Abstract
Cdc25, the dual-specificity phosphatase that dephosphorylates the Cdc2–cyclin B complex at mitosis, is highly regulated during the cell cycle. In Xenopus egg extracts, Cdc25 is associated with two isoforms of the 14-3-3 protein. Cdc25 is complexed primarily with 14-3-3ε and to a lesser extent with 14-3-3ζ. The association of these 14-3-3 proteins with Cdc25 varies dramatically during the cell cycle: binding is high during interphase but virtually absent at mitosis. Interaction with 14-3-3 is mediated by phosphorylation of Xenopus Cdc25 at Ser-287, which resides in a consensus 14-3-3 binding site. Recombinant Cdc25 with a point mutation at this residue (Cdc25-S287A) is incapable of binding to 14-3-3. Addition of the Cdc25-S287A mutant to Xenopus egg extracts accelerates mitosis and overrides checkpoint-mediated arrests of mitotic entry due to the presence of unreplicated and damaged DNA. These findings indicate that 14-3-3 proteins act as negative regulators of Cdc25 in controlling the G2–M transition.
INTRODUCTION
In cycling eukaryotic cells, the entry into mitosis (M-phase) is orchestrated by a cyclin-dependent kinase consisting of the catalytic subunit Cdc2 and a B-type cyclin partner. The Cdc2–cyclin B complex, also known as MPF (maturation or M-phase promoting factor), acts by phosphorylating a myriad of mitotic substrates (Coleman and Dunphy, 1994; King et al., 1994; Morgan, 1995). After MPF-catalyzed phosphorylation, these substrates directly or indirectly participate in mitotic processes such as nuclear envelope breakdown (NEB), chromosome condensation, and spindle assembly. The proper execution of mitotic events ultimately results in the faithful segregation of replicated chromosomes to daughter cells.
It is essential that MPF be active for only a brief period during the cell cycle (Elledge, 1996). For this reason, there are elaborate posttranslational mechanisms regulating both the activation of MPF at the G2–M boundary and its subsequent inactivation at the metaphase–anaphase transition. In the case of the G2–M transition, the phosphorylation of Cdc2 plays an important role in proper mitotic timing (Morgan, 1995). Cdc2 absolutely requires phosphorylation on Thr-161 for catalytic activity. However, the Thr-161-phosphorylated Cdc2–cyclin B complex is kept inactive throughout interphase by dominantly inhibitory phosphorylations on the Tyr-15 and Thr-14 residues of Cdc2. These phosphorylations are carried out collectively by the inhibitory kinases Wee1 and Myt1 (Featherstone and Russell, 1991; Igarashi et al., 1991; Parker and Piwnica-Worms, 1992; Mueller et al., 1995a,b). When the conditions are appropriate for mitosis, a dual-specificity phosphatase called Cdc25 activates Cdc2–cyclin B by removing these inhibitory phosphates (Dunphy and Kumagai, 1991; Gautier et al., 1991; Strausfeld et al., 1991).
Cdc25, Wee1, and Myt1 are all highly regulated during the cell cycle. For example, Cdc25 is virtually inactive during interphase but undergoes a strong activation at mitosis due to phosphorylation of its N-terminal regulatory domain (Izumi et al., 1992; Kumagai and Dunphy, 1992). This stimulatory phosphorylation process is carried out by at least two kinases, including Cdc2–cyclin B itself and a Xenopus homologue of the kinase Polo called Plx1 (Hoffmann et al., 1993; Izumi and Maller, 1995; Kumagai and Dunphy, 1996). In parallel with the activation of Cdc25 at mitosis, Wee1 and Myt1 are shut-off at mitosis by multiple regulatory kinases, one of which may be Cdc2–cyclin B (McGowan and Russell, 1995; Mueller et al., 1995a; Watanabe et al., 1995).
The events leading to the activation of Cdc25 and inactivation of Wee1/Myt1 at mitosis are fundamental problems in cell cycle control. Recently, 14-3-3 proteins have been implicated in mitotic regulation (al-Khodairy and Carr, 1992; Ford et al., 1994; Aitken, 1996; Elledge, 1996). In the fission yeast Schizosaccharomyces pombe, mutations in the Rad24 and Rad25 proteins, both of which are 14-3-3 homologues, disrupt mitotic timing and the checkpoint response to damaged DNA (Ford et al., 1994). In humans, two-hybrid analysis has revealed that 14-3-3 proteins associate with certain forms of the Cdc25 protein (Conklin et al., 1995). In recent studies, 14-3-3 proteins have been shown to bind to a phosphorylated serine (Ser-216) of human Cdc25C and thereby negatively regulate its function (Peng et al., 1997; Sanchez et al., 1997).
In this report, we have explored the regulation of Cdc25C (hereafter referred to simply as Cdc25) in Xenopus egg extracts. In particular, we have focused on the inactive form of Cdc25 found during interphase. We have identified two 14-3-3 proteins that bind to the inactive but not the active form of Cdc25. The properties of this interaction strongly suggest that these 14-3-3 proteins serve to suppress the activation of Cdc25 throughout interphase in Xenopus egg extracts.
MATERIALS AND METHODS
Isolation of Cdc25-binding Proteins
Nickel-agarose beads containing His6-Cdc25 were incubated for 30 min in interphase Xenopus egg extracts or in cytostatic factor-arrested (M-phase) extracts. The beads were isolated by centrifugation (Kumagai and Dunphy, 1997) and washed once in buffer A (10 mM HEPES-KOH [pH 7.5], 20 mM β-glycerolphosphate, 500 mM NaCl, 5 mM 2-mercaptoethanol, 5 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 0.1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, 0.1 mM sodium orthovanadate, 10 μM phosphoserine, 10 μM phosphothreonine, and 10 μM phosphotyrosine) containing 1 μM microcystin, washed three times with buffer A lacking microcystin, and washed twice with HBS (10 mM HEPES-KOH [pH 7.5] and 150 mM NaCl). Bound proteins were eluted with 150 mM imidazole in HBS, separated by SDS-PAGE, and silver-stained.
Peptide Sequencing
The eluted 28-kDa and 31-kDa Cdc25-binding proteins were alkylated with iodoacetamide, separated by SDS-PAGE, and stained with Coomassie blue. Gel pieces containing p28 and p31 were washed with 50% acetonitrile, dried in a SpeedVac, and digested with lysyl endopeptidase (Wako Bioproducts, Richmond, VA) or with trypsin (Boehringer Mannheim, Indianapolis, IN), respectively (Hellman et al., 1995). The peptides were eluted and separated by reverse-phase chromatography. Peptide sequence analysis was performed with an ABI 476A sequencer in the Caltech Protein/Peptide Micro Analytical Laboratory.
Cloning of Xenopus 14-3-3ε and 14-3-3ζ
14-3-3ε.
