Abstract
We compared the muscular anatomy of the distal front limb in terrestrial and aquatic chelonians to test whether observed differences between the two groups are associated with their divergent lifestyles and locomotor modes. Given the different use of the forelimb in the two environments (body support and propulsion on land vs. mainly propulsion in water) we expected that: (1) aquatic and terrestrial turtles would show differences in their muscular anatomy, with aquatic species having more individualized muscle bundles to allow for the complex forearm movements observed during swimming, and (2) that terrestrial turtles would have more robust muscles to support their body weight against gravity. To address these questions, we examined the forelimb myology and associated tissues in six aquatic or semi-aquatic turtles (Phyrnops hilarii, Podocnemis unifilis, Trachemys scripta, Sacalia bealei, Cuora amboinensis and Mauremys caspica) and six terrestrial or semi-terrestrial turtles (Geochelone chilensis, Testudo graeca, Cuora galbinifrons, Glyptemys insculpta, Terrapene carolina and Rhinoclemmys pulcherrima). This paper describes the general structure of the forelimb musculature in all species, and quantifies muscle masses in those species with more than five specimens available (Ph. hilarii, Po. unifilis and Ge. chilensis). The general structure of the forelimb muscles in the strictly terrestrial species Ge. chilensis and Tes. graeca was found to be notably different from the pattern of the aquatic and semi-aquatic species examined, showing a distinct fusion of the different muscular bodies. Ter. carolina also show a distinctly terrestrial pattern, but a less extensive tendon development. R. pulcherrima and Gl. insculpta were found to be morphologically intermediate; in the geoemydids the strictly terrestrial bauplan never appears. Quantitative differences in the robustness or mass of the distal forelimb muscles were also observed for the species investigated, supporting our prediction that the extensor muscles are more robust in terrestrial turtles. However, in contrast to our expectations, not only the extensor muscles of the distal forelimb (which are crucial in providing both body support and propulsion), but all muscles acting around the wrist were found to be heavier in terrestrial turtles.
Keywords: constraint, forelimb, locomotion, myology, turtle
Introduction
It has been proposed that variation in locomotor mode results in differences in the anatomy of the structures involved in locomotion (Collette, 1961; Odendaal, 1979; Russell & Bauer, 1989; Carrillo de Espinoza et al. 1990; Hamrick, 1996; Payne et al. 2004, 2005, 2006; Smith et al. 2006; Williams et al. 2007) as well as in the way the muscles are coordinated during movement (Biewener & Gillis, 1999; Gillis & Blob, 2001). In particular, locomotion in media with radically differing physical properties such as water and land probably imposes stringent demands on the structure and control of the musculoskeletal system (Gillis, 1998; Biewener & Gillis, 1999; Gillis & Blob, 2001).
Turtles are an interesting group to study in this respect, as they have a short, broad trunk encased in a rigid shell, which has had extensive consequences for the morphology and function of their locomotor apparatus (Walker, 1971). Because of their rigid bodies, turtles must rely entirely on their appendicular system for locomotion and thus constraints imposed by different locomotor environments (i.e. water versus air) should be directly reflected in its structure and function. Indeed, the use of the limbs during terrestrial versus aquatic locomotion is radically different. During terrestrial locomotion, the limbs must ensure body support against gravity, must provide stability and must generate the propulsive forces for forward locomotion (Zani & Claussen, 1995; Wren et al. 1998; Zani et al. 2005). In water, the limbs are no longer used for body support and mainly need to provide propulsion. Interestingly, the forelimbs have become the dominant propulsive limb pair in some aquatic turtles such as sea turtles, generating lift and propulsion using a flapping motion (Renous & Bels, 1993). Other aquatic turtles use drag-based rowing motions to generate propulsion with the front and hindlimbs (Pace et al. 2001).
Given the different use of the forelimb in the two environments (body support and propulsion on land vs. mainly propulsion in water) we expected that: (1) aquatic and terrestrial turtles would show differences in their muscular anatomy, with aquatic species having more individualized muscle bundles to allow for the more complex forelimb movements observed during swimming (Renous & Bels, 1993; Pace et al. 2001); and (2) that terrestrial turtles would have more robust muscles to support their body weight against gravity. More specifically, we expected predominantly the wrist extensor muscles to be heavier in terrestrial species, and rotators to be better developed in aquatic species given the importance of wrist rotations during swimming.
However, our understanding of the muscular anatomy of turtle limbs is currently still limited. The most complete description of chelonian limb musculature was provided by Walker (1973), who described the limb muscles in some cryptodiran and pleurodiran chelonians. Beyond this, only a few anatomical descriptions dealing with chelonian limb musculature are available (Bojanus, 1819; Walther, 1922; George & Patel, 1957; Yasukawa & Hikida, 1999; Wyneken 2001). Thus, the first goal of the present paper was to provide a detailed description of the forelimb musculature in a variety of turtles that use primarily aquatic or terrestrial habitats. As differences in forelimb movements are most prominent at the wrist (Pace et al. 2001; Zani et al. 2005; our personal observations) we focused on the distal forelimb muscles only. In a first approach to test quantitatively for differences among species living in different environments, we measured the mass of the distal forelimb muscles and tested whether terrestrial species do indeed have more robust (heavier) muscles for their body size than aquatic species.
Material and methods
Forty-two alcohol-preserved specimens were used in this study: 13 Geochelone chilensis, eight Phrynops hilarii, six Podocnemis unifilis, two Trachemys scripta, two Testudo graeca, one Rhinoclemmys pulcherrima, three Terrapene carolina, two Sacalia bealei, one Cuora galbinifrons, one Cuora amboinesis, one Glyptemys insculpta and two Mauremys caspica (see Appendix 2).
Special attention was paid to general muscle architecture (origin, insertion, fibre orientation, etc.) as well as the structure and position of associated tendons. Basic details of the musculature of the chelonian forelimb were obtained by dissections. The specimens were observed and dissected under a binocular microscope and drawings were made using a camera lucida. Muscles were classified according to the terminology proposed by Haines (1939, 1950), Walker (1973) and Russell (1988). Romer (1956) and Sheil (2003) were followed for the osteological nomenclature.
For the quantitative analysis we used only those species for which more than five specimens were available. Muscle of all specimens of Ph. hilarii, Po. unifilis and Ge. chilensis were dissected out, dried in an oven at 60 °C and weighed with an analytical balance (±1 mg). Fifteen morphometric variables including body mass and the mass of all muscles were recorded (Table 1). Additionally, the biceps muscle and its tendon length were measured using digital calipers (± 0.01 mm).
Table 1.
