi: 10.1128/jvi.29.2.753-762.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning E., Burger C., Gurney E. G. Comparison of T antigen-associated host phosphoproteins from SV40-infected and -transformed cells of different species. J Gen Virol. 1981 Aug;55(Pt 2):367–378. doi: 10.1099/0022-1317-55-2-367. [DOI] [PubMed] [Google Scholar]
- Fanning E., Nowak B., Burger C. Detection and characterization of multiple forms of simian virus 40 large T antigen. J Virol. 1981 Jan;37(1):92–102. doi: 10.1128/jvi.37.1.92-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning E., Westphal K. H., Brauer D., Cörlin D. Subclasses of simian virus 40 large T antigen: differential binding of two subclasses of T antigen from productively infected cells to viral and cellular DNA. EMBO J. 1982;1(9):1023–1028. doi: 10.1002/j.1460-2075.1982.tb01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidoni D., Scheller A., Barnet B., Hantzopoulos P., Oren M., Prives C. Different forms of simian virus 40 large tumor antigen varying in their affinities for DNA. J Virol. 1982 May;42(2):456–466. doi: 10.1128/jvi.42.2.456-466.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan D. S., Carroll R. B. Complex of simian virus 40 large tumor antigen and 48,000-dalton host tumor antigen. Proc Natl Acad Sci U S A. 1981 Jan;78(1):105–109. doi: 10.1073/pnas.78.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Krokan H., Schaffer P., DePamphilis M. L. Involvement of eucaryotic deoxyribonucleic acid polymerases alpha and gamma in the replication of cellular and viral deoxyribonucleic acid. Biochemistry. 1979 Oct 2;18(20):4431–4443. doi: 10.1021/bi00587a025. [DOI] [PubMed] [Google Scholar]
- McCormick F., Harlow E. Association of a murine 53,000-dalton phosphoprotein with simian virus 40 large-T antigen in transformed cells. J Virol. 1980 Apr;34(1):213–224. doi: 10.1128/jvi.34.1.213-224.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenarh M., Deppert W., Henning R. Mapping of a DNA-binding domain of simian virus 40 T-antigen using non-defective adenovirus 2--simian virus 40 hybrid viruses. FEBS Lett. 1982 Jun 1;142(1):129–132. doi: 10.1016/0014-5793(82)80235-4. [DOI] [PubMed] [Google Scholar]
- Montenarh M., Henning R. Disaggregation and reconstitution of oligomeric complexes of simian virus 40 large T-antigen. J Gen Virol. 1983 Jan;64(Pt 1):241–246. doi: 10.1099/0022-1317-64-1-241. [DOI] [PubMed] [Google Scholar]
- Montenarh M., Kohler M., Henning R. Oligomerization of simian virus 40 large T antigen is not necessarily repressed by temperature-sensitive A gene lesions. J Virol. 1984 Mar;49(3):658–664. doi: 10.1128/jvi.49.3.658-664.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Williams R. C., Tjian R. Oligomeric structure of a simian virus 40 T antigen in free form and bound to DNA. J Mol Biol. 1981 Jun 5;148(4):347–353. doi: 10.1016/0022-2836(81)90180-7. [DOI] [PubMed] [Google Scholar]
- Pintel D., Bouck N., di Mayorca G. Separation of lytic and transforming functions of the simian virus 40 A region: two mutants which are temperature sensitive for lytic functions have opposite effects on transformation. J Virol. 1981 May;38(2):518–528. doi: 10.1128/jvi.38.2.518-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C., Barnet B., Scheller A., Khoury G., Jay G. Discrete regions of simian virus 40 large T antigen are required for nonspecific and viral origin-specific DNA binding. J Virol. 1982 Jul;43(1):73–82. doi: 10.1128/jvi.43.1.73-82.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C., Beck Y., Gidoni D., Oren M., Shure H. DNA binding and sedimentation properties of SV40 T antigens synthesized in vivo and in vitro. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):123–130. doi: 10.1101/sqb.1980.044.01.014. [DOI] [PubMed] [Google Scholar]
- Scheller A., Covey L., Barnet B., Prives C. A small subclass of SV40 T antigen binds to the viral origin of replication. Cell. 1982 Jun;29(2):375–383. doi: 10.1016/0092-8674(82)90154-4. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V. G., Tevethia M. J., Lewton B. A., Tegtmeyer P. DNA binding properties of simian virus 40 temperature-sensitive A proteins. J Virol. 1982 Nov;44(2):458–466. doi: 10.1128/jvi.44.2.458-466.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N., Kuchino T. Temperature-sensitive mutants of simian virus 40 selected by transforming ability. J Virol. 1975 Jun;15(6):1297–1301. doi: 10.1128/jvi.15.6.1297-1301.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]