Abstract
The vomeronasal organ is the primary olfactory organ that detects sexual pheromones in mammals. We investigated the anatomy of the vomeronasal organ of the tammar wallaby (Macropus eugenii), a small macropodid marsupial. Pheromones may be important for activation of the hypothalamo-pituitary axis of tammar males at the start of the breeding season because plasma testosterone and luteinizing hormone concentration in males rise concurrently with pregnancy and the post-partum ovulation in females. The gross anatomy and the connection to the brain of the vomeronasal organ were examined by light and electron microscopy in adult male and female tammars. The vomeronasal organ was well developed in both sexes. The vomeronasal organ is a tubular organ connected at the rostral end via the nasopalatine duct (incisive duct) to the mouth and nasal cavity. At the rostral end the lumen of the vomeronasal organ was crescent shaped, changing to a narrow oval shape caudally. Glandular tissue associated with the vomeronasal organ increased towards the blind end of the organ. The tammar has the typical pattern of mammalian vomeronasal organs with electron-dense supporting cells and electron-lucent receptor cells. Microvilli were present on the surface of both epithelia while cilia were only found on the surface of the non-receptor epithelium. Some non-receptor epithelial cells appeared to secrete mucus into the vomeronasal organ lumen. The vomeronasal organ shows a high degree of structural conservation compared with eutherian mammals. The degree of vomeronasal organ development makes it likely that, as in other mammals, pheromones are important in the reproduction of the tammar.
Keywords: accessory olfactory bulb, pheromone, tammar wallaby, vomeronasal organ
Introduction
The mammalian olfactory system consists of the main olfactory system and the vomeronasal system (Brennan, 2001; Halpern & Martinez-Marcos, 2003). In air-breathing animals the main olfactory system can discriminate an enormous variety of volatile chemicals present in the environment (Guo et al. 1997) while the vomeronasal system detects volatile and non-volatile chemicals in a fluid phase (Keverne, 2004). The chemicals that activate the olfactory system are called odours and the chemicals known as pheromones are those used for intraspecies communication (Johnston, 1998; Meredith, 2001; Rodrigues, 2004). The vomeronasal organ (VNO) is a paired tubular organ that is found in most tetrapods and is a part of the vomeronasal system (Halpern, 1987; Halpern & Martinez-Marcos, 2003). Whereas the VNO is thought to detect pheromones or pheromone blends (Johnston, 1998), the main olfactory epithelium (MOE) is thought to detect ‘general odorants’ that tell the animal about its environment (Restrepo et al. 2004). However, the VNO also responds to odorants that normally act as pheromones (Sam et al. 2001; Trinh & Storm, 2003) while some pheromones can be detected by the MOE (Hudson & Distel, 1986).
In mammals, the VNO lies at the base of each nasal cavity stretching along the sides of the septum. The development and relative size vary substantially between species (Estes, 1972; Dawley, 1998). Humans have a small VNO of undetermined function (Halpern & Martinez-Marcos, 2003), whilst it is large, and has well-defined functions in rodents, equids and bovids (Broom, 1896a; Minett, 1925; Taniguchi & Mikami, 1985; Mendoza, 1993).
The morphology of the eutherian VNO has been intensively studied, especially in rodents (Mendoza, 1993; Garrosa et al. 1998). In most mammals there are two major epithelial types, the medial receptor epithelium and the lateral non-receptor epithelium (Takami, 2002). Both epithelia consist of pseudostratified columnar cells that are easily distinguished by their structure and thickness. The receptor epithelium is thicker than the non-receptor epithelium and contains the receptor, supporting and basal cells.
There are few data available on the VNO of marsupial mammals. The most detailed information is on the grey short-tailed opossum, Monodelphis domestica (Poran, 1998; Freyer, 1999). Studies on other marsupials have been mostly limited to light microscopy of small numbers of specimens (Broom, 1896b; Kratzing, 1982a, 1984). The available data indicate that there is a well-developed VNO in marsupials that may be important in detecting pheromones.
The marsupial best understood in terms of its reproductive physiology is the macropodid, the tammar wallaby (Macropus eugenii) (Tyndale-Biscoe & Renfree, 1987). The tammar has a distinct breeding season and most animals give birth at the end of January after a 26.5-day pregnancy (Renfree et al. 1989). Mating occurs about 1 h after birth (Rudd, 1994a) and the resulting conceptus develops only to the blastocyst stage whilst there is a sucking young in the pouch (Tyndale-Biscoe & Renfree, 1987). At the onset of the breeding season, luteinizing hormone and testosterone levels increase sharply in males (Catling & Sutherland, 1980; Inns, 1982) when oestradiol and progesterone levels are rising in females (Renfree, 1979; Flint & Renfree, 1982). However, this rise in testosterone does not occur in the absence of oestrous females (Catling & Sutherland, 1980). The increase of sexual hormone levels in the presence of oestrous females and associated behaviour shown by males, such as investigation of the females’ urogenital opening and pouch (Renfree et al. 1989; Rudd, 1994a), suggests that pheromones secreted by females during pregnancy might induce a physiological response in males. Furthermore, many male macropodids show flehmen-like behaviour (Gansloßer, 1979; Coulson & Croft, 1981; Croft, 1982). This behaviour involves retraction of the split upper lip exposing the upper incisors when investigating the urogenital opening of oestrous females (Coulson & Croft, 1981; Renfree et al. 1989).
In many species where pheromones are important for reproduction or behaviour, the VNO is well developed (Estes, 1972; Ladewig & Hart, 1980; Keverne, 2004). The extent of VNO development in tammars may therefore give insight into the function of the VNO in marsupials and will help to understand the co-evolution of the chemosensory system and reproductive behaviour in mammals generally. This study provides the first detailed investigation of the VNO in a macropodid marsupial, the tammar wallaby.
Methods
Animals
Tammar wallabies from our breeding colony were killed by an overdose of sodium pentabarbitone or by cervical dislocation, or were wild-shot animals from Kangaroo Island. Various observations of flehmen behaviour by male tammars interacting with female tammars were made by filming (Renfree et al. 1989) and by direct observation in our wallaby colony and in the wild. All samples of animals were collected using techniques approved by The University of Melbourne Animal Experimentation Ethics Committee.
Tissues
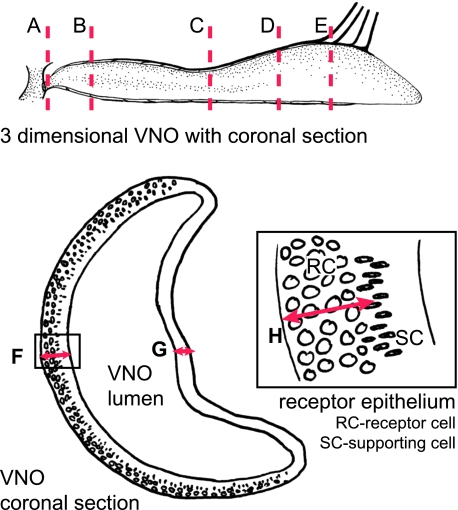
The gross anatomy of five adult male and five adult female tammars was examined to determine the relationship between external openings, the rostral palatine area and the nasopalatine duct opening. Two skulls of adult tammars were cut midsagittally to expose the structures of the nasal septum, the VNO and the cribriform plate. Nose samples of six male and eight female adult tammar wallabies were collected for light microscopy. All tammar adult tissue was fixed for at least 2 weeks and for one night under vacuum with 10% neutral buffered formalin before being decalcified with RDO (Rapid Decalcifier solution, Grale Scientific, Ringwood, Victoria, Australia) for 3 days under vacuum. Coronal sections were taken in four different areas (plane A to E, Fig. 1) of six males and six females. Sagittal sections were taken through VNOs from two females. Most sections were stained with Mallory's trichrome stain. Two coronal sections (plane B and D) of the VNO from three males and three females were stained with periodic acid-Schiff's reagent (PAS) and Alcian Blue (AB). The epithelial height of the left and the right VNO was measured in two sections at the rostral end of the VNO posterior to the opening to the nasopalatine duct (plane B, Fig. 1) in five females and five males. Digital images were taken with an Olympus BX51 light microscope with a 10× objective and triplicate measurements were made using the measure tool in Adobe Photoshop in the middle of the receptor epithelium and the non-receptor epithelium. The height of the receptor cell nuclei layer above the basement membrane was also measured in triplicate (Fig. 1). The differences in epithelium height and receptor cell nucleus height of males and females were assessed by nested analysis of variance with sex (male versus female) and side (right versus left) as main factors and section and replicate within section as within-subject factors.
Fig. 1.
Areas of coronal sections and measurements of the vomeronasal epithelia. The graphic on the top shows where coronal sections A to E through the VNO were taken. Underneath is an illustration showing where measurements in the receptor (F) and non-receptor epithelia (G) were taken. The enlargement shows where the measurement for the height of the receptor cell nuclei layer (H) was taken.
Transmission electron microscopy
Samples of the VNO from three adult female tammars and a sample from the respiratory epithelium of one adult female were taken for electron microscopy. To obtain the VNO samples, the skin from the septum was loosened and the organ was pulled as intact as possible out of its cartilage support. The organ was cut into 2 × 4-mm pieces and was fixed with Superfix (0.04 m glutaraldehyde, 0.96 m cacodylate buffer, 5.2 mm picric acid, 5.36 mm CaCl2 and 40 mm paraformaldehyde) at room temperature for 4 h or overnight at 4 °C. The samples were then washed three times for 10 min with 0.1 m cacodylate buffer at room temperature, post-fixed in 1% osmium tetroxide in 0.2 m cacodylate buffer and washed with 0.1 m cacodylate buffer. Finally, the samples were dehydrated and embedded in epon araldite resin. Sections were cut with an ultramicrotome (Reichert Ultracut S). Thick sections (1 µm) were stained with 1% toluidine blue for light microscopy, and thin sections (90 nm) were mounted on copper grids, stained with uranyl acetate and Reynolds lead citrate and examined by transmission electron microscopy (Phillips CM 100).
Results
Observations of flehmen behaviour
Males retracted their split upper lip when investigating the urogenital opening of oestrous females. This flehmen-like behaviour observed in tammar wallaby males exposed the gap between the two first incisors (Fig. 2A). This behaviour was only seen when males investigated the urogenital opening of females and they never raised their heads.
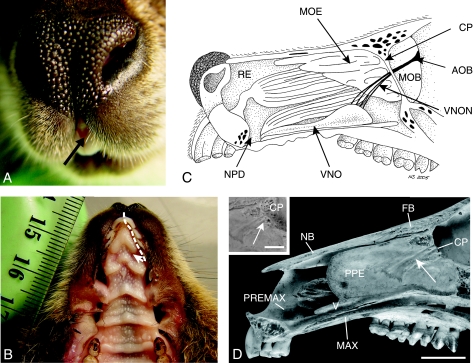
Fig. 2.
Gross morphology and neural connection of the VNO. (A) The gap between the two first incisors (arrow) becomes visible if the upper lips are retracted. (B) Under the two wing-like excrescences of the papilla incisiva lie the entrance to the nasopalatine ducts (NPDs) (path of the pheromone marked by a dotted arrow). (C) The right nasal cavity after removal of the nasal septum. The vomeronasal organ (VNO) opens into the NPD. The VNO is connected to the accessory olfactory bulb (AOB) through nerves (vomeronasal nerves, VNON) that cross the cribriform plate (CP) near the septum and lead medially along the main olfactory bulb. The VNO is connected to the nasal and the oral cavity via the NPD. The main olfactory epithelium (MOE) fills large parts of the caudal nasal cavity while the rest of the nasal cavity is lined by the respiratory epithelium (RE). The nerves of the MOE connect to the main olfactory bulb (MOB). (D) The vomeronasal nerve bundles lie in special grooves (arrows) in the perpendicular plate of the ethmoid bone (PPE). They penetrate the cribriform plate (CP) central medially (arrows) (scale for skull 1 cm and 0.5 cm for the enlargement of the cribriform plate); FB – frontal bone, NB – nasal bone, MAX – maxilla, PREMAX – premaxilla, V – vomer.
Gross anatomy
Behind the incisors lie the papilla incisiva, which have two wing-like folds at the level of the third incisor pair just before the diastema (Fig. 2B). These covered the entrances to the paired nasopalatine ducts (NPDs) or incisive duct. Grooves led along both sides of the papilla towards the entrances. The NPDs connected the oral and the nasal cavities (Fig. 2C,D). The opening of the NPD in the oral cavity was more rostral than the opening to the nasal cavity. The paired VNOs lay in the tissue on either side of the nasal septum, stretching along its sides towards the back of the nasal cavity. These tubular structures opened to the NPDs near its opening to the nasal cavity. Bundles of the vomeronasal nerves pass along the ventral and dorsal side of the organ, increasing in thickness and abundance towards the caudal end, from where three or more bundles of nerves connected to the accessory olfactory bulb (AOB). Those bundles left the vomeronasal structure at the caudal end and led along the side of the nasal septum, embedded in grooves in the perpendicular plate of the ethmoid bone, to the cribriform plate. These bundles formed the nervus vomeronasalis (Seifert, 1971). They traversed the cribriform plate near the septum and ran medially along the inner sides of the main olfactory bulbs (MOBs) towards their caudal ends, where they entered the brain dorso-medial to the MOBs at the area of the AOB.
The vomeronasal complex consisted of the vomeronasal tube, the soft tissue associated with it and the cartilaginous supporting tissue. The whole complex measured around 40 mm in length in males and females, varying in size depending on the animals overall head size. The paraseptal cartilage (or vomeronasal cartilage) enclosed the VNO near its entrance to the NPD. It became open dorso-laterally and gradually declined in size towards the caudal end of the VNO and eventually disappeared. Caudally the medial surface of the VNO was supported by a vertical process of the maxilla.
Microscopic anatomy
The lumen of the VNO tube was crescent shaped at its rostral end (Fig. 3A), changing to an elongated oval shape with a narrow lumen towards the caudal end of the organ (Fig. 3C,D). The receptor epithelium lay on the medial side, whilst the lateral side was non-receptor epithelium. Caudally the lumen is surrounded by glandular tissue (Fig. 3). It was not possible from the sections to establish whether the VNO lumen was fully filled with liquid or air in the live animal, as the tissue processing would have removed any air from the VNO lumen. However, some mucus could be seen in the lumen in most sections indicating that the lumen was at least partially fluid filled.
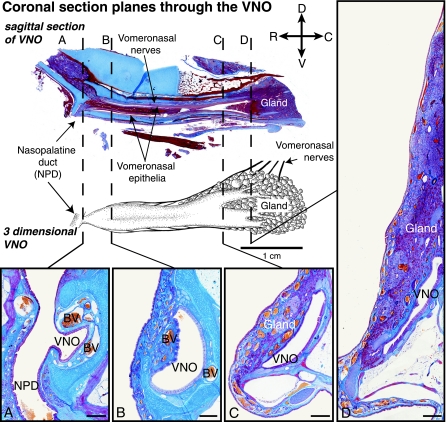
Fig. 3.
Light microscopic sections through the VNO. On the top left a sagittal section through the vomeronasal complex, showing the epithelia, the nerves running along the organ, glands at the caudal end and the nasopalatine duct. The drawing beneath shows where sections A, B, C and D were cut. (A) The VNO opening into the NPD. Two blood vessels (BV) are prominent on either side of the lumen in the first two rostral sections (A and B). The gland tissue increases towards the caudal end of the vomeronasal complex and the lumen changes from crescent shaped to a longitudinal oval shape (C and D) (Mallory's trichrome stain; scale 500 µm for all coronal sections) (orientation indicators: D – dorsal, C – caudal, R – rostral, V – ventral).
Blood vessels were situated ventro-medially and lateral to the VNO (Fig. 4A). At the rostral end of the VNO, two thick veins lay on either side of the opening of the VNO into the NPD.
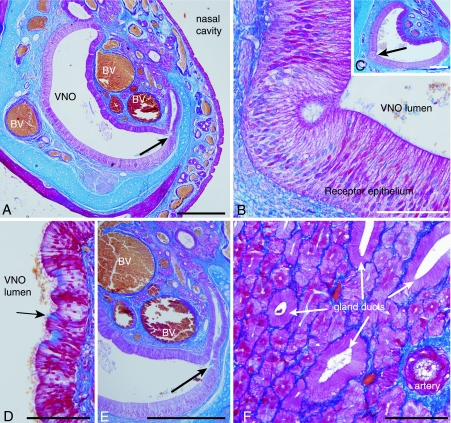
Fig. 4.
Glands and blood vessels. (A) Large blood vessels (BV) surround the VNO lumen in its rostral part (scale 500 µm). The arrow shows the opening of a gland to the VNO lumen. (B,C) Some grooves in the receptor epithelia which were found in a few specimens (scale is 100 µm in B and 500 µm in C). (D) Unicellular glands in the non-receptor epithelium. The arrow marks a unicellular gland which is secreting mucus into the VNO lumen (scale 100 µm). (E) Gland opening into the VNO lumen (arrow) (scale 500 µm). (F) The gland tissue consists of mucus-producing cells and gland ducts (scale 100 µm) (all sections stained with Mallory's trichrome stain).
Vomeronasal glands entered the VNO lumen through ducts that opened to the lumen dorsal and ventral to where the receptor epithelium passed into the non-receptor epithelium (Fig. 4A,E). Some groove-like structures were found in the receptor epithelium in some of the specimens (Fig. 4B,C). They looked similar to the ‘rosette’ formations described in the grey short-tailed opossum (Poran, 1998). Glandular tissue lay lateral to the non-receptor epithelium only. Towards the caudal end, the VNO lumen divided into branches that finally became gland ducts. The vomeronasal glands were component glands that consisted of mucus-producing cells and transporting channels (Fig. 4F). They produced PAS-positive and AB-negative mucus in their centre, although a few cells were found to be AB-positive. There were also individual cells scattered in the non-receptor epithelium that appeared similar to goblet cells, which presumably secreted mucus. These cells are referred to as unicellular glands (Fig. 4D). They were both strongly PAS- and AB-positive. Diastase digestion did not remove the PAS-positive staining in any of the tissues, which showed that it was not glycogen.
The receptor epithelium started at the opening of the VNO to the NPD and formed the convex portion of the crescent shape surrounding the lumen on the medial side. It consisted of a microvillar, pseudostratified, columnar epithelium (Fig. 5). The receptor epithelium was smooth along its surface with a regular height of 85 ± 13.5 µm (mean ± SD) in the female and 94 ± 15.1 µm in the male. The receptor cell nuclei layer covered 48 ± 11.4 µm of the epithelia height in the female VNO and 57 ± 9.9 µm in the male. At its highest point in transverse section, the non-receptor epithelium was 70 ± 7 µm high in females and 65 ± 4 µm in males. The epithelium height decreased in both epithelia where the non-receptor and receptor epithelium joined. There was no significant difference (P > 0.05) between male and female or left and right sides for any of the measured heights.
Fig. 5.
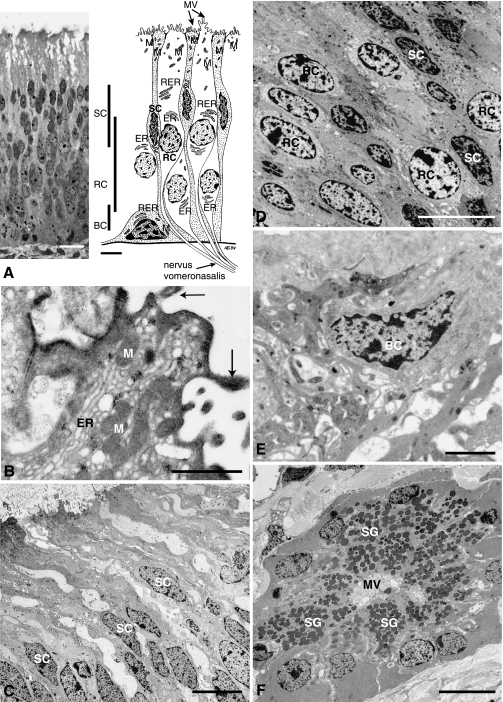
Ultrastructure of the receptor epithelium and the vomeronasal gland. The ultrastructure of the vomeronasal receptor epithelium and glands of the respiratory system. (A) A 1-µm section through the receptor epithelium revealing three different layers of nuclei. The top layer near the cell surface is formed by the supporting cell (SC) nuclei. Underneath the receptor cell (RC) nuclei follow. The most basal layer is formed by the basal cells (BC) (scale 10 µm; Toluidine blue staining). The figure on the right shows the structure of the cells in the epithelium as identified under the transmission electron microscope. The more electron-dense supporting cells are shown dotted. Microvilli (MV) are found on the surface of the epithelium. The dendrites of the receptor cells form the nervus vomeronasalis when they leave the epithelium. Mitochondria (M) are mainly concentrated near the epithelia surface while rough (RER) and smooth endoplasmic reticulum (ER) is more concentrated around the nuclei in all cell types. (B) The epithelia surface of the olfactory knob-like structure is lined by microvilli (arrows) and parts of receptor as well as supporting cells protrude into the lumen (ER – smooth endoplasmic reticulum, M – mitochondria) (scale 1 µm). (C) While the supporting cell nuclei have accumulated in the upper level of the epithelia and appear ellipsoid, the more rounded receptor nuclei (see D) form a layer underneath and are less electron dense (scale 10 µm in C and D). (E) The basal cells (BC) are the smallest cell type and are found at the base of the epithelia (scale 2 µm) (all sections beside A stained with uranyl acetate and Reynolds lead citrate). (F) The vomeronasal glands show microvilli (MV) in the lumen of the gland and secretory granules (SG) filling up to 80 % of the cell lumen (scale 10 µm).
The receptor epithelium had three layers where nuclei appeared in high density (Fig. 5A). The layer at the base of the epithelium contained the nuclei of basal cells, above which was a relatively high layer of the rounded nuclei of the receptor cells. The oval nuclei of the supporting cells formed the third layer. Towards the lumen was a layer composed of the cell body extensions of receptors and supporting cells lined by microvilli and with occasional knob-like structures which showed microvilli but no cilia on their surface. We assumed that these were olfactory structures given that except for the lack of cilia they appeared to be similar to the olfactory knobs in the MOE (Matsuoka et al. 2001) (Fig. 5B). The supporting cells (Fig. 5D) have nuclei with an oval shape with heterochromatin accumulated in small particles. Usually one nucleolus was visible. Mitochondria were abundant in the cytoplasm. Rough endoplasmic reticulum and ribosomes are also found. The cell surface had microvilli that protruded into the vomeronasal lumen but showed no olfactory knob-like structures. The supporting cells separated the receptor cells from each other.
Under the electron microscope, the receptor cell cytoplasm and nuclei were less electron dense than those of the supporting cells (Fig. 5D). The nuclei were circular in appearance with heterochromatin accumulated in small particles mainly along the nuclear membrane. Most nuclei had a single nucleolus. Smooth endoplasmic reticulum and mitochondria were found throughout the cytoplasm. Free ribosomes and small vesicles were located near the surface to the lumen. The cell surface sometimes bulged into the vomeronasal lumen, forming olfactory knob-like structures.
Basal cells (Fig. 5E) had electron-dense cell bodies and nuclei. The nuclei included accumulations of heterochromatin. The nucleus filled most of the cells. The cytosol included mitochondria, free ribosomes and rough endoplasmic reticulum.
The typically rectangular-shaped gland cells of the vomeronasal glands (Fig. 5F) were filled to 80% with electron-dense secretory granules leaving little room for cell organelles. The nuclei were situated near the cell membrane away from the lumen of the gland. On the surface of the gland cells microvilli protruded into the lumen of the glandular duct. The non-receptor epithelium formed the lateral, concave portion of the crescent-shaped section of the VNO. It was nearly as high as the receptor epithelium. This ciliated and microvillar pseudostratified columnar epithelium was folded and of irregular thickness. Mucus-secreting unicellular glands were often located in grooves of the epithelium, but could also be found at any area throughout the epithelial surface (Fig. 4D) and deep in the epithelium. Abundance of these gland cells decreased towards the caudal end of the VNO. At the same time the overall structure of the non-receptor epithelium changed to a non-folded epithelium at the caudal end of the VNO.
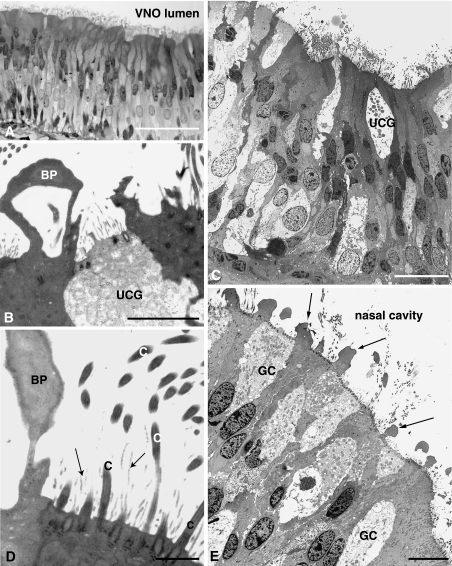
Three types of cells could be found under the electron microscope: unicellular glands (Fig. 6B,C), which could readily be distinguished from the other epithelial cells as they included secretory vesicles accumulated mainly in their apical region; an electron-lucent cell type with cilia and microvilli (Fig. 6C,D); and an electron-dense cell type showing bleb-like protrusions with no cilia or microvilli on their surface projecting into the lumen of the VNO (Fig. 6B,D) which looked similar to structures found on the surface of the respiratory epithelium (Fig. 6E).
Fig. 6.
The ultrastructure of the vomeronasal non-receptor epithelium and the respiratory epithelium of the nose. (A–C) The non-receptor epithelium of the VNO includes, besides electron-dense and electron-lucent epithelia cells, unicellular glands (UCG) which release mucus into the VNO lumen. This mucus is stored as secretory granules in these cells (scales: A, 50 µm; B, 2 µm; C, 20 µm). (D) The surface of the non-receptor epithelium is lined by cilia (C) and microvilli (marked with arrows). Each cilium is anchored with a basal body in the cell. Bleb-like protrusions (BP) are found on the epithelial surface (scale 1 µm). (E) The respiratory epithelium contains goblet cells (GC) similar in structure to the unicellular glands of the VNO. Bleb-like protrusions (arrows) are present on the surface of the epithelium (scale 10 µm) (all sections stained with uranyl acetate and Reynolds lead citrate).
Discussion
Tammar wallabies have a large, well-developed VNO, suggesting that it is an important organ for the detection of pheromonal signals. Tammars also show flehmen-like behaviour, supporting this suggestion. The receptor epithelium of the tammar at 85–94 µm is higher than the receptor epithelium in cattle (65–70 µm; Taniguchi & Mikami, 1985), although it is thinner than in the rat (140 µm; Vaccarezza et al. 1981), which is thought to have one of the most developed mammalian VNOs (Table 1). The tammar has the typical pattern of mammalian VNOs with electron-dense supporting cells and electron-lucent receptor cells. The olfactory knob-like structures on the surface of the receptor epithelium seen in the tammar are found in many other mammals (Kolnberger, 1971; Kolnberger & Altner, 1971; Loo & Kanagasuntheram, 1972; Ciges et al. 1977). The microvilli on these knob-like structures in the tammar VNO may fulfil the function of pheromone detection as was shown in mice (Zufall et al. 2002).
Table 1.
Evidence for VNO-related behaviour and anatomical features of the VNO in ungulates, rodents and marsupials.
| Rats | Cattle | Grey short-tailed opossum | Tammar wallaby | |
|---|---|---|---|---|
| Nuzzling | ✗? | ✗ | ✓10 | ✗ |
| Flehmen/flehmen-like behaviour | ✗ | ✓ | ✗ | ✓ |
| Entrance to nasal and oral cavity via NPD | ✓*1 | ✓†6,8 | ✓11 | ✓ |
| Large blood vessels | 1 lateral1,2 | 1 lateral6,7 | 1 lateral & 1 medial11 | 1 lateral & 1 medial |
| VNO capsule | cartilagenous capsule not totally closed5/ bony capsule3,4 | cartilagenous capsule not totally closed6,7 | VNO surrounded by not closed cartilagenous capsule while gland encased in bone11 | cartilagenous capsule not totally closed |
| Receptor epithelium thickness (µm) | 1405 | 65–708 | 15011 | 85–94 |
| Vomeronasal complex length (mm) | 6–102,5 | 80–909 | 7–911 | 40 |
| Unicellular glands in non-receptor epithelium | ✗ | ✗ | ✗ | ✓ |
| References | 1, Addison & Rademaker, 1927;2, Breipohl et al. 1979;3, Mendoza & Szabo, 1988;4, Salazar & Sanchez Quinteiro, 1998;5, Vaccarezza et al. 1981 | 6, Broom, 1897;7, Jacobs et al. 1981;8, Taniguchi & Mikami, 1985;9, Minett, 1925 | 10, Poran et al. 1993;11, Poran, 1998 | This study |
✓, yes; ✗, no; ✗/✓, some yes some no.
VNO entrance into nasal cavity rostral to NPD opening.
NPD is described as closed in the cattle in Broom (1897) and Jacobs et al. (1981) but as open in Taniguchi & Mikami (1985).
The tammar non-receptor epithelium has a ciliated surface, as found in many other species (Taniguchi & Mikami, 1985). These cilia may be involved in slow movement of mucus laden with pheromones over the receptor epithelium. There are groove-like depressions in the epithelium. Similarly shaped structures in sheep (Kratzing, 1971) and rat (Breipohl et al. 1979) facilitate the changes in shape needed for contractions and expansion of the VNO lumen to suck pheromones into the lumen (Kratzing, 1971). The contraction and expansion of the VNO lumen could be achieved through dilation and contraction of the two large blood vessels on opposite sides of the VNO through pulsation of the blood or regulation of the blood supply. The groove-like depressions would also increase the surface area that can bear cilia, which is especially important in a large tube-like organ like the VNO of the tammar to help to move mucus in and out.
The cell types found in the non-receptor epithelium are similar to the cell types found in the respiratory epithelium of the bandicoot (Kratzing, 1982b) and the respiratory epithelium of the tammar. Unicellular glands in the non-receptor epithelium have been reported in various eutherians (Arnautovic et al. 1970; Kratzing, 1971; Adams & McFarland, 1972; Breipohl et al. 1979; Adams & Wiekamp, 1984; Nagpal et al. 1988; Roslinski et al. 2000). The unicellular glands of the VNO are often described as goblet or goblet-like cells as their structure is similar (Fig. 6B,C,E). In the tammar the unicellular glands stain PAS- and AB-positive, as in other mammals (Roslinski et al. 2000). They include periodate-reactive carbohydrates in their acidic mucus. This great similarity between the structure of the main nasal cavity and the VNO makes it likely that both structures have similar functions, providing mucus production through glands and movement of this mucus by cilia, as discussed by Ciges et al. (1977) in their comparative study of the VNO and the olfactory mucosa. In the tammar the unicellular glands stain PAS- and AB-positive, as in other mammals (Roslinski et al. 2000).
Different types of vomeronasal glands can be distinguished in most mammals. There are usually one or two ‘end glands’ (Enddrüsen in German; Broman, 1918) at the caudal end of the organ and smaller glands along the sides of the organ (Broman, 1918). The tammar VNO has mucus glands along the side of the organ and at its caudal end the lumen of the VNO divides into glandular tubes. These vomeronasal glands stain PAS-positive and mainly AB-negative, the same staining pattern as found in most other mammals (Roslinski et al. 2000). The grey short-tailed opossum appears to be an exception as its VNO glands are PAS-negative (Poran, 1998). This difference in staining characteristics is surprising. It may reflect differences in mucus characteristics to accommodate differences in pheromones transported, and further investigation is needed to clarify this.
Secretions from the VNO have three possible functions. First, it is important to keep the receptor cell and their microvilli moist so that they do not stick together. Secondly, mucus is important to dissolve molecules and transport them to receptors. Thirdly, molecules need to be removed after they have been perceived and mucus may aid this action. The large gland at the caudal end of the tammar VNO might produce mucus to clean it after the perception of a pheromone, while the unicellular glands and glands along the lateral side of the lumen might produce mucus to dissolve molecules and might include transport molecules similar to vomeromodulin found in the rat (Khew-Goodall et al. 1991; Krishna et al. 1994) or aphrodisin found in the hamster (Henzel et al. 1988).
How pheromones are conveyed to the sensory epthelium of the VNO varies between species. In rats, the VNO opens into the nasal cavity caudal to the NPD opening, making it likely that pheromones enter the VNO through the nose. The VNO capsule and one large blood sinus may generate a strong pumping mechanism (Salazar & Sanchez Quinteiro, 1998). In cattle, the VNO opens rostrally on the NPD (Taniguchi & Mikami, 1985) and the very obvious flehmen behaviour must play a role in transferring pheromones into the VNO. During flehmen, intake of breath with partially closed nares forces pheromone-laden air into the NPDs (Estes, 1972). The venturi effect of air flow in the NPD will also help to draw mucus from the NPD, and it would return, laden with odorants, at the end of the inspiration. This process would be assisted by muscular movements of the mouth and tongue and pulsation of the medial blood vessels to move pheromones to the sensory epithelium efficiently (Meredith, 1994). The rat is dependent on a strong pumping mechanism via contractions of the blood sinus in order to take pheromones into the VNO lumen, as are hamsters and goats (Melese-d’Hospital & Hart, 1985; Meredith, 2001). Another behaviour that appears to serve a similar function to flehmen in transfer of pheromones, the ‘nuzzling behaviour’, has so far only been described in the hamster (Powers et al. 1979) and the grey short-tailed opossum (Poran et al. 1993). The flehmen-like behaviour of tammars presumably has a similar role in transfer of pheromones, but further functional studies are needed to establish this. Cattle and rats have a single medial blood vessel beside the VNO, but in both marsupials studied so far, there are both medial and lateral blood vessels (Table 1). How these two vessels interact in the transfer of pheromones needs further study.
There is considerable between-species variation in the relative thickness of the receptor and non-receptor epithelia of the VNO. In rat and grey short-tailed opossum the VNO receptor epithelium is much thicker than the non-receptor epithelium [rats: 30 µm for non-receptor versus 140 µm for the receptor epithelium (Vaccarezza et al. 1981) grey short-tailed opossum: 25–30 µm for the non-receptor versus 150 µm for receptor epithelium (N. Schneider, unpublished observations and Poran, 1998), Table 1]. In the bovids, equids and camelids investigated and in the tammar both epithelia are similar in absolute thickness (tammar non-receptor epithelium 65–70 µm thickness versus 85–94 µm for the receptor epithelium). The non-receptor epithelium of the tammar wallaby also contains unicellular glands. The function of the relatively thick non-receptor epithelium in tammars and cattle is unclear but may be a consequence of different structural constraints given the overall larger size of the VNO, which is around 40 mm in length in the tammar and 9 mm in the grey short-tailed opossum (Poran, 1998).
The VNO of the tammar is thus anatomically similar to that of other mammals with some minor differences. That it is well developed suggests that it is functionally important. In males, it is likely to play a role in the endocrine responses seen in males at the start of the breeding season in response to reproductively active females. We found no significant differences in the heights of the VNO epithelia between the two sexes and Rudd (1994b) found no sex differences in the AOB development. It is likely that pheromone communication takes place in both directions, and probably between animals of the same sex. Renfree et al (1989) and Rudd (1994a) note that oestrous females show mounting behaviour towards other oestrous females, possibly as a result of the same pheromonal cues that attract the males to the female.
The findings of this study show in an Australian marsupial that the structure and probably function of the VNO are similar to that in eutherian mammals, and combined with the previous studies on the South American marsupial, the grey short tailed opossum, suggests a high degree of conservation of the VNO in marsupials. Pheromones are important for sexual behaviour and reproductive endocrine control both in modern-day mammals (both eutherian and marsupial) and reptiles. In addition, there is evidence that pheromones are important in some eutherian mammals for the detection of the teat (e.g. rabbit: Schaal et al. 2003). It is interesting to speculate that pheromones may be important for neonatal marsupials, which despite a highly altricial state of development, manage to climb unaided from the birth canal to the pouch/teat area, locate the teat and attach to it. With the development of milk as a source of nutrition in the ancestral therian mammal, which probably also had altricial young, pheromones may have assisted the young to find their source of nutrition. A study of the developmental of the VNO during fetal and postnatal life is in progress and may help to clarify these ideas. The findings make it likely that a shared ancestor of all mammals possessed a well-developed VNO which adapted throughout evolution in the different species to sense pheromonal signals between individuals.
Acknowledgments
We thank Joan Clark for assistance with electron microscopy, Bruce Abaloz for histological techniques and David Paul for photography of skulls. This study was supported by the Holsworth Wildlife Research Endowment (ACTA) to N.Y.S. and an Australian Research Council Federation Fellowship to M.B.R.
References
- Adams DR, McFarland LZ. Morphology of the nasal fossae and associated structures of the hamster (Mesocricetus auratus. J Morphol. 1972;137:161–179. doi: 10.1002/jmor.1051370204. [DOI] [PubMed] [Google Scholar]
- Adams DR, Wiekamp MD. The canine vomeronasal organ. J Anat. 1984;138:771–787. [PMC free article] [PubMed] [Google Scholar]
- Addison EM, Rademaker LA. The postnatal growth of the vomeronasal organ of the albino rat (Mus norvegius albinus. J Comp Neurol. 1927;44:69–86. [Google Scholar]
- Arnautovic I, Abdalla O, Fahmy MF. Anatomical study of the vomeronasal organ and the nasopalatine duct of the one-humped camel. Acta Anat (Basel) 1970;77:144–154. doi: 10.1159/000143537. [DOI] [PubMed] [Google Scholar]
- Breipohl W, Bhatnagar KP, Mendoza A. Fine structure of the receptor-free epithelium in the vomeronasal organ of the rat. Cell Tissue Res. 1979;200:383–395. doi: 10.1007/BF00234850. [DOI] [PubMed] [Google Scholar]
- Brennan PA. The vomeronasal system. Cell Mol Life Sci. 2001;58:546–555. doi: 10.1007/PL00000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman I. Organon Vomero-Nasale Jacobsoni – Ein Wassergeruchsorgan! Anat Hefte. 1918;174:140–191. [Google Scholar]
- Broom R. Observations of the relations of the organ of jacobson in the horse. Proc Linn Soc NSW. 1896a;21:9–13. [Google Scholar]
- Broom R. The comparative anatomy of the organ of Jacobson in marsupials. Proc Linn Soc NSW. 1896b;21:591–623. [Google Scholar]
- Broom R. A contribution to the comparative anatomy of the mammalian organ of Jacobson. Transactions of the Royal Society of Edinburgh. 1897;39:231–255. [Google Scholar]
- Catling PC, Sutherland RL. Effect of gonadectomy, season and the presence of female tammar wallabies (Macropus eugenii) on concentrations of testosterone, luteinizing hormone and follicle-stimulating hormone in the plasma of male tammar wallabies. J Endocrinol. 1980;86:25–33. doi: 10.1677/joe.0.0860025. [DOI] [PubMed] [Google Scholar]
- Ciges M, Labella T, Gayoso M, Sanchez G. Ultrastructure of the organ of Jacobson and comparative study with olfactory mucosa. Acta Otolaryngol. 1977;83:47–58. doi: 10.3109/00016487709128812. [DOI] [PubMed] [Google Scholar]
- Coulson GM, Croft DB. Flehmen in Kangaroos. Aust Mammal. 1981;4:139–140. [Google Scholar]
- Croft DB. Some observations on the behaviour of the Antilopine Wallaroo, Macropus antilopinus. Aust Mammal. 1982;5:5–13. [Google Scholar]
- Dawley EM. Species, sex, and seasonal differences in vno size. Microsc Res Techn. 1998;41:506–518. doi: 10.1002/(SICI)1097-0029(19980615)41:6<506::AID-JEMT6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Estes RD. The role of the vomeronasal organ in mammalian reproduction. Mammal. 1972;36:315–341. [Google Scholar]
- Flint AP, Renfree MB. Oestradiol-17 beta in the blood during seasonal reactivation of the diapausing blastocyst in a wild population of tammar wallabies. J Endocrinol. 1982;95:293–300. doi: 10.1677/joe.0.0950293. [DOI] [PubMed] [Google Scholar]
- Freyer C. Die Regio ethmoidalis in der Ontogenese von Monodelphis domestica (Didelphidae: Marsupialia) und Thylacinus cynocephalus (Thylacinidae: Marsupialia. Institut für Systematische Zoologie des Museums für Naturkunde der Humboldt-Universität zu Berlin. Diplom Thesis.
- Gansloßer U. Soziale Interaktionen des Doria-Baumkängaruhs, Dendrolagus dorianus Ramsay, 1833 (Marsupialia, Macropodidae. Z Säugetierkunde. 1979;44:1–18. [Google Scholar]
- Garrosa M, Gayoso MJ, Esteban FJ. Prenatal development of the mammalian vomeronasal organ. Microsc Res Techn. 1998;41:456–470. doi: 10.1002/(SICI)1097-0029(19980615)41:6<456::AID-JEMT2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Guo J, Zhou A, Moss RL. Urine and urine-derived compounds induce c-fos mRNA expression in accessory olfactory bulb. Neuroreport. 1997;8:1679–1683. doi: 10.1097/00001756-199705060-00024. [DOI] [PubMed] [Google Scholar]
- Halpern M, Martinez-Marcos A. Structure and function of the vomeronasal system: an update. Prog Neurobiol. 2003;70:245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- Halpern M. The organization and function of the vomeronasal system. Annu Rev Neurosci. 1987;10:325–362. doi: 10.1146/annurev.ne.10.030187.001545. [DOI] [PubMed] [Google Scholar]
- Henzel WJ, Rodriguez H, Singer AG, Stults JT, Macrides F, Agosta WC, Niall H. The primary structure of aphrodisin. J Biol Chem. 1988;263:16682–16687. [PubMed] [Google Scholar]
- Hudson R, Distel H. Pheromonal release of suckling in rabbits does not depend on the vomeronasal organ. Physiol Behav. 1986;37:123–128. doi: 10.1016/0031-9384(86)90394-x. [DOI] [PubMed] [Google Scholar]
- Inns RW. Seasonal changes in the accessory reproductive system and plasma testosterone levels of the male tammar wallaby, Macropus eugenii, in the wild. J Reprod Fertil. 1982;66:675–680. doi: 10.1530/jrf.0.0660675. [DOI] [PubMed] [Google Scholar]
- Jacobs VL, Sis RF, Chenoweth PJ, Klemm WR, Sherry CJ. Structures of the bovine vomeronasal complex and its relationships to the palate: tongue manipulation. Acta Anat (Basel) 1981;110:48–58. doi: 10.1159/000145412. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Pheromones, the vomeronasal system, and communication – from hormonal responses to individual recognition. Ann NY Acad Sci. 1998;855:333–48. doi: 10.1111/j.1749-6632.1998.tb10592.x. [DOI] [PubMed] [Google Scholar]
- Keverne EB. Importance of olfactory and vomeronasal systems for male sexual function. Physiol Behav. 2004;83:177–187. doi: 10.1016/j.physbeh.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Khew-Goodall Y, Grillo M, Getchell ML, Danho W, Getchell TV, Margolis FL. Vomeromodulin, a putative pheromone transporter: cloning, characterization, and cellular localization of a novel glycoprotein of lateral nasal gland. FASEB J. 1991;5:2976–2982. doi: 10.1096/fasebj.5.14.1752363. [DOI] [PubMed] [Google Scholar]
- Kolnberger I. Vergleichende Untersuchungen am Riechepithel, insbesondere des Jacobsonschen Organs von Amphibien, Reptilien und Säugetieren. Z Zellfor mikroskop Anatomie. 1971;122:53–67. [PubMed] [Google Scholar]
- Kolnberger I, Altner H. Ciliary-structure precursor bodies as stable constituents in the sensory cells of the vomeronasal organ of reptiles and mammals. Z Zellfor mikroskop Anatomie. 1971;118:254–262. doi: 10.1007/BF00341569. [DOI] [PubMed] [Google Scholar]
- Kratzing JE. The structure of the vomeronasal organ in the sheep. J Anat. 1971;108:247–260. [PMC free article] [PubMed] [Google Scholar]
- Kratzing JE. The anatomy of the rostral nasal cavity and vomeronasal organ in Tarsipes rostratus(Marsupialia: Tarsipedidae) Aust Mammal. 1982a;5:211–219. [Google Scholar]
- Kratzing JE. Regional variation in respiratory epithelium of the nasal cavity of the bandicoot (Isoodon macrourus. J Anat. 1982b;134:1–9. [PMC free article] [PubMed] [Google Scholar]
- Kratzing JE. The epithelium of the vomeronasal organ in the agile wallaby (Macropus agilis. Chem Senses. 1984;8:254–254. [Google Scholar]
- Krishna NS, Getchell ML, Getchell TV. Expression of the putative pheromone and odorant transporter vomeronmodulin mRNA and protein in nasal chemosensory mucosae. J Neurosci Res. 1994;39:243–259. doi: 10.1002/jnr.490390303. [DOI] [PubMed] [Google Scholar]
- Ladewig J, Hart BL. Flehmen and vomeronasal organ function in male goats. Physiol Behav. 1980;24:1067–1071. doi: 10.1016/0031-9384(80)90049-9. [DOI] [PubMed] [Google Scholar]
- Loo SK, Kanagasuntheram R. The vomeronasal organ in tree shrew and slow loris. J Anat. 1972;112:165–172. [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M, Yoshida-Matsuoka J, Iwasaki N, Norita M, Costanzo RM, Ichikawa M. Immunocytochemical study of G(i)2alpha and G(o)alpha on the epithelium surface of the rat vomeronasal organ. Chem Senses. 2001;26:161–166. doi: 10.1093/chemse/26.2.161. [DOI] [PubMed] [Google Scholar]
- Melese-d’Hospital PY, Hart BL. Vomeronasal organ cannulation in male goats: evidence for transport of fluid from oral cavity to vomeronasal organ during flehmen. Physiol Behav. 1985;35:941–944. doi: 10.1016/0031-9384(85)90263-x. [DOI] [PubMed] [Google Scholar]
- Mendoza AS. Morphological studies on the rodent main and accessory olfactory systems: the regio olfactoria and vomeronasal organ. Ann Anat. 1993;175:425–446. doi: 10.1016/s0940-9602(11)80110-x. [DOI] [PubMed] [Google Scholar]
- Mendoza AS, Szabo K. Developmental studies on the rat vomeronasal organ: vascular pattern and neuroepithelial differentiation. II. Electron microscopy. Brain Res. 1988;467:259–268. doi: 10.1016/0165-3806(88)90030-2. [DOI] [PubMed] [Google Scholar]
- Meredith M. Chronic recording of vomeronasal pump activation in awake behaving hamsters. Physiol Behav. 1994;56:345–354. doi: 10.1016/0031-9384(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Meredith M. Human vomeronasal organ function: a critical review of best and worst cases. Chem Senses. 2001;26:433–445. doi: 10.1093/chemse/26.4.433. [DOI] [PubMed] [Google Scholar]
- Minett FC. The organ of Jacobson in the horse, ox, camel and pig. J Anat. 1925;60:110–118. [PMC free article] [PubMed] [Google Scholar]
- Nagpal SK, Sudhakar LS, Singh Y. Anatomy of the vomeronasal organ in camel. Indian J Anim Sci. 1998;58:218–220. [Google Scholar]
- Poran NS. Vomeronasal organ and its associated structure in the opossum Monodelphis domestica. Microsc Res Techn. 1998;43:500–510. doi: 10.1002/(SICI)1097-0029(19981215)43:6<500::AID-JEMT3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Poran NS, Vandoros A, Halpern M. Nuzzling in the gray short-tailed opossum I: Delivery of odours to vomeronasal organ. Phys Behav. 1993;53:959–967. doi: 10.1016/0031-9384(93)90275-k. [DOI] [PubMed] [Google Scholar]
- Powers JB, Fields RB, Winans SS. Olfactory and vomeronasal system participation in male hamsters’ attraction to female vaginal secretions. Physiol Behav. 1979;22:77–84. doi: 10.1016/0031-9384(79)90407-4. [DOI] [PubMed] [Google Scholar]
- Renfree MB, Fletcher TP, Blanden DR. Physiological and behavioural events around the time of birth in macropodid marsupials. In: Grigg G, Jarman P, Hume I, et al., editors. Kangaroos, Wallabies and Rat-Kangaroos. New South Wales, Australia: Surrey Beatty & Sons Pty; 1989. pp. 323–337. [Google Scholar]
- Renfree MB, Green SW, Young IR. Growth of the corpusluteum and its progesterone during pregnancy in the tammar wallaby, Macropus eugenii. J Repro Fert. 1979;57:131–136. doi: 10.1530/jrf.0.0570131. [DOI] [PubMed] [Google Scholar]
- Restrepo D, Arellano J, Oliva AM, Schaefer ML, Lin W. Emerging views on the distinct but related roles of the main and accessory olfactory systems in responsiveness to chemosensory signals in mice. Horm Behav. 2004;46:247–256. doi: 10.1016/j.yhbeh.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Rodrigues I. Pheromone receptors in mammals. Horm Behav. 2004;46:219–230. doi: 10.1016/j.yhbeh.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Roslinski DL, Bhatnagar KP, Burrows AM, Smith TD. Comparative morphology and histochemistry of glands associated with the vomeronasal organ in humans, mouse lemurs, and voles. Anat Rec. 2000;260:92–101. doi: 10.1002/1097-0185(20000901)260:1<92::AID-AR100>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Rudd CD. Sexual behaviour of male and female tammar wallabies (Macropus eugenii) at post-partum oestrus. J Zool. 1994a;232:151–162. [Google Scholar]
- Rudd CD. Sex differences in the tammar wallaby. Department of Antomy, Monash University; 1994b. PhD thesis. [Google Scholar]
- Salazar I, Sanchez Quinteiro P. Supporting tissue and vasculature of the mammalian vomeronasal organ: the rat as a model. Microsc Res Techn. 1998;41:492–505. doi: 10.1002/(SICI)1097-0029(19980615)41:6<492::AID-JEMT5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Sam M, Voran S, Malnic B, Ma WD, Novotny MV, Buck LB. Neuropharmacology – Odorants may arouse instinctive behaviours. Nature. 2001;412:142. doi: 10.1038/35084137. [DOI] [PubMed] [Google Scholar]
- Schaal B, Coureaud G, Langlois D, Ginies C, Semon E, Perrier G. Chemical and behavioural characterization of the rabbit mammary pheromone. Nature. 2003;424:68–72. doi: 10.1038/nature01739. [DOI] [PubMed] [Google Scholar]
- Seifert K. Licht- und elektronenmikroskopische Untersuchungen am Jacobsonschen Organ (Organon vomero nasale) der Katze. Arch klin exp Ohr-, Nas- u Kehlk Heilk. 1971;200:223–251. [PubMed] [Google Scholar]
- Takami S. Recent progress in the neurobiology of the vomeronasal organ. Microsc Res Techn. 2002;58:228–250. doi: 10.1002/jemt.10094. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Mikami S. Fine structure of the epithelia of the vomeronasal organ of horse and cattle. A comparative study. Cell Tissue Res. 1985;240:41–48. doi: 10.1007/BF00217556. [DOI] [PubMed] [Google Scholar]
- Trinh K, Storm DR. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nat Neurosci. 2003;6:519–525. doi: 10.1038/nn1039. [DOI] [PubMed] [Google Scholar]
- Tyndale-Biscoe CH, Renfree MB. ) Reproductive Physiology of Marsupials. Cambridge: Cambridge University Press; 1987. [Google Scholar]
- Vaccarezza OL, Sepich LN, Tramezzani JH. The vomeronasal organ of the rat. J Anat. 1981;132:167–185. [PMC free article] [PubMed] [Google Scholar]
- Zufall F, Kelliher KR, Leinders-Zufall T. Pheromone detection by mammalian vomeronasal neurons. Microsc Res Techn. 2002;58:251–260. doi: 10.1002/jemt.10152. [DOI] [PubMed] [Google Scholar]