Two oligonucleotides (GTIGCIGGIATGGATGTIGA and GCIGCIGGIATIAIITGTTTITC) were designed on the basis of the peptide sequences. A polymerase chain reaction (PCR) was performed with these oligonucleotides by using Xenopus oocyte cDNA as the template (Kumagai and Dunphy, 1996). The reaction yielded a single 250-bp fragment. A Xenopus oocyte cDNA library was screened with the 250-bp fragment as a probe. 5′ truncated and 3′ truncated overlapping clones were isolated. PCR primers (a, GGAATTCCATATGGAAGAGCGAGAGGATTTAG; b, GGAATTCCCCAGTCAGATATCCAGTAGTAC) were designed for the 5′ and 3′ ends of the open reading frame. A PCR was performed with oligonucleotides a and b by using Xenopus oocyte cDNA to obtain the complete coding sequence flanked by NdeI and EcoRI sites. The PCR product was cloned into the pET9His6 vector that had been digested with NdeI and EcoRI. The insert was sequenced by standard methods.
14-3-3ζ.
Two oligonucleotides containing an NdeI site at the start codon and an EcoRI site after the stop codon were designed by using the published Xenopus 14-3-3ζ sequence (GenBank accession number X95519; Kousteni et al., 1997). These primers (c, GGAATTCCATATGGATAAAAATGAACTGGTCCAG; d, GGAATTCCTTAGTTCTCCCCTCCTTCTCCTTG) were used to amplify a fragment from Xenopus oocyte cDNA, which was then cloned into pET9His6 vector. The insert was sequenced by standard methods. The sequence matched the published sequence of the Xenopus 14-3-3ζ protein except for a small stretch from amino acids 172 to 187. Because this region is highly conserved among 14-3-3 proteins, the previously published sequence is most likely erroneous in this area.
Production of Antibodies to His6-14-3-3ε and His6-14-3-3ζ
Escherichia coli BL21DE3(LysS) was transformed with either pET9His6-14-3-3ε or pET9His6-14-3-3ζ. Proteins were expressed by adding 0.4 mM isopropyl β-d-thiogalactoside at 20°C for 3 h. Cells were harvested and stored frozen at −80°C. Cells were suspended in 0.2 M Tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl, pH 7.5), 0.5 M NaCl, 0.1% Triton X-100, 5 mM 2-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml pepstatin, 10 μg/ml chymostatin, and 10 μg/ml leupeptin and sonicated. The lysate was centrifuged at 10,000 rpm for 10 min in an HB-4 rotor (DuPont, Newtown, CT). The supernatant was mixed with nickel-agarose beads for 30 min at 4°C. Bound proteins were washed three times with 0.2 M Tris-HCl (pH 7.5), 0.5 M NaCl, 0.1% Triton X-100, and 5 mM 2-mercaptoethanol and three times with HBS. The His6-14-3-3 proteins were eluted with 150 mM imidazole in HBS. Rabbit polyclonal antibodies were raised at a commercial facility. Antibodies were affinity-purified by using His6-14-3-3 protein that had been coupled to CNBr-activated Sepharose (Pharmacia, Piscataway, NJ). The anti-14-3-3ε antibodies used in this article could immunoprecipitate both 14-3-3ε and 14-3-3ζ but could detect only 14-3-3ε in immunoblots. The anti-14-3-3ζ antibodies did not cross-react with 14-3-3ε in immunoblots and could not immunoprecipitate 14-3-3ε.
Production of Wild-Type and S287A His6-Cdc25 Proteins in Insect Cells
The NcoI–XhoI fragment of Xenopus Cdc25 was produced by PCR using Pfu DNA polymerase (Stratagene, La Jolla, CA), pBluescript SK-Cdc25-1 (Kumagai and Dunphy, 1992) as the template, and the following two oligonucleotides: Cdc25-NcoI, CATGCCATGGCAGAGAGTCACATAATG; Cdc25-XhoI, TTCGGCTCGAGTTAAAGCTTCATTATGCGGGC. The fragment was digested with NcoI and XhoI and cloned into pFastBacHTa. The His6-Cdc25-S287A mutant was created by PCR using two oligonucleotides (CTAGTCTAGAGCGGTTTAAAGATATATTGTTTACATTG and CTAGTCTAGACTTTACCGATCGCCTGCTATGCC) in addition to the two oligonucleotides described above. The resulting two PCR fragments were digested with either NcoI and XbaI or XbaI and XhoI and then cloned into pFastBacHTa that had been digested with NcoI and XhoI. Baculoviruses were produced by using the Bac-to-Bac baculovirus expression system (Life Technologies, Gaithersburg, MD). Proteins were harvested from Sf9 insect cells after 48 h of infection and purified by using nickel-agarose beads (Pharmacia) as described (Kumagai and Dunphy, 1997).
Phosphopeptide Mapping
Nickel-agarose beads (5 μl) containing either wild-type His6-Cdc25 or His6-Cdc25-S287A were incubated for 50 min in 100 μl of Xenopus interphase egg extract containing 100 μg/ml cycloheximide and 0.1 mCi of [32P]orthophosphate. Beads were washed once with buffer A containing 1 μM microcystin, three times with buffer A, and twice with HBS. Proteins were eluted with 150 mM imidazole in HBS. Samples were boiled in SDS sample buffer containing 15 mM dithiothreitol for 2 min. After cooling the sample to room temperature, 50 mM iodoacetamide was added and samples were incubated at room temperature for 15 min in the dark. Samples were separated by SDS-PAGE, and radioactive bands corresponding to the His6-Cdc25 protein were excised. The gel pieces were swollen in 25 mM ammonium bicarbonate and then washed in 50% acetonitrile/25 mM ammonium bicarbonate for three 30-min periods under agitation. The gel piece was dried in a SpeedVac. Trypsin (1 μg) dissolved in 25 mM ammonium bicarbonate was added to the dried gel piece. After swelling, additional 25 mM ammonium bicarbonate was added to immerse the gel piece. After an overnight incubation at 37°C, the supernatant was collected, and the gel piece was incubated once in 25 mM ammonium bicarbonate and twice in 50% acetonitrile/0.1% trifluoroacetic acid to elute digested peptides. The eluted peptides were pooled, dried in a SpeedVac, and subjected to thin layer electrophoresis and chromatography as described (Boyle et al., 1991).
Preparation of Xenopus Egg Extracts
Xenopus egg extracts were prepared as described previously (Murray, 1991; Mueller et al., 1995a). To impose the replication checkpoint, interphase egg extracts containing 1000 demembranated sperm nuclei per microliter were treated with 100 μg/ml aphidicolin (Dasso and Newport, 1990). Demembranated sperm nuclei were prepared from Xenopus testis as described (Smythe and Newport, 1991). To prepare UV-damaged nuclei, sperm nuclei were spread on Parafilm at a concentration of 105 nuclei per μl and treated with UV light (254 nm) in a Stratalinker (Stratagene) at a dose of 888 J/m2. Endogenous Cdc25 was immunodepleted from Xenopus egg extracts with anti-Cdc25 antibodies and Affi-prep protein A beads (Bio-Rad, Richmond, CA) that had been incubated in 1 mg/ml bovine serum albumin as described (Carpenter et al., 1996; Coleman et al., 1996). Cdc25 was immunodepleted from M-phase extracts prior to activation with calcium ion to avoid removal of endogenous 14-3-3 proteins.
Assay of the Activity of the Cdc25 Protein
A 32P-labeled Cdc2–cyclin B1 complex was prepared by phosphorylating Cdc2 with Myt1 as described (Kumagai and Dunphy, 1997). Nickel-agarose beads containing either His6-Cdc25 or His6-Cdc25-S287A were mixed with interphase extracts containing 100 μg/ml cycloheximide for 30 min at room temperature to allow the phosphorylation of Ser-287 and subsequent binding of Xenopus 14-3-3 proteins. Beads were washed as described above. The 32P-phosphorylated Cdc2–cyclin B1 complex was mixed with His6-Cdc25 protein or His6-Cdc25-S287A protein in the presence of 50 μg/ml His6-14-3-3ε protein in phosphatase buffer containing 5 mM dithiothreitol (Kumagai and Dunphy, 1997). Aliquots were taken at various times and the reaction was stopped by the addition of the gel sample buffer. Samples were separated by SDS-PAGE and the 32P remaining in Cdc2 was quantitated with a PhosphorImager.
RESULTS
Xenopus Cdc25 Associates with Two 14-3-3 Proteins
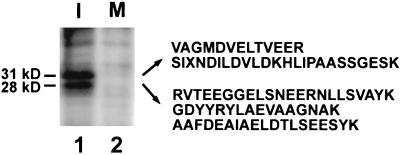
To explore the mechanisms underlying the activation of Cdc25 at mitosis, we asked whether potential regulatory proteins would associate with Cdc25 in Xenopus egg extracts during the course of the cell cycle. For this purpose, we first added nickel-agarose beads containing a histidine-tagged version of Cdc25 (His6-Cdc25) to interphase or M-phase egg extracts. After a 30-min incubation at 23°C, we reisolated the nickel agarose beads. Subsequently, the beads were washed extensively and bound proteins were eluted with imidazole. Gel electrophoresis and silver staining revealed the presence of two proteins with molecular masses of 28 kDa and 31 kDa (p28 and p31) that appeared to associate with the interphase form of His6-Cdc25 (Figure 1, lane 1). In control experiments, there was no binding of these two proteins to nickel-agarose lacking His6-Cdc25, indicating that these proteins do not bind nonspecifically to the nickel resin. Interestingly, the 28- and 31-kDa proteins did not associate with the M-phase hyperphosphorylated version of His6-Cdc25 (Figure 1, lane 2), suggesting that the interaction of these proteins with Cdc25 might vary during the cell cycle.
Figure 1.
The 28-kDa and 31-kDa proteins bind to the interphase form of Xenopus Cdc25. Nickel-agarose beads containing His6-Cdc25 protein were incubated in interphase (lane 1) or M-phase (lane 2) egg extracts. Bound proteins were eluted with 150 mM imidazole in HBS, separated by SDS-PAGE, and silver stained. From the 31-kDa protein, we obtained the following peptide sequences: 1, VAGMDVELTVEER; 2, SIXNDILDVLDKHLIPAASSGESK. From the 28-kDa protein, we obtained the following three sequences: 3, RVTEEGGELSNEERNLLSVAYK; 4, GDYYRYLAEVAAGNAK; 5, AAFDEAIAELDTLSEESYK. X denotes unreadable residues.
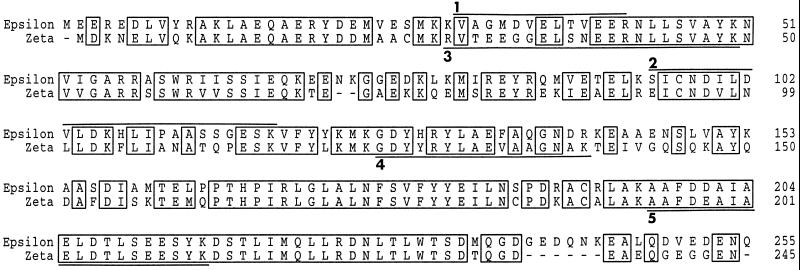
Next, we excised gel pieces containing the 28- and 31-kDa proteins and extensively digested both proteins in situ with lysyl endopeptidase or trypsin. Sequencing of three peptides from p28 revealed that it is a previously cloned Xenopus 14-3-3 protein (Kousteni et al., 1997). More specifically, p28 appears to correspond to the ζ isoform of Xenopus 14-3-3. Peptide sequencing suggested that p31 is also a 14-3-3 protein, but this particular isoform had not been identified previously from Xenopus. Therefore, we set out to clone the cDNA encoding this protein from a Xenopus oocyte library. With oligonucleotides based on the peptide sequences, we were able to amplify a single 250-bp fragment in a PCR. After obtaining and sequencing a full-length clone, we established that p31 corresponds to the ε isoform of Xenopus 14-3-3 (Figure 2), because it is 97% identical to human 14-3-3ε (Conklin et al., 1995). Among vertebrate 14-3-3 proteins, the ε isoform bears the greatest similarity to the Rad24 and Rad25 gene products of S. pombe (Ford et al., 1994).
Figure 2.
Comparison of the amino acid sequences of the Xenopus 14-3-3ε and 14-3-3ζ proteins. Peptides from the amino acid sequencing analysis in Figure 1 are underlined. The GenBank accession numbers for the DNA sequences of Xenopus 14-3-3ε and 14-3-3ζ are AF033311 and AF033312, respectively.
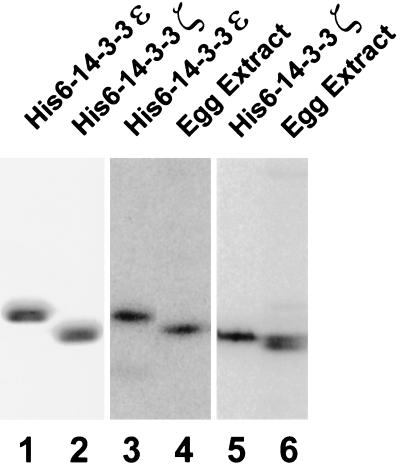
To examine the functional properties of p28 (14-3-3ζ) and p31 (14-3-3ε) in Xenopus egg extracts, we prepared histidine-tagged versions of both proteins (Figure 3) and then raised polyclonal antibodies in rabbits against each polypeptide. By immunoblotting, the anti-14-3-3ε antibodies recognize a single band of the anticipated size (Figure 3). The anti-14-3-3ζ antibodies detect a doublet of proteins in egg extracts, with the lower band matching the expected size of 14-3-3ζ (Figure 3). In principle, the upper band of the doublet could represent a modified form of 14-3-3ζ or a distinct isoform of 14-3-3 with which the anti-14-3-3ζ antibodies cross-react in immunoblots. We also used the anti-14-3-3 antibodies to measure the endogenous concentrations of 14-3-3ε and 14-3-3ζ in Xenopus extracts. With known amounts of recombinant His6-14-3-3ε and His6-14-3-3ζ as standards, we established that 14-3-3ε and 14-3-3ζ are each present at a concentration of approximately 40 μg/ml in egg extracts. This value corresponds to a molar concentration of 1.3 μM and 1.4 μM for the 14-3-3ε and 14-3-3ζ polypeptides, respectively. Because 14-3-3 proteins are known to form dimers (Aitken, 1996), the effective concentration of homodimeric 14-3-3ε and 14-3-3ζ would be 0.65 μM and 0.7 μM. By comparison, the concentration of endogenous Xenopus Cdc25C in such extracts is approximately 10 μg/ml or 0.14 μM (Kumagai and Dunphy, 1992).
Figure 3.
The proteins 14-3-3ε and 14-3-3ζ are abundant in Xenopus egg extracts. Bacterially expressed His6-14-3-3ε (lane 1, 2.5 μg of protein; lane 3, 40 ng of protein), His6-14-3-3ζ (lane 2, 2.5 μg of protein; lane 5, 40 ng of protein), and 1 μl of interphase egg extract (lanes 4 and 6) were subjected to SDS-PAGE and either stained with Coomassie blue (lanes 1 and 2) or immunoblotted with anti-14-3-3ε antibodies (lanes 3 and 4) or anti-14-3-3ζ antibodies (lanes 5 and 6). Note that the electrophoretic mobilities of the recombinant 14-3-3 proteins are reduced due to the presence of a six-histidine tag.
14-3-3 Proteins Bind to Ser-287 of Xenopus Cdc25
14-3-3 proteins have been shown to bind to a phosphoserine-containing motif. For example, 14-3-3 associates with the sequence RSTSTP in the protein kinase Raf, in which the underlined serine is phosphorylated (Muslin et al., 1996). Human Cdc25C contains a similar sequence (RSPS216MP), and it has been established that phosphorylation of Ser-216 is required for the interaction of 14-3-3 with human Cdc25C (Ogg et al., 1994; Peng et al., 1997).
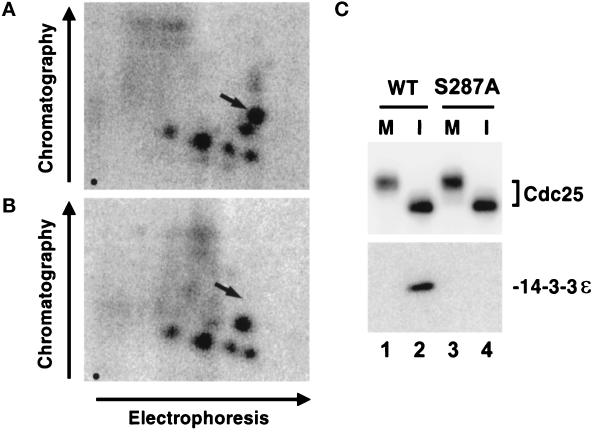
Xenopus Cdc25 contains an identical sequence (RSPS287MP) at a similar location within the polypeptide. To evaluate whether Xenopus Cdc25 is phosphorylated on Ser-287 in egg extracts, we changed this residue to Ala by site-directed mutagenesis to generate the His6-Cdc25-S287A mutant. Next, we added either wild-type His6-Cdc25 or the mutant His6-Cdc25-S287A to interphase extracts that had been equilibrated with [32P]orthophosphate to radiolabel endogenous ATP. Subsequently, the radiolabeled wild-type and S287A forms of His6-Cdc25 were subjected to tryptic phosphopeptide analysis. We observed that the map of the S287A mutant was missing a single major tryptic phosphopeptide (Figure 4), which suggests strongly that Ser-287 is a physiological site of phosphorylation in the Xenopus system.
Figure 4.
Phosphorylation of Ser-287 in Xenopus Cdc25 is essential for the binding of 14-3-3 proteins. (A and B) Tryptic phosphopeptide mapping of His6-Cdc25 (A) and His6-Cdc25-S287A (B) that had been radiolabeled in interphase extracts. Arrows indicate the expected position of the phosphopeptide containing Ser287. Origins are indicated by a dot in the lower left of each map. (C) The His6-Cdc25-S287A mutant does not bind to 14-3-3ε in egg extracts. His6-Cdc25 (lanes 1 and 2) and His6-Cdc25-S287A (lanes 3 and 4) were incubated in either M-phase (lanes 1 and 3) or interphase (lanes 2 and 4) extracts. The proteins were reisolated as described in MATERIALS AND METHODS and subjected to SDS-PAGE and immunoblotting with anti-Cdc25 antibodies (top) or anti-14-3-3ε antibodies (bottom).
To ask whether phosphorylation at Ser-287 mediates the interaction of 14-3-3 with Xenopus Cdc25, we added the wild-type and S287A forms of His6-Cdc25 to both interphase and M-phase egg extracts. Subsequently, we recovered the wild-type and mutant proteins with nickel-agarose and examined their association with 14-3-3 by immunoblotting with anti-14-3-3 antibodies. In particular, immunoblotting with anti-14-3-3ε antibodies indicated that 14-3-3ε binds to the interphase form of wild-type His6-Cdc25 but not the S287A mutant (Figure 4). By this analysis, neither the wild-type nor mutant Cdc25 could be found in a complex with 14-3-3ε during M-phase. In parallel studies, we demonstrated by immunoblotting with the anti-14-3-3ζ antibodies that Xenopus 14-3-3ζ also binds to the interphase but not M-phase form of wild-type His6-Cdc25, whereas it cannot associate with the His6-Cdc25-S287A mutant at either interphase or M-phase (our unpublished data). Collectively, these experiments establish that phosphorylation at Ser-287 is required for the binding of the ε and ζ forms of 14-3-3 to Xenopus Cdc25.
Endogenous Cdc25 in Xenopus Egg Extracts Is Associated Quantitatively with 14-3-3
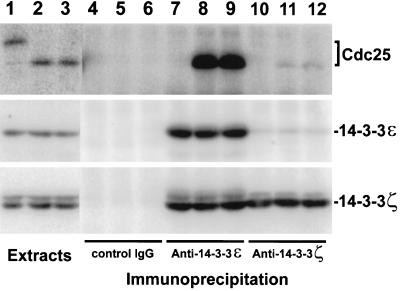
To characterize the interaction between Cdc25 and 14-3-3 in greater detail, we used the anti-14-3-3 antibodies to immunoprecipitate endogenous Cdc25 from Xenopus egg extracts. In addition to verifying that 14-3-3ε and -ζ bind to endogenous Cdc25 in egg extracts, these studies allowed us to examine the stoichiometry and regulation of this association. Immunoprecipitation with anti-14-3-3ε and -ζ antibodies revealed that endogenous Cdc25 in interphase extracts is associated mainly with the ε form of 14-3-3 (Figure 5). By quantitating the amount of Cdc25 that could be immunoprecipitated with the anti-14-3-3 antibodies, we estimate that 86% of the Cdc25 is associated with 14-3-3ε; most or all of the remaining Cdc25 appears to be bound to 14-3-3ζ. These findings suggest that endogenous Cdc25 has a higher affinity for 14-3-3ε than for 14-3-3ζ. Our observation that both 14-3-3ε and 14-3-3ζ bind with nearly equal efficiency to recombinant His6-Cdc25 (see Figure 1) may be due to the fact that this protein was added to the extract at a concentration that is higher than that of the endogenous Cdc25 (1 μM versus 0.14 μM). Consistent with the results described above, neither the anti-14-3-3ε nor the anti-14-3-3ζ antibodies could immunoprecipitate the M-phase form of endogenous Cdc25 (Figure 5).
Figure 5.
Xenopus Cdc25 binds mainly to 14-3-3ε in interphase extracts. Two microliters of M-phase extract (lane 1), interphase extract containing no sperm nuclei (lane 2), and interphase extract containing 3000 UV-damaged sperm nuclei per μl (lane 3) were subjected to SDS-PAGE and immunoblotted with anti-Cdc25 antibodies (top), anti-14-3-3ε antibodies (middle), or anti-14-3-3ζ antibodies (bottom). One hundred microliters of M-phase extract (lanes 4, 7, and 10), interphase extract containing no sperm nuclei (lanes 5, 8, and 11), and interphase extract containing 3000 UV-damaged sperm nuclei/μl (lanes 6, 9, and 12) were immunoprecipitated with control antibodies (lanes 4–6), anti-14-3-3ε antibodies (lanes 7–9), or anti-14-3-3ζ antibodies (lanes 10–12). Immunoprecipitated proteins were separated by SDS-PAGE and immunoblotted with anti-Cdc25 antibodies (top), anti-14-3-3ε antibodies (middle), or anti-14–3-3ζ antibodies (bottom). All extracts contained 100 μg/ml cycloheximide.
In fission yeast and humans, 14-3-3 proteins have been implicated in the G2–M checkpoint (Ford et al., 1994; Peng et al., 1997; Sanchez et al., 1997). In the Xenopus system, a putative DNA damage checkpoint response can be elicited by the addition of UV-treated sperm chromatin (Kumagai and Dunphy, unpublished data). The replication checkpoint can be triggered by the addition of the DNA polymerase inhibitor aphidicolin and sperm chromatin (Dasso and Newport, 1990).
To examine the effect of damaged DNA on the interaction between 14-3-3 and Cdc25, we immunoprecipitated Xenopus egg extracts containing UV-damaged nuclei with antibodies against the ε and ζ forms of 14-3-3. These experiments indicated that the binding of both 14-3-3 proteins to Cdc25 is similar in the absence and presence of damaged DNA (Figure 5). In other experiments, we observed that the association of both 14-3-3 proteins with Cdc25 is essentially identical in aphidicolin-treated extracts containing unreplicated DNA and in control extracts lacking this replication inhibitor (Yakowec and Dunphy, unpublished data). Collectively, these experiments indicate that the inactive form of Cdc25 found during interphase is quantitatively associated with 14-3-3 proteins. Furthermore, this interaction is maintained in the presence of damaged and unreplicated DNA.
Effect of the S287A Mutant of Cdc25 on Cell Cycle Progression in Xenopus Egg Extracts
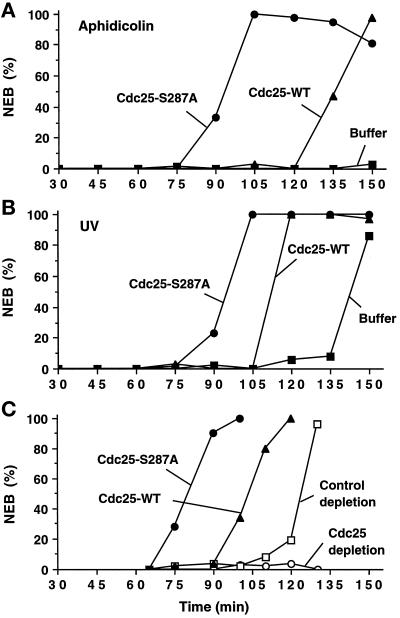
The above studies indicate that the inactive form of Cdc25 in interphase extracts is associated with 14-3-3 proteins, whereas the mitotically active version of Cdc25 is devoid of 14-3-3 proteins. To explore the possibility that 14-3-3 proteins play a causal role in suppressing Cdc25 during interphase, we asked whether the S287A version of Cdc25 with a mutation in its 14-3-3 binding site would affect the transit through the cell cycle in Xenopus egg extracts (Figure 6). For this purpose, we introduced either the wild-type or S287A form of His6-Cdc25 into various types of Xenopus egg extracts and then examined the effect on mitotic timing, as observed by the breakdown of reconstituted nuclei in the extracts. Because the fission yeast 14-3-3 homologues Rad24 and Rad25 had been implicated in G2 checkpoint control (Ford et al., 1994), we examined the effect of the S287A mutant on egg extracts containing unreplicated or damaged DNA. In one set of experiments, we treated extracts containing sperm chromatin (1000 nuclear equivalents of DNA per μl of extract) with aphidicolin to impose the replication checkpoint. Subsequently, we added wild-type His6-Cdc25, the His6-Cdc25-S287A mutant, or control buffer to the extracts and then monitored the timing of NEB. Typically, we introduced the recombinant His6-Cdc25 proteins to a final concentration of 10 μg/ml (0.14 μM). Because the concentration of endogenous Cdc25 is also 10 μg/ml (0.14 μM), this procedure doubles the total concentration of Cdc25 in the extracts. As expected, the aphidicolin-treated extracts containing added buffer alone remained in interphase for at least 150 min (Figure 6A). For comparison, control extracts lacking unreplicated DNA entered mitosis 75–90 min after activation. Both the wild-type and S287A mutant His6-Cdc25 proteins triggered mitosis in the absence of DNA replication and thus overrode the replication checkpoint. Significantly, the effect of the S287A mutant was much more pronounced. This mutant elicited half-maximal NEB at 90–95 min, which is approximately 40–45 min earlier than the time of half-maximal NEB in the extract containing wild-type His6-Cdc25 (Figure 6A). In parallel experiments, we found that exogenously added wild-type His6-Cdc25 and His6-Cdc25-S287A proteins could override the checkpoint-induced delay of mitosis in the presence of UV-damaged DNA (Figure 6B). As in the aphidicolin-treated extracts, the S287A mutant was more effective, eliciting mitosis approximately 20 min earlier than wild-type His6-Cdc25.
Figure 6.
Cdc25-S287A mutant induces mitosis more efficiently than the wild-type Cdc25 protein. His6-Cdc25 (▴), His6-Cdc25-S287A (•), or buffer alone (▪) were added to the interphase egg extracts containing either aphidicolin (A) or UV-damaged sperm nuclei (B). (C) Xenopus egg extracts were immunodepleted with anti-Cdc25 antibodies (▴, •, or ○) or control rabbit anti-mouse IgG antibodies (□). We verified by immunoblotting with anti-Cdc25 antibodies that Cdc25 was quantitatively removed by this procedure. To the Cdc25-depleted extracts, we added His6-Cdc25 (▴), His6-Cdc25-S287A (•), or buffer alone (○). Buffer alone was added to the control-depleted extract (□). (A–C) Recombinant His6-Cdc25 and His6-Cdc25-S287A were added to a final concentration of 10 μg/ml (0.14 μM). Aliquots were taken at the indicated times and NEB was monitored by microscopy.
In other experiments, we asked whether the His6-Cdc25-S287A would affect mitotic timing in extracts lacking a replication inhibitor or damaged DNA. For these experiments, we first immunodepleted the endogenous Cdc25 in the egg extracts with anti-Cdc25 antibodies (Figure 6C). As expected, Cdc25-depleted extracts were unable to enter mitosis. In control extracts that had undergone a mock immunodepletion with an irrelevant antibody (rabbit anti-mouse IgG), NEB occurred at about 120 min. We found that both wild-type His6-Cdc25 and His6-Cdc25-S287A mutant could restore the ability of Cdc25-depleted extracts to proceed into M-phase. However, consistent with the results described above, NEB was consistently 20–30 min earlier in extracts containing the S287A mutant. Collectively, these experiments suggest that 14-3-3 proteins provide an inhibitory constraint on Cdc25. When this inhibitory mechanism is abolished by destruction of the 14-3-3 binding site in Cdc25, Xenopus egg extracts enter mitosis at an accelerated pace and are unable to arrest effectively in interphase in response to unreplicated and UV-damaged DNA.
Effect of 14-3-3 Proteins on Cdc25 Activity
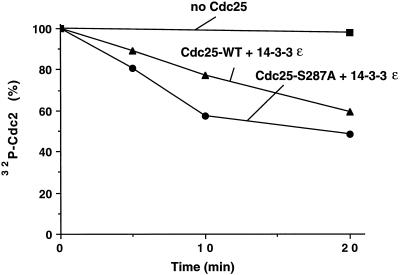
The above experiments implicate 14-3-3 proteins as negative regulators of Cdc25. In principle, 14-3-3 proteins could inhibit the catalytic activity of Cdc25. Alternatively, 14-3-3 proteins could influence the action of Cdc25 by inhibiting its interaction with positive regulators, enhancing its recognition by negative regulators, or by physically preventing its contact with the Cdc2–cyclin B complex. As a first step to distinguish between the possibilities, we asked whether binding of 14-3-3 would affect the in vitro phosphatase activity of Cdc25 (Figure 7). For this purpose, we preincubated the wild-type His6-Cdc25 and His6-Cdc25-S287A proteins in Xenopus egg extracts. During this incubation, the wild-type but not the mutant protein became phosphorylated on Ser-287 and bound to 14-3-3. Next, we isolated the wild-type and mutant Cdc25 proteins with nickel-agarose and incubated them with both recombinant His6-14-3-3ε and a Cdc2–cyclin B complex that had been phosphorylated with 32P in vitro on both Tyr-15 and Thr-14 by treatment with recombinant Myt1 kinase. Dephosphorylation of the radiolabeled Cdc2 protein was monitored by SDS gel electrophoresis. We observed that under these experimental conditions the phosphatase activity of the His6-Cdc25-S287A mutant that cannot bind to 14-3-3 was only slightly higher (less than twofold) than that of the wild-type His6-Cdc25 containing 14-3-3.
Figure 7.
Effect of 14-3-3 proteins on Cdc25 activity. Nickel-agarose beads containing His6-Cdc25 protein and the His6-Cdc25-S287A mutant protein were incubated in interphase extracts. Beads were isolated and bound proteins were eluted with 150 mM imidazole in HBS. A 32P-phosphorylated Cdc2–cyclin B complex phosphorylated in vitro by Myt1 was mixed with His6-Cdc25 (▴), His6-Cdc25-S287A (•), or control buffer (▪) in the presence of 50 μg/ml His6-14-3-3ε protein. Aliquots were taken at the times indicated and subjected to SDS-PAGE. 32P remaining on Cdc2 was quantitated by using a PhosphorImager. In control experiments, His6-Cdc25 and His6-Cdc25-S287A purified directly from baculovirus-infected insect cells showed very similar Cdc2-specific phosphatase activity.
DISCUSSION
In this report, we have examined the regulation of the mitotic inducer Cdc25C by 14-3-3 proteins during the cell cycle in Xenopus egg extracts. We have observed that the inactive hypophosphorylated form of Cdc25 present during interphase is quantitatively associated with two 14-3-3 proteins (p28 and p31). p31 is most similar to the ε form of 14-3-3 and appears to be the major partner of Cdc25: approximately 86% of Cdc25 is bound to 14-3-3ε during interphase. The other binding partner (p28) most closely resembles the 14-3-3ζ protein. It appears that most, if not all, of the inactive Cdc25 in Xenopus extracts is bound to either the ε or ζ form of 14-3-3. Furthermore, both the ε and ζ homodimers would each be present in an approximately fivefold molar excess over Cdc25 in egg extracts. Thus, the abundance of 14-3-3 proteins and the stoichiometry of their interaction with Cdc25 could account for the suppression of endogenous Cdc25 in Xenopus egg extracts during interphase.
A variety of observations strongly suggest that these 14-3-3 proteins act as negative regulators of Cdc25. First, the binding of 14-3-3 proteins to Cdc25 is highly regulated during the cell cycle. 14-3-3 proteins bind only to the interphase form of Cdc25 that displays weak activity toward the Cdc2–cyclin B complex. In contrast, the mitotic form of Cdc25 that can efficiently dephosphorylate Cdc2–cyclin B contains no detectable 14-3-3 protein. Another argument that 14-3-3 proteins negatively regulate Cdc25 is that a mutant of Cdc25 containing a single amino acid change that completely abrogates 14-3-3 binding shows a strongly enhanced ability to induce mitosis. This Cdc25-S287A mutant can compromise the checkpoints involving unreplicated and damaged DNA. In the case of the replication checkpoint, Xenopus egg extracts containing unreplicated DNA and the Cdc25-S287A mutant enter mitosis efficiently even though DNA synthesis has been completely abolished by the treatment with aphidicolin. Significantly, in such extracts, mitosis takes place at about 90 min after activation of the extract, which corresponds to the time that extracts lacking aphidicolin would normally enter mitosis. It appears that the presence of the Cdc25-S287A mutant largely abolishes the responsiveness of Xenopus egg extracts to unreplicated/damaged DNA. Recently, Peng et al. (1997) have shown that a mutant of human Cdc25C (S216A) with a defective 14-3-3 binding site overrides G2 checkpoint controls in human cells. Thus, the regulation of Cdc25 by binding of 14-3-3 proteins appears to be a conserved mechanism of checkpoint control in vertebrates.
The molecular mechanism by which 14-3-3 proteins suppress the action of Cdc25 remains to be established. Because 14-3-3 proteins are known to form dimers (Aitken, 1996), it is plausible that the binding of 14-3-3 may serve to oligomerize Cdc25 in egg extracts during interphase. As described herein, the binding of 14-3-3 appears to result in only a modest suppression (less than twofold) of the ability of Cdc25 to dephosphorylate a recombinant Cdc2–cyclin B complex, but we cannot be certain that these in vitro assays faithfully recapitulate the conditions found in vivo. Notwithstanding this caveat, it is conceivable that such a small effect on Cdc25 activity could tip the balance between the competing actions of Cdc25 and Wee1/Myt1 so that the Tyr-15 and Thr-14 dephosphorylation of Cdc2 could not proceed as long as 14-3-3 proteins remain bound, but other possibilities must also be considered. For example, the binding of 14-3-3 could preclude the interaction of Cdc25 with positive regulators. At least two kinases, including Cdc2–cyclin B and Plx1, phosphorylate Cdc25 in its N-terminal regulatory domain and stimulate its phosphatase activity at mitosis (Izumi and Maller, 1995; Kumagai and Dunphy, 1996). Perhaps binding of 14-3-3 could hinder the ability of these kinases to carry out their stimulatory phosphorylations. Alternatively, 14-3-3 proteins could enhance the ability of Cdc25 to interact with negative regulators such as the PP2A-like, Cdc25-inhibitory phosphatase (Kumagai and Dunphy, 1992; Clarke et al., 1993).
Another type of explanation for the function of 14-3-3 would be that these proteins might preclude the ability of Cdc25 to interact physically with the Cdc2–cyclin B complex. For example, 14-3-3 proteins could directly affect the recognition of Cdc2–cyclin B by Cdc25 or could indirectly prevent this interaction by keeping Cdc25 at an intracellular location where it would not have access to Cdc2–cyclin B. Xenopus Cdc25 contains an excellent putative bipartite nuclear localization signal with the sequence KRPVRPLDSETPVRVKRRR (the two basic clusters are underlined). Intriguingly, this putative nuclear localization sequence resides at amino acid residues 298 to 315 in Cdc25 and thus lies in close proximity to the 14-3-3 binding site around Ser-287, raising the possibility that binding of 14-3-3 could influence the intracellular localization of Cdc25. The localization of Cdc25C varies somewhat depending on the cell type. In human and fission yeast cells, Cdc25C is a nuclear protein during G2-phase (Millar et al., 1991; Girard et al., 1992). In hamster cells, Cdc25C is cytoplasmic during G2 and enters the nucleus at about the beginning of mitosis (Seki et al., 1992). At this time, it is not known whether the intracellular localization of Cdc25C plays a causal role in mitotic entry.
Recent studies have implicated Chk1 as the kinase that phosphorylates Ser-216 in the 14-3-3 binding site of human Cdc25C (Peng et al., 1997; Sanchez et al., 1997). A Xenopus homologue of Chk1 has not been described, but clearly it will be valuable to ask whether a putative Chk1 homologue can phosphorylate Ser-287 of Xenopus Cdc25 to allow the binding of 14-3-3ε and 14-3-3ζ. Interestingly, the binding of 14-3-3ε and -ζ to Xenopus Cdc25 is similar whether or not the replication/damage checkpoint has been activated. This observation suggests that a kinase that phosphorylates Ser-287 is active in the absence of a checkpoint-triggered delay of mitosis. Thus, the function of Chk1 could be to maintain this critical phosphorylation of Cdc25 past the time at which mitosis would normally occur in an extract lacking unreplicated or damaged DNA.
In previous studies, we have analyzed the activities of the various enzymes controlling the tyrosine phosphorylation of Cdc2 in the absence and presence of unreplicated DNA (Kumagai and Dunphy, 1992, 1995; Mueller et al., 1995a,b). These studies have indicated that both Wee1 and Myt1 are highly active during interphase and that their kinase activities toward Cdc2–cyclin B as measured in vitro are not detectably altered by the presence of unreplicated DNA. In the case of Cdc25, its phosphatase activity is maintained in the same inactive state in the presence and absence of unreplicated DNA. It is apparent that this inactive form of Cdc25 is complexed with 14-3-3 proteins and that imposition of the replication checkpoint would keep Cdc25 in this state. As a consequence, the dephosphorylation of Tyr-15 and Thr-14 on Cdc2 would be precluded. A similar mechanism appears to account for the interphase arrest of egg extracts containing UV-damaged DNA. According to this scheme, the inhibitory phosphorylation of Cdc2 would be the ultimate target of the unreplicated and damaged DNA checkpoints in Xenopus egg extracts, as is the case in fission yeast and humans (Enoch and Nurse, 1990; Lundgren et al., 1991; Jin et al., 1996; Blasina et al., 1997; Furnari et al., 1997; O’Connell et al., 1997; Peng et al., 1997; Rhind et al., 1997; Sanchez et al., 1997).
In conclusion, we have found that 14-3-3 proteins negatively regulate the ability of Cdc25 to induce mitosis in Xenopus egg extracts. When this negative regulatory system is compromised by a mutation that prevents Cdc25 from interacting with 14-3-3, the unreplicated and damaged DNA checkpoints are unable to operate properly. In the future, it will be important to elucidate the molecular mechanisms controlling the association of 14-3-3 proteins with Cdc25 and how these regulatory mechanisms are modulated by unreplicated and damaged DNA.
ACKNOWLEDGMENTS
We thank the other members of our laboratory for comments on the manuscript. This work was supported in part by a grant from the NIH (GM43974). W.G.D. is an investigator of the Howard Hughes Medical Institute.
REFERENCES
- Aitken A. 14-3-3 and its possible role in co-ordinating multiple signalling pathways. Trends Cell Biol. 1996;6:341–347. doi: 10.1016/0962-8924(96)10029-5. [DOI] [PubMed] [Google Scholar]
- al-Khodairy F, Carr AM. DNA repair mutants defining G2 checkpoint pathways in Schizosaccharomyces pombe. EMBO J. 1992;11:1343–1350. doi: 10.1002/j.1460-2075.1992.tb05179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasina A, Paegle ES, McGowan CH. The role of inhibitory phosphorylation of CDC2 following DNA replication block and radiation-induced damage in human cells. Mol Biol Cell. 1997;8:1013–1023. doi: 10.1091/mbc.8.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WJ, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Carpenter PB, Mueller PR, Dunphy WG. Role for a Xenopus Orc2-related protein in controlling DNA replication. Nature. 1996;379:357–360. doi: 10.1038/379357a0. [DOI] [PubMed] [Google Scholar]
- Clarke PR, Hoffmann I, Draetta G, Karsenti E. Dephosphorylation of cdc25-C by a type-2A protein phosphatase: specific regulation during the cell cycle in Xenopus egg extracts. Mol Biol Cell. 1993;4:397–411. doi: 10.1091/mbc.4.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman TR, Carpenter PB, Dunphy WG. The Xenopus Cdc6 protein is essential for the initiation of a single round of DNA replication in cell-free extracts. Cell. 1996;87:53–63. doi: 10.1016/s0092-8674(00)81322-7. [DOI] [PubMed] [Google Scholar]
- Coleman TR, Dunphy WG. Cdc2 regulatory factors. Curr Opin Cell Biol. 1994;6:877–882. doi: 10.1016/0955-0674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Conklin DS, Galaktionov K, Beach D. 14-3-3 proteins associate with cdc25 phosphatases. Proc Natl Acad Sci USA. 1995;92:7892–7896. doi: 10.1073/pnas.92.17.7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasso M, Newport JW. Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: studies in Xenopus. Cell. 1990;61:811–823. doi: 10.1016/0092-8674(90)90191-g. [DOI] [PubMed] [Google Scholar]
- Dunphy WG, Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991;67:189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- Enoch T, Nurse P. Mutation of fission yeast cell cycle control genes abolishes dependence of mitosis on DNA replication. Cell. 1990;60:665–673. doi: 10.1016/0092-8674(90)90669-6. [DOI] [PubMed] [Google Scholar]
- Featherstone C, Russell P. Fission yeast p107wee1 mitotic inhibitor is a tyrosine/serine kinase. Nature. 1991;349:808–811. doi: 10.1038/349808a0. [DOI] [PubMed] [Google Scholar]
- Ford JC, al-Khodairy F, Fotou E, Sheldrick KS, Griffiths DJ, Carr AM. 14-3-3 protein homologs required for the DNA damage checkpoint in fission yeast. Science. 1994;265:533–535. doi: 10.1126/science.8036497. [DOI] [PubMed] [Google Scholar]
- Furnari B, Rhind N, Russell P. Cdc25 mitotic inducer targeted by Chk1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- Girard F, Strausfeld U, Cavadore JC, Russell P, Fernandez A, Lamb NJ. cdc25 is a nuclear protein expressed constitutively throughout the cell cycle in nontransformed mammalian cells. J Cell Biol. 1992;118:785–794. doi: 10.1083/jcb.118.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman U, Wernstedt C, Gonez J, Heldin CH. Improvement of an “in-gel” digestion procedure for the micropreparation of internal protein fragments for amino acid sequencing. Anal Biochem. 1995;224:451–455. doi: 10.1006/abio.1995.1070. [DOI] [PubMed] [Google Scholar]
- Hoffmann I, Clarke PR, Marcote MJ, Karsenti E, Draetta G. Phosphorylation and activation of human cdc25-C by cdc2–cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J. 1993;12:53–63. doi: 10.1002/j.1460-2075.1993.tb05631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, Nagata A, Jinno S, Suto K, Okayama H. Wee1(+)-like gene in human cells. Nature. 1991;353:80–83. doi: 10.1038/353080a0. [DOI] [PubMed] [Google Scholar]
- Izumi T, Maller JL. Phosphorylation and activation of the Xenopus Cdc25 phosphatase in the absence of Cdc2 and Cdk2 kinase activity. Mol Biol Cell. 1995;6:215–226. doi: 10.1091/mbc.6.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Walker DH, Maller JL. Periodic changes in phosphorylation of the Xenopus cdc25 phosphatase regulate its activity. Mol Biol Cell. 1992;3:927–939. doi: 10.1091/mbc.3.8.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Gu Y, Morgan DO. Role of inhibitory CDC2 phosphorylation in radiation-induced G2 arrest in human cells. J Cell Biol. 1996;134:963–970. doi: 10.1083/jcb.134.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RW, Jackson PK, Kirschner MW. Mitosis in transition. Cell. 1994;79:563–571. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- Kousteni S, Tura F, Sweeney GE, Ramji DP. Sequence and expression analysis of a Xenopus laevis cDNA which encodes a homologue of mammalian 14-3-3 zeta protein. Gene. 1997;190:279–285. doi: 10.1016/s0378-1119(97)00013-9. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992;70:139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Control of the Cdc2/cyclin B complex in Xenopus egg extracts arrested at a G2/M checkpoint with DNA synthesis inhibitors. Mol Biol Cell. 1995;6:199–213. doi: 10.1091/mbc.6.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Regulation of Xenopus Cdc25 protein. Methods Enzymol. 1997;283:564–571. doi: 10.1016/s0076-6879(97)83044-3. [DOI] [PubMed] [Google Scholar]
- Lundgren K, Walworth N, Booher R, Dembski M, Kirschner M, Beach D. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell. 1991;64:1111–1122. doi: 10.1016/0092-8674(91)90266-2. [DOI] [PubMed] [Google Scholar]
- McGowan CH, Russell P. Cell cycle regulation of human WEE1. EMBO J. 1995;14:2166–2175. doi: 10.1002/j.1460-2075.1995.tb07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar JB, Blevitt J, Gerace L, Sadhu K, Featherstone C, Russell P. p55CDC25 is a nuclear protein required for the initiation of mitosis in human cells. Proc Natl Acad Sci USA. 1991;88:10500–10504. doi: 10.1073/pnas.88.23.10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Dunphy WG. Cell cycle regulation of a Xenopus Wee1-like kinase. Mol Biol Cell. 1995a;6:119–134. doi: 10.1091/mbc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Kumagai A, Dunphy WG. Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science. 1995b;270:86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Muslin AJ, Tanner JW, Allen PM, Shaw AS. Interaction of 14–3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell. 1996;84:889–897. doi: 10.1016/s0092-8674(00)81067-3. [DOI] [PubMed] [Google Scholar]
- O’Connell MJ, Raleigh JM, Verkade HM, Nurse P. Chk1 is a wee1 kinase in the G2 DNA damage checkpoint inhibiting cdc2 by Y15 phosphorylation. EMBO J. 1997;16:545–554. doi: 10.1093/emboj/16.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg S, Gabrielli B, Piwnica-Worms H. Purification of a serine kinase that associates with and phosphorylates human Cdc25C on serine 216. J Biol Chem. 1994;269:30461–30469. [PubMed] [Google Scholar]
- Parker LL, Piwnica-Worms H. Inactivation of the p34cdc2–cyclin B complex by the human WEE1 tyrosine kinase. Science. 1992;257:1955–1957. doi: 10.1126/science.1384126. [DOI] [PubMed] [Google Scholar]
- Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- Rhind N, Furnari B, Russell P. Cdc2 tyrosine phosphorylation is required for the DNA damage checkpoint in fission yeast. Genes Dev. 1997;11:504–511. doi: 10.1101/gad.11.4.504. [DOI] [PubMed] [Google Scholar]
- Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, Elledge SJ. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- Seki T, Yamashita K, Nishitani H, Takagi T, Russell P, Nishimoto T. Chromosome condensation caused by loss of RCC1 function requires the cdc25C protein that is located in the cytoplasm. Mol Biol Cell. 1992;3:1373–1388. doi: 10.1091/mbc.3.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe C, Newport JW. Systems for the study of nuclear assembly, DNA replication, and nuclear breakdown in Xenopus laevis egg extracts. Methods Cell Biol. 1991;35:449–468. doi: 10.1016/s0091-679x(08)60583-x. [DOI] [PubMed] [Google Scholar]
- Strausfeld U, Labbe JC, Fesquet D, Cavadore JC, Picard A, Sadhu K, Russell P, Doree M. Dephosphorylation and activation of a p34cdc2/cyclin B complex in vitro by human CDC25 protein. Nature. 1991;351:242–245. doi: 10.1038/351242a0. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Broome M, Hunter T. Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 1995;14:1878–1891. doi: 10.1002/j.1460-2075.1995.tb07180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]