Means ± standard deviations, and confidence limits of the 17 variables measured in the specimens of each turtle species for the quantitative analysis
| Ge. chilensis | Ph. hilarii | Po. unifilis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | CL | Mean | SD | CL | Mean | SD | CL | |
| SVL (mm) | 175.64 | 35.38 | 28.31 | 132.87 | 7.61 | 6.09 | 57.80 | 2.29 | 1.83 |
| BITL (mm) | 12.47 | 3.00 | 2.63 | 8.42 | 0.67 | 0.54 | 5.02 | 0.90 | 0.72 |
| BIML (mm) | 23.10 | 5.18 | 4.54 | 17.00 | 2.72 | 2.17 | 8.92 | 2.12 | 1.70 |
| Ratio T/M | 1.8580 | 0.0986 | 0.0864 | 2.0350 | 0.3973 | 0.3179 | 1.8150 | 0.4802 | 0.3842 |
| EXDILO (mg) | 287.9 | 440.9 | 352.8 | 13.7 | 2.3 | 1.9 | 1.2 | 0.1 | 0.1 |
| EXCARA (mg) | 92.0 | 49.8 | 39.8 | 6.6 | 2.6 | 2.1 | 1.2 | 0.5 | 0.4 |
| EXCAUL (mg) | 54.1 | 33.9 | 27.1 | 6.1 | 5.3 | 4.2 | 0.5 | 0.3 | 0.2 |
| TRRA (mg) | 70.2 | 57.8 | 50.7 | 3.8 | 2.0 | 1.6 | 0.9 | 0.4 | 0.4 |
| PRTE (mg) | 34.6 | 20.0 | 16.0 | 7.4 | 0.7 | 0.6 | 0.4 | 0.2 | 0.2 |
| FLCARA (mg) | 115.5 | 97.4 | 77.9 | 6.7 | 1.7 | 1.3 | 0.6 | 0.2 | 0.2 |
| FLDILO (mg) | 209.2 | 49.8 | 39.9 | 19.6 | 12.5 | 10.0 | 1.8 | 0.6 | 0.5 |
| PALO (mg) | 51.1 | 30.0 | 29.4 | 12.2 | 3.9 | 3.1 | 1.2 | 0.3 | 0.2 |
| FLCAUL (mg) | 98.3 | 73.3 | 58.7 | 8.3 | 1.6 | 1.3 | 1.4 | 0.9 | 0.7 |
| PRPR (mg) | 25.5 | 13.5 | 11.8 | 3.9 | 1.7 | 1.4 | 0.5 | 0.2 | 0.2 |
| PRAC (mg) | 19.3 | 13.6 | 12.0 | 3.3 | 1.2 | 1.0 | 0.6 | 0.5 | 0.4 |
| ABPOLO (mg) | 6.7 | 5.1 | 4.5 | 2.4 | 0.9 | 0.7 | 0.3 | 0.3 | 0.2 |
| Mass (mg) | 987,88 | 617,44 | 494,04 | 127,30 | 23,52 | 18,82 | 15,77 | 1,67 | 1,34 |
ABPOLO: abductor pollici longus, BIML: biceps muscular length, BITL: biceps tendon length, EXCARA: extensor carpi radialis, EXCAUL: extensor carpi ulnaris, EXDILO: extensor digitorum longus, FLCARA: flexor carpi radialis, FLCAUL: flexor carpi ulnaris mass, FLDILO:flexor digitorum longus, PALO: palmaris longus, PRAC: pronator accesorius, PRPR: pronator profundus, PRTE: pronator teres mass, Ratio T/M: ratio biceps tendon/muscular length, SD: standard deviation, SVL: snout–vent length, TRRA: tractor radii mass.
Data were log10-transformed before analysis, and normality and homoscedasticity were tested with Shapiro-Wilks and Levenes tests, respectively (Sokal & Rolph, 1995). As morphometric data were correlated with overall animal size (Pearson correlations, all P < 0.05), analyses of co-variance (with body mass as a covariate) were used to test for differences between terrestrial and aquatic turtles. We performed different tests for the extensor, flexor and rotator muscle groups.
Results
Wrist extensors
This group consisted of m. extensor digitorum longus, m. extensor carpi radialis, m. tractor radii and m. extensor carpi ulnaris. Quantitative analysis showed that terrestrial species have heavier wrist extensors than aquatic species (mancova: Wilk's Lambda F4,12 = 0.42; P = 0.03). Subsequent univariate F-tests showed that this difference was significant for all muscles (all P < 0.05).
The m. extensor digitorum longus in Ph. hilarii (Fig. 1a), Po. unifilis, Tr. scripta, C. amboinensis, M. caspica, S. bealei and R. pulcherrima originates in the central and dorsal region of the distal condyle of the humerus and extends distally, covering the dorsal surface of the manus. At the base of the metacarpals it divides into four short, wide and flat slips reaching each digit. These insert at the distal end of each metacarpal, there being two slips to each digit except for the first and fifth, which lack slips on their outer surface. In Ter. carolina and C. galbinifrons the muscle splits into three slips only and, distally, it is fused with the ventral slip of the m. extensor carpi radialis. In Ge. chilensis (see Fig. 3a), Gl. insculpta, C. galbinifrons and Tes. graeca, the muscle has the same origin although by means of a very wide and ribbon-like tendon. A tendinous layer joins the dorsal digital tendons and covers the distal portion of the m. extensor digitorum longus. Some of its fibres are closely associated with the proximal part of the m. extensor carpi radialis.
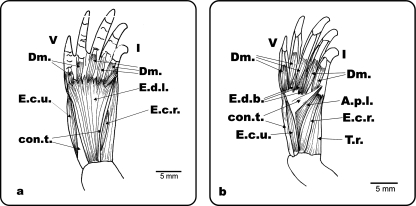
Fig. 1.
Ph. hilarii. Dorsal view of the left antebrachium and hand. (a) Superficial musculature of the antebrachium and hand; (b) deep musculature of the antebrachium and hand. E.d.l.: m. extensor digitorum longus; E.c.r.: m. extensor carpi radialis; E.c.u.: m. extensor carpi ulnaris, T.r.: m. tractor radii; con.t.: connective tissue; E.d.b.: m. extensor digiti brevis, A.p.l.: m. abductor pollici longus; Dm.: m. dorsometacarpalis; I and V: digits I and V.
Fig. 3.
Ge. chilensis. Dorsal view of the left antebrachium and hand. (a) Dorsal musculature and connective tissue of the antebrachium and hand; (b) extensor digitorum longus and most of the superficial connective tissue removed. E.d.l.: m. extensor digitorum longus; E.c.r.: m. extensor carpi radialis; E.c.u.: m. extensor carpi ulnaris; T.r.: m. tractor radii; con.t.: connective tissue; E.d.b.: m. extensor digiti brevis; A.p.l.: m. abductor pollici longus; Dm.: mm. dorsometacarpalis; E.c.r.t.: m. extensor carpi radialis; I and V: digits I and V.
The extensor carpi radialis muscle in Ph. hilarii (Fig. 1a,b), Po. unifilis, Tr. scripta, Gl. insculpta, C. amboinensis, M. caspica, S. bealei and R. pulcherrima originates via a tendon on the dorsolateral surface of the distal epicondyle of the humerus. This muscle has two bellies, one dorsal and the other ventral, which envelop the m. extensor digitorum longus. Both muscle bellies insert fleshy (not tendinous) on the distal head of the radius, the ventral one also inserting along the lateral edge of the radius. In Ge. chilensis (see Fig. 3a,b), Tes. graeca, C. galbinifrons and Ter. carolina, this muscle has three bellies, superficialis, intermedius, and profundus, which originate together from the humerus via a tendinous structure described below. The superficialis belly envelops the lateral edge of the m. extensor digitorum longus inserting fleshy at the distal extreme of the radius. In Ter. carolina, this branch is very slender and inserts onto the radial bone. The intermedius and the superficialis bellies have a common origin on the humerus, but run separately along the surface of the radius. The third branch (profundus) is the deepest of the three and is attached fleshy along the medial edge of the radius, reaching the distal epicondyle.
The most lateral muscle of the radial muscular group corresponds to m. tractor radii. In Ph. hilarii, Po. unifilis, Tr. scripta, S. bealei, Ter. carolina and R. pulcherrima this is a bulky and well-developed muscle (Fig. 1b). It originates broadly from the medial edge of the distal epicondyle of the humerus. It inserts fleshy along the lateral edge of the radius, ending in a short tendon on its distal extreme. In M. caspica the insertion is slightly displaced to the dorsal surface. In Ge. chilensis (see Fig. 3b), Gl. insculpta, C. galbinifrons, C. amboinensis and Tes. graeca the muscle is thin and narrow, and arises from the most external edge of the lateral epicondyle of the humerus. It extends along the lateral surface of the radius and inserts on the lateral side of its condyle.
In Ph. hilarii, S. bealei, C. galbinifrons, C. amboinensis, M. caspica and Tr. scripta the m. extensor carpi ulnaris (Fig. 1a,b), a narrow and flat muscle, originates from the lateral epicondyle of the humerus, and inserts via a tendon on the laterodistal surface of the ulna. Its fleshy insertion covers nearly the entire lateral surface of the ulna, and runs along the m. extensor digitorum brevis of digit V. In Gl. insculpta there are three branches. In Po. unifilis, the m. extensor carpi ulnaris shows a pattern similar to that mentioned for Phrynops and the other aquatic species, except for the insertion, which covers the distal third of the ulna in this species. In Ge. chilensis (see Fig. 3a,b), Tes. greca, R. pulcherrima and Ter. carolina, this is a very thick and broad muscle originating on the distal condyle of the humerus by means of a long and strong tendon. In Tr. scripta both lateral muscle complexes (mm. extensor carpi ulnaris and radialis) have been shifted slightly to the dorsal surface.
Wrist flexors
This group consists of the m. flexor carpi radialis, the m. flexor carpi ulnaris, the m. palmaris longus, the m. flexor digitorum longus and the m. brachialis inferior. Quantitative analysis showed that terrestrial turtles have heavier wrist flexors than aquatic turtles (mancova: Wilk's Lambda F4,10 = 0.11; P < 0.01). Subsequent univariate F-tests showed that this pattern holds for all muscles (all P < 0.01).
In Ph. hilarii (Fig. 2a,b,c), Po. unifilis, S. bealei, Tr. scripta, C. galbinifrons, C. amboinensis, M. caspica and R. pulcherrima the m. flexor carpi radialis originates from the lateral epicondyle of the humerus and envelops the m. flexor digitorum longus. It inserts on the aponeurosis that covers the distal condyle of the radius. The muscle is notably modified in Ge. chilensis (Fig. 4b,c), Tes. graeca, Gl. insculpta and Ter. carolina. In these species, it is a fusiform, thick, and short muscle, originating from a broad area on the lateral distal epicondyle of the humerus. In Ge. chilensis and Tes. graeca, halfway through the radius length, the muscle continues into a very long and thick tendon that merges with the aponeurotic tissue, and reaches the ungual phalanx of digit I. In Ter. carolina, it inserts fleshy along most of the length of the radius.
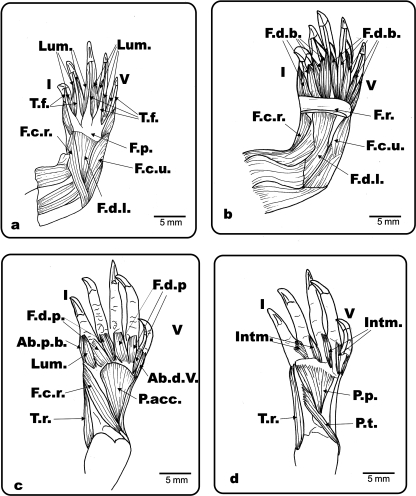
Fig. 2.
Ph. hilarii. Ventral view of the left antebrachium and hand. (a) Mm. flexor digiti brevis superficialis and palmar aponeurosis removed showing the flexor plate and flexor tendons; (b) flexor plate removed; (c) flexor digitorum longus, flexor carpi ulnaris and superficial branch of m. flexor carpi radialis removed; (d) deeper layer of the ventral hand and antebrachium. Lum.: m. lumbricalis; T.f.: flexor tendons; F.d.l.: m. flexor digitorum longus; F.d.b.: m. flexor digiti brevis; F.p.: flexor plate; F.c.r.: m. flexor carpi radialis; F.c.u.: m. flexor carpi ulnaris; F.d.p.: m. flexor digiti brevis profundi; P.t.: m. pronator teres; P.acc.: m. pronator accesorius; P.p.: m. pronator profundus; m. flexor carpi radialis; Ab.p.b.: m. abductor pollici brevis; Ab.d.V.: m. abductor digiti V; Intm.: mm. intermetacarpalis; T.r.: m. tractor radii; I and V: digits I and V.
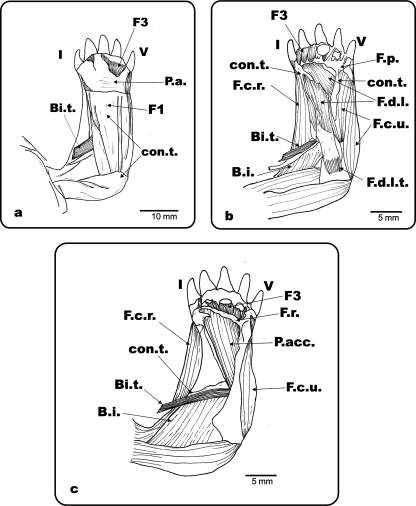
Fig. 4.
Ge. chilensis. Ventral view of the left antebrachium and hand. (a) Superficial musculature and connective tissue of the antebrachium and hand; (b) palmar aponeurosis, Fusion 1 and most of the superficial connective tissue removed; (c) flexor digitorum longus and flexor plate removed. F.d.l.: m. flexor digitorum longus; F.d.l.t.: m. flexor digitorum longus tendon; P.a.: palmar aponeurosis; con.t.: connective tissue; F.p.: flexor plate; F.c.r.: m. flexor carpi radialis; F.c.u.: m. flexor carpi ulnaris; Bi.: m. biceps; Bi.t.: m. biceps tendon; P.t.: m. pronator teres; B.i.: m. brachialis inferior; P.acc.: m. pronator accesorius; F.r.: flexor retinaculum; F1: fusion 1; F3: fusion 3; I and V: digits I and V.
The flexor carpi ulnaris muscle is divided into two or three branches in the chelonians examined here. In Ph. hilarii (Fig. 2b), Po. unifilis, S. bealei and Tr. scripta the muscle has a superficial and a deep branch. The superficial branch originates by means of a short tendon at the laterodistal epicondyle of the humerus. It is fixed to the lateral edge of the ulna and inserts on the os pisiformis. The deep branch has the same origin as the superficial one. It is triangular, long, and inserts fleshy along the medial edge of the ulna. In R. pulcherrima, M. caspica, C. galbinifrons and C. amboinensis, this muscle has three branches. The external and internal branches arise from the distal extreme of the humerus and insert onto the distalmost part of the ulna. The internal branch is bulky; the external one is flat and envelops the internal one. The third branch lies deep and originates from the distal extreme of the humerus, and inserts fleshy along the entire length of the ulna (except in M. caspica where it covers only the proximal third of the ulna). In C. galbinifrons and C. amboinensis, the internal and deep branches are fused with the m. flexor digitorum longus. The distal fibres of the third branch are joined with the flexor plate. In M. caspica many fibres of the deep branch are distally joined with the m. flexor digitorum longus and with the flexor plate. In Ge. chilensis(Fig. 4b), Tes. graeca, Gl. insculpta and Ter. carolina, this muscle has two branches, an internal (medial) and an external (lateral) one. Both branches arise from a broad tendon that covers the lateral epicondylus of the humerus, and fleshy from the ulna. The medial branch originates also fleshy from the distal two-thirds of the ulna, and the lateral one also proximally from the ulna. Distally, the muscle merges with the tendinous layer covering the m. abductor digitorum V.
The forearm of Ge. chilensis (Fig. 4a,c), Tes. graeca, Gl. insculpta and Ter. carolina has many muscular fusions that we have classified and described as follows:Fusion 1 (Fig. 4a): m. palmaris longus + m. flexor digitorum longus. Despite the fusion, both muscles can still be distinguished. Fusion 2 (Fig. 4b): m. flexor carpi ulnaris (medial branch) + Fusion 3.Fusion 3 (Fig. 4a,c): the flexor muscles of the digits were fused in an undifferentiated muscular mass. Superficially, mm. flexor brevis superficialis appear as a single reduced muscular mass located at the base of digits IV and V. The medial layer, which is a prolongation of m. flexor digitorum longus, included the flexor plate, which is mineralized. In the deepest layer another undifferentiated muscular mass could be observed, probably corresponding to the mm. flexores brevis profundi, and the mm. contrahentes.
In the chelonians analysed here (with the exception of R. pulcherrima that has no m. palmaris longus) the m. palmaris longus originates via a short tendon on the lateral epicondyle of the humerus, lateral to m. flexor carpi radialis. It is a flat, broad, and subtriangular muscle that inserts onto the palmar aponeurosis, which includes the flexor retinaculum at the wrist. The palmaris longus muscle is wide and well developed in Ge. chilensis (Fig. 4a), Tes. graeca, C. galbinifrons, C. amboinensis and Ter. carolina; and it is almost indistinguishable in M. caspica and Gl. insculpta.
The m. flexor digitorum longus, in Ph. hilarii(Fig. 2a,b), Po. unifilis, C. galbinifrons and Tr. scripta has two branches, a superficial one and a large deep one. The superficial branch originates from the lateral surface of the distal epicondyle of the humerus. It is triangular in shape and inserts onto the flexor plate. The deep branch originates along the lateral edge of the ulna, and also inserts onto the flexor plate. In C. galbinifrons and S. bealei, the ulnar head is fused with the m. flexor carpi ulnaris and the entire muscle is separated from the m. palmaris longus. Tr. scripta has some of the muscle fibres merged with those of the m. palmaris longus. In R. pulcherrima, M. caspica and C. amboinensis, this muscle has only one branch. It is a bulky, triangular muscle that inserts onto the flexor plate. In Ge. chilensis (Fig. 4b), Tes. graeca, Gl. insculpta and Ter. carolina this bulky muscle has a well-developed carpal head, inserting onto the flexor plate and which is incorporated to Fusion 3. The main body of the muscle is part of Fusion 1.
In Ph. hilarii (Fig. 5b), Po. unifilis, S. bealei, Tr. scripta and R. pulcherrima the m. brachialis inferior originates from the base of the proximolateral condyle of the humerus. It is a large, wide muscle that extends along the humerus, and inserts by means of a short, wide and strong tendon on the base of the proximal condyle of the radius. It is strongly associated with the tendon of the m. biceps. In Ge. chilensis, (Fig. 5a), Ter. carolina, Gl. insculpta, M. caspica, C. galbinifrons, C. amboinensis and Tes. graeca the m. brachialis inferior is quite large and originates along the distal half of the humerus. It inserts fleshy on the lateral and proximal quarter of the ulna, enveloping the laterodistal epicondyle of the humerus. The insertion tendons of the two mm. biceps pass through the m. brachialis inferior, which is thus separated into two overlapping layers.
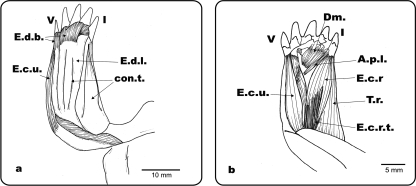
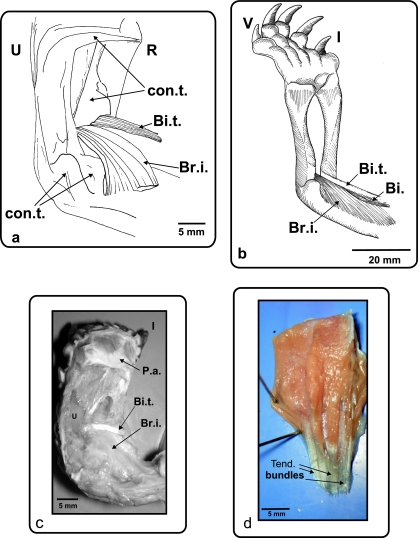
Fig. 5.
(a) Ge. chilensis, ventral view of the right antebrachium and hand. (b) Ph. hilarii, ventral view of the left antebrachium and hand. (c) Ge. chilensis, photograph of the biceps tendon insertion. A ventral view of the right antebrachium and hand (d) Muscle extensor digitorum longus removed from the dorsal side of the forelimb. Colour photograph showing the ventral face of the muscle with the tendinous bundles of its head. Bi.: m. biceps; Bi.t.: m. biceps tendon; Br.i.: m. brachialis inferior; R: radius; U: ulna; con.t.: connective tissue; P.a.: palmar aponeurosis; Tend. Bundles: tendinous bundles; I and V: digits I and V.
Pronator muscles of the forearm
This group consists of: m. pronator teres, m. pronator accesorius and m. pronator profundus. Quantitative analysis showed that terrestrial turtles again have heavier muscles than aquatic ones (mancova: Wilk's Lambda F3,12 = 0.47; P = 0.02). Subsequent univariate F-tests showed that this pattern holds for all muscles (all P < 0.05).
A large muscle, the m. pronator teres (Fig. 2d) extends obliquely, close to the m. flexor digitorum longus of the forearm. The former originates via a strong and long tendinous bundle, from the laterodistal epicondyle of the humerus, and inserts along the distal edge of the radius. In Ge. chilensis, Ter. carolina, Tes. graeca and Gl. insculpta, the m. pronator teres is well developed, with a broad origin on the distal condyle of the humerus, and a fleshy insertion along the ventral surface of the radius.
In all turtles examined, the m. pronator accesorius (Figs 2c, 4c) is a thick, big muscle, originating by means of a strong and long tendon from the distal extreme of the humerus between the origin of the m. flexor carpi ulnaris and the m. flexor carpi radialis. The muscle runs obliquely between the radius and the ulna and is partly covered by the deepest branch of the m. flexor digitorum longus. The muscle inserts fleshy on the medial surface of the distal extreme of the radius.
In Ph. hilarii(Fig. 2d), Po. unifilis and Tr. scripta the m. pronator profundus is a double muscle with a proximal and a distal branch. The proximal branch arises fleshy from the radius and inserts on the distal extreme of the ulna. The distal branch arises fleshy from the distal condyle and the distal two-thirds of the ulna. It inserts on the distal extreme of the radius, along its medial surface, and on the distal aspect of carpals 1, 2 and 3. In Ge. chilensis (Fig. 4c), Tes. graeca, Ter. carolina, M. caspica, C. galbinifrons, C. amboinensis, S. bealei and R. pulcherrima this is a single muscle and its insertion is moved slightly to the medial edge of the dorsal surface of the ulna.
Abductor muscles of the hand
This group consists of m. abductor pollici longus, m. abductor pollici brevis and m. abductor digitorum V. A univariate ancova testing for differences in the mass of the m. abductor pollici longus revealed no differences between terrestrial and aquatic species (F1,14 = 0.01; P = 0.93).
The abductor pollici longus muscle (Fig. 1b) is a triangular-like muscle that originates from the distal two-thirds or the entire length of the medial surface of the ulna. It inserts by means of a tendon on the distal extreme of metacarpal I. In Po. unifilis the muscle is shorter and the insertion point is on the proximal region of metacarpal I. In Ge. chilensis (Fig. 3b), Tes. graeca, Ter. carolina, Gl. insculpta, C. amboinensis, M. caspica and R. pulcherrima, however, the insertion is fleshy at the base of metacarpal I.
There is an m. abductor pollici brevis in Ph. hilarii (Fig. 2c), S. bealei, Po. unifilis, Tr. scripta, C. galbinifrons, C. amboinensis, M. caspica, Ter. carolina and R. pulcherrima. The muscle originates from the external edge of the distal condyle of the radius. It is a short and thick muscle, located on the lateral edge of the carpus, and inserting on the base of the first phalanx of digit I. The muscle has some fibres attached on the internal face of the skin at the dorsal and ventral sides of the manus (some specimens of Ter. carolina have no fibres attached to the skin). In Ge. chilensis, Tes. graeca and Gl. insculpta the lateral muscles of digits I and V (m. abductor digiti minimi and m. abductor pollici brevis) show anastomoses with the mm. extensor carpi, forming a continuous structure that runs on both sides of the forearm and manus. No fibres are attached to the skin in these species.
In all turtles examined, the m. abductor digitorum V (Fig. 2c) is long and narrow and originates from the lateral edge of the carpus on the os pisiformis. It inserts on the first phalanx of digit V. It extends laterally on the carpal area and many of its fibres are interconnected with the ventral flexor musculature.
Digital extensors
This group consists of mm. extensores digiti brevis and mm. dorsometacarpalis.
Each digit in Ph. hilarii, Po. unifilis, S. bealei, Tr. scripta, Ter. carolina and R. pulcherrima has a bicipital m. extensor digitorum brevis (Fig. 1b), which originates from the base of the corresponding metacarpal and inserts onto the base of the first phalanx via a short tendon. These muscles have fibres attached to the internal face of the skin. In M. caspica and Ter. carolina this muscle also originates from the ulnar bone but there are no fibres attaching to the skin. In Gl. insculpta, C. galbinifrons and C. amboinensis, these muscles have only one belly, arise from the ulnar bone and insert on the basal portion of the proximal phalanges. In Ge. chilensis (Fig. 3a) and Tes. graeca, mm. extensores digitii brevis form an undifferentiated muscular mass, which is associated with the ventral surface of the carpal portion of m. extensor digitorum longus. This muscular mass originates via tendons from os intermedium and inserts on the base of each ungual phalanx.
A group of muscles, the mm. Dorsometacarpalis, covers the sides of each digit in Ph. hilarii (Fig. 1a,b), Po. unifilis, Tr. scripta, S. bealei, C. amboinensis and R. pulcherrima. In Gl. insculpta, C. galbinifrons, M. caspica and Ter. carolina they cover the dorsal surface of each digit. They have a broad origin, with fibres arising from the distal carpal of each corresponding digit, and from the base of the corresponding metacarpal. They insert on the medial edge of each metacarpal bone, and extend distally via a wide and flat tendon that is attached from the second phalanx to the ungual one. In Ter. carolina this muscle is proximally undifferentiated from the mm. extensores digitii brevis. In S. bealei the insertion tendon is indistinguishable. In Ge. chilensis (Fig. 3b) and Tes. graeca, this group forms a continuous and undifferentiated layer with mm. extensores digitii brevis and extends on the dorsal surface of the digits.
Digital flexors
This group consists of mm. flexores digiti brevis superficialis, mm. lumbricalis, mm. flexores digiti brevis profundus, mm. intermetacarpalis and mm. contrahentes.
In Ph. hilarii (Fig. 2b), Po. unifilis, S. bealei and Tr. scripta the fan-shaped mm. flexores digiti brevis superficiales arise from the flexor retinaculum covering the palm of the manus, and split off into five slips running toward each digit. Each slip inserts fleshy on the base of the corresponding proximal phalanx. The dorsal fibres of the muscle are attached to the flexor plate. In R. pulcherrima, M. caspica, C. galbinifrons and C. amboinensis, these muscles are all undifferentiated in the proximal part of the hand, and only split toward each digit in the distal part of the hand. In Ge. chilensis(Fig. 4a–c), Tes. graeca and Gl. insculpta, these muscles are very reduced, forming an undifferentiated muscular mass close to the base of digits IV and V. These muscles are part of Fusion 3. In Ter. carolina the undifferentiated muscular mass is not reduced.
The flexores digiti brevis profundus (Fig. 2c) arise from the distal carpals, forming a flat layer that continues on each digit as a wide and flat muscle, which inserts on the distal extreme of the corresponding metacarpal. In Ge. chilensis (Fig. 4a–c), Tes. graeca, Gl. insculpta, R. pulcherrima and Ter. carolina these muscles form an undifferentiated muscular mass that is part of Fusion 3.
The mm. intermetacarpalis (Fig. 2d) are triangular with a broad origin on the lateral edge of each metacarpal. The muscle between digits V and IV has its origin on the fifth metacarpal; the muscle between digits IV and III has its origin on the metacarpal of digit IV, and so on. Each muscle inserts via a short tendon on the distal extreme of the next metacarpal. In M. caspica, C. amboinensis and S. bealei the mm. intermetacarpalis are tightly associated with the inter-digital webbing. In Ge. chilensis (Fig. 4a–c) and Tes. graeca these muscles are part of Fusion 3. In R. pulcherrima, Ter. carolina and Gl. insculpta these persist individually and do not take part in Fusion 3.
In Ph. hilarii (Fig. 2a), Po. unifilis, M. caspica, C. galbinifrons, C. amboinensis and Tr. scripta, the mm. lumbricalis arise from the tendinous flexor plate on both sides of each flexor tendon and from the os intermedium. The muscles insert on the base of the ungual phalanx of each digit. Some fibres of mm. lumbricalis arise from an underlying muscular layer (probably corresponding to the carpal head of m. flexor digitorum longus). This extremely flat muscular layer extends over the medial surface of the hand and is dorsally fused to the flexor plate. In S. bealei these muscles are very reduced. In Ge. chilensis, Tes. graeca Gl. insculpta, R. pulcherrima and Ter. carolina these muscles are part of Fusion 3 (Fig. 4a–c).
The mm. contrahentes form the deepest muscular layer of the manus in Ph. hilarii, Po. unifilis, Gl. insculpta, M. caspica and Tr. Scripta, forming a very flat and thin layer that arises from the distal carpals, and inserting on the base of the proximal phalanx of each digit. No mm. contrahentes are evident in C. galbinifrons, C. amboinensis or S. bealei. In Ge. chilensis, Tes. graeca, R. pulcherrima and Ter. carolina they probably form part of Fusion 3.
Associated tendinous structures
These are mainly represented by tendinous bundles, the flexor retinaculum, the palmar aponeurosis and the flexor plate.
Tendinous bundles (Fig. 5d) are composed of many tendons forming a very thick bundle. These structures are maximally developed in the tendons of the m. biceps that inserts onto the different regions of the proximal half of the ulnar shaft. They are present near the origin and sometimes also near the insertion of the following muscles: the flexor digitorum longus (origin and insertion in both types of chelonians), the flexor carpi ulnaris (origin in both types of chelonians), the flexor carpi radialis (origin in both types of chelonians), the pronator accesorius (origin in Ge. chilensis and Tes. graeca) and the extensor carpi ulnaris (incipient development in the origin of the muscle in P. hilarii).
The m. biceps is one of the main flexor muscles of the forearm, and also acts as a retractor of the humerus. It is commonly divided into two bundles, a superficial and a deep one. The latter inserts by means of a long tendon on the forearm. The tendinous bundles in the m. biceps profundus tendon in turtles examined here are remarkably large, and resemble cables arranged around a lumen. The insertion of the m. biceps tendon in Ge. chilensis and Tes. graeca is via a fan-shaped long tendon on the base of the proximal condyle of the radius and ulna. In Ph. hilarii, S. bealei, Po. unifilis, Gl. insculpta, C. galbinifrons, C. amboinensis, M. caspica, R. pulcherrima, Ter. carolina and Tr. scripta, the insertion is at the base of the proximal condyle of the radius, separated from m. brachialis inferior. A quantitative analysis of the length of the m. biceps tendon shows that the tendon is significantly longer in the terrestrial species analysed (ancova: F1,14 = 4.76; P = 0.045).
The flexor retinaculum (Figs 2b, 4c) is an annular ligament that crosses the wrist and is generally present in all the members of the Amniota (our personal observations). It originates on the radial bone and inserts on the ulnar bone. In both aquatic and terrestrial chelonians the flexor retinaculum is strongly embedded in the m. palmaris longus superficially, as well as in the m. flexor digitorum longus.
An aponeurotic layer, the palmar aponeurosis (Figs 4a, 5c), covers the surface of mm. flexores digiti brevis superficialis, and originates on the flexor retinaculum. It extends over the palmar surface of the manus, and inserts on each digit. In the species studied here, there is a well-developed and tendinous flexor plate (Figs 2a, 4b), completely covered by the mm. flexor digiti brevis superficialis. The flexor plate originates on the muscle fibres of the m. flexor digitorum longus, and splits distally into five, corresponding to the five digits and continuing in the digital flexor tendons. These tendons insert on the ungual phalanx of each digit, inside the ungual sheath. In Ge. chilensis (Fig. 4b) and Tes. graeca, the tendinous flexor plate also splits into five and inserts by means of five very short extensions at the base of each digit.
Discussion
Most of the turtles analysed here presented a conservative muscular pattern of the forelimbs already described for other turtles and reptiles (Bojanus, 1819; Walther, 1922; Haines, 1939, 1950; Walker, 1973; Moro & Abdala, 2004). Departures from this typical pattern appear in extremely terrestrial turtles such as Geochelone, and in the flippers of aquatic turtles (Walther, 1922). Wyneken (2001) stressed that the musculature of the flipper of sea turtles is obscured by extensive connective tissue. Thus, these derived patterns are reached with the same basic resources: muscular fusion and tendon development.
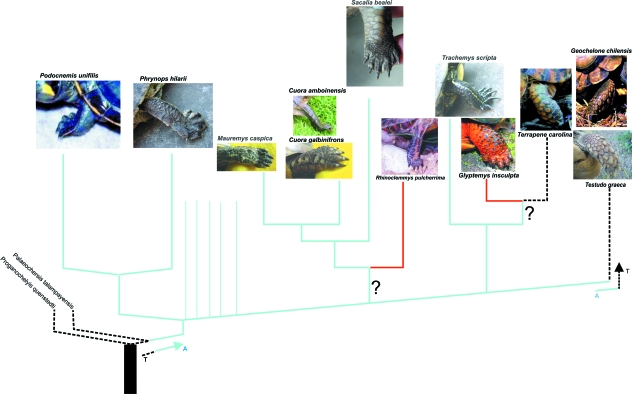
Our analysis of the distal forelimb muscles in terrestrial and aquatic turtles (not including marine turtles, which have derived flippers) shows distinct qualitative differences between the two groups mainly revolving around muscular fusion and the reinforcement of muscular fibres by tendinous tissue. In general, terrestrial species tend to show increased fusion and an increase in the amount of tendinous tissue. Based on our data, we would argue that these differences can in some cases be ascribed to constraints imposed by the locomotor environment. This link is very clear when terrestrial turtles included in the emydid and testudinid clades are considered (Joyce & Gauthier, 2004; Spinks et al. 2004). Thus, Ge. chilensis, Tes. graeca and Ter. carolina all present different grades of modification leading to the terrestrial pattern. This pattern is extreme in Ge. Chilensis and Tes. graeca, but not so divergent and specialized in Ter. carolina.
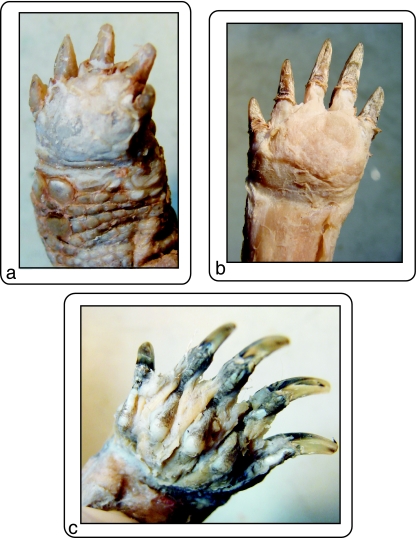
The morphologically intermediate condition displayed by Rhinoclemmys and Gl. insculpta, on the other hand, shows that the nature of the changes in the musculature related to differences in locomotor environment are also dependent on the ancestral bauplan of the species. In geoemydids the ecomorphological links are less clear. Although we dissected only one species of terrestrial geoemydid, C. galbinifrons, its muscular pattern is more similar to the typical bauplan as observed in the aquatic species than to the terrestrial one. Moreover, it appears that the completely terrestrial pattern has never appeared within the geoemydid clade. The only concession made to terrestriality is the tendency to develop a ‘pad’ in the palmar surface of the manus of the terrestrial geoemydid dissected. A typical ‘pad’ implies the presence of Fusion 3, and can also be seen in the terrestrial forms as Geochelone (Fig. 5c), Testudo (Fig. 6a) or Ter. carolina(Fig. 6b). It is possible that the fusion described by Walther (1922) in Carettochelys insculpta also corresponds to this type of structure.
Fig. 6.
Ventral view of the palmar surface of the hands, showing the ‘pads’: (a) Tes. Graeca, (b) Ter. Carolina, (c) Tr. scripta, showing no pad.
In general, there appears to be a common chelonian pattern that was probably acquired early in their phylogenetic history (Walker, 1973; present study). The same pattern, exemplified by the tendency to preserve a proximal insertion of m. extensor longus in comparison with other vertebrates, can be found in the lizard forearm (Abdala & Moro, 2006). This may provide additional support for the proposed relationship between turtles and squamates (de Braga & Rieppel, 1997; Rieppel, 2000). This typical pattern is perfectly suitable to perform all movements required in aquatic, semiterrestrial or terrestrial locomotion, as it is present in all geoemydid species analysed independent of their locomotor habits or lifestyle. The phylogenetic distribution of the typical forelimb muscular bauplan (Fig. 7) supports Joyce & Gauthier's (2004) interpretation of ancestral terrestrial habits with a derived acquisition of an aquatic lifestyle in crown turtles and a subsequent reversal to terrestrial habits in Testudinidae. Our data also suggest that adaptations to terrestrial habits and lifestyles were independently acquired within the emydids (Fig. 7).
Fig. 7.
Cladogram showing the hypothesis of character transition from terrestrial to aquatic morphology of the species included in this study (redrawn after Joyce & Gauthier 2004; Spinks et al. 2004). T: terrestrial; A: aquatic; ?: ambiguous optimization. Red: morphologically intermediate forms.
In reptiles the major flexor muscle of the forearm, represented by the m. flexor digitorum longus, is usually divided into several branches and is frequently fused with the neighbouring muscles such as the mm. flexor carpi radialis and/or flexor carpi ulnaris. When Haines (1950) and Landsmeer (1984) described the musculature of Varanuslizards, they found that m. flexor digitorum longus has three branches, one fused with both flexor carpi muscles, the other with the m. pronator teres, and the deepest one (the carpal head) being the origin of the tendinous flexor plate of the manus. This carpal head is also present in other lizards (Moro & Abdala, 2004). In the turtles the carpal head is also evident and can be separated from the main muscular body. However, in Ge. chilensis, although identifiable, it is incorporated into Fusion 3 (see Results). In most turtles examined, the m. flexor digitorum longus is strongly attached to m. palmaris longus (except for, for example, R. pulcherrima), even to such an extent that it is difficult to separate them from each other. This strong relationship suggests the possibility of the m. palmaris longus being just one more layer of the m. flexor digitorum longus. However, we follow Haines (1950) and suggest that the m. palmaris longus ends in the superficial palmar aponeurosis and that the m. flexor digitorum longus ends in the tendinous plate. McMurrich (1905) and Walker (1973), however, consider the palmar aponeurosis and flexor plate as the same structure onto which both muscles are attached. We disagree with their interpretation as the mm. flexores digiti brevis superficialis separate both tendinous layers. In R. pulcherrima the m. palmaris longus is entirely absent.
The extensor carpi radialis muscle has two branches in Ph. hilarii instead of the three branches observed in Podocnemis and Ge. chilensis. The intermedius and profundus branches in other chelonians seem to be fused in Ph. hilarii. Walther (1922) stressed that in Tryonix there is only one portion, and two in Caretochelys insculpta. We observed that the lateralmost muscle of the radial muscular group corresponds to the m. tractor radii, thus agreeing with Walker (1973) that this muscle is always present in chelonians. In most of aquatic turtles analysed, the insertion of this muscle is ventrally displaced possibly allowing the m. tractor radii to act like a pronator muscle. McMurrich (1905) considers the presence of strong tendinous bands between the digits, rather than the presence of the mm. Intermetacarpalis, as a generalized character for Reptilia. However, in turtles the mm. intermetacarpalis are well developed (Walther, 1922; Walker 1973; present study), thus contradicting this statement.
With respect to the intrinsic extensor muscles of the hands, the mm. extensores digiti brevis were described by Walker (1973) as absent in testudinids like Geochelone elephantopus. Moreover, he interpreted the fusion of the mm. extensores digiti brevis and the m. interossei into a single muscle group as being related to the less independent digital movements in sea turtles. We observed the same condition in Ge. chilensis, Tes. graeca and Ter. carolina for both muscles, and agree with Walker (1973) that the fusion of both muscles prevents independent digit movements. We have also observed that the aponeurotic tissue of both flexor and extensor surfaces of the forelimb in G. chilensis and Tes. graeca probably contributes to the limited mobility of the hand. Ter. carolina, however, shows an increased mobility of the digits. The aponeurotic tissue of the flexor and extensor surfaces is highly expanded, forming a thick and firm layer that superficially covers the muscles of the manus and is strongly associated with the underlying muscular structures. In Ph. hilarii the aponeurotic tissue is more loosely attached to the underlying musculature, and is associated with the internal face of the skin, in a pattern probably related to the movement of the forelimb webbing. Wyneken (2001) described a similar attachment of muscular fibres to the skin of the flipper in sea turtles, and related this condition to their swimming behaviour. This link between muscular fusion, tendon development and less movement flexibility has already been suggested by Walther (1922) in relation to the structure of the flipper in Caretochelys insculpta.
The muscular structure of Ge. chilensis and Tes. graeca is clearly different from that of the other turtles analysed. R. pulcherrima, Gl. insculpta and Ter. carolina, also terrestrial turtles, do not completely share the same pattern as the other two species especially because the tendinous system in their forelimbs is not as strongly developed as in Ge. chilensis or Tes. graeca. Interestingly, Wilbur & Morin (1994) stressed that the Emydidae in general are a predominantly aquatic family, but showing secondarily trends towards terrestriality in the genera Terrapene, Glyptemys and Rhinoclemmys. The forelimb musculature also shows this tendency. Rhinoclemmys presents fusion of some muscles: the ventral musculature of the hand, the m. flexor digitorum longus, and the m. pronator profundus, where only one branch can be discerned. In Ter. carolina and Gl. insculpta the tendency to terrestriality is more acute, as they present Fusions 1, 2, and 3, although the last one to a lesser degree. Auffenberg & Iverson (1979) suggest that testudinids have evolved from emydids with intermediate forms similar to extant Terrapene. The myology of Rhinoclemmys, showing forearm muscles to some degree similar to the aquatic turtles, supports the idea of these turtles being morphologically intermediate forms. By contrast, the presence of Fusion 3 in most of the terrestrial forms analysed probably indicates that in the manus some type of reinforcement is necessary to allow an efficient terrestrial locomotion. However, it should be noted that this trait also appears, although less pronounced, in all geoemydid turtles, making its relationship with ecology spurious.
In fact, only in aquatic turtles such as Ph. hilarii, Po. unifilis and Tr. scripta (Fig. 6c) is it possible to recognize the individual intrinsic muscles of the ventral portion of the hand. The other extreme is presented in testudines, with this palmar zone having the aspect of a fatty pad covered by a hard tendinous tissue (Figs 5c and 6a). In terrestrial emydids the pad is present (Fig. 6b), although not as well developed, and the tendinous tissue rather has the consistency of a hard aponeurosis. This portion of the hand is very simple in the aquatic turtles, as also suggested by Bojanus's (1819) work on Emys orbicularis in which all muscles can be easily identified, no fusion being indicated or drawn.
Ge. chilensis presents well-developed muscles and tendinous structures, although with a remarkable tendency to a fusion of the different muscular bodies. This tendency can be observed in Fusions 1, 2, and 3, as described in the Results. As burrowing is prevalent in tortoises, it could be hypothesized that the forelimb musculature is modified in relation to these habits. Morphological adaptations for forelimb digging are rare, however, and are only known to occur in taxa that undertake extensive tunnelling, such as the North American gopher tortoises (Kley & Kearney, 2007). Although Ge. chilensis shares this burrowing habit, its digging does not involve extensive tunnelling (Cabrera, 1998). In contrast, this species uses tunnels dug by other animals, including Lagostomus maximus holes (Richard, 1999). Moreover, analysis of the myology of dedicated fossorial forms such as moles (Whidden, 2000) shows no presence of fusion of the muscles, nor a pronounced development of tendinous structures.
Intuitively, more and more independent muscular masses suggest a more flexible locomotor repertoire. Wyneken (2001) suggests that in young sea turtles, the muscle divisions of the forearm and the flipper, in particular, are more obvious than in older animals. In the former, less connective tissue is present and the digits have rudimentary flexion and extension capabilities. Ph. hilarii has distinct muscle divisions, and these are present also in older animals. Ge. chilensis, by contrast, presents the extreme condition with no possibility of digit movements in any ontogenetic stage. Thus, whereas aquatic freshwater turtles appear to keep their digit movement abilities in their adult life, marine turtles suffer a decrease of their movement capacities as they get older, and terrestrial turtles are unable to move their digits at all.
In addition to these qualitative differences, quantitative differences in the robustness or mass of the distal forelimb muscles were observed. Although these differences have to be interpreted cautiously as only a single terrestrial representative from only one family was included in the analysis, they suggest that in accordance with our a priori predictions the extensor muscles are more robust in terrestrial turtles. However, in contrast to our expectations, not only are the extensor muscles of the distal forelimb (which are crucial in providing both body support and propulsion) heavier in terrestrial turtles, but so too are all muscles acting around the wrist, and even the forearm rotators. This suggests an important function for the flexors and rotators in stabilizing the wrist and forearm during the stance phase. Electromyographic analyses of the forearm muscles are needed to test this prediction, however. Although more species of a wider range of families clearly have to be examined in a quantitative manner, our data suggest that functionally relevant differences exist in the distal forelimb muscles in aquatic versus terrestrial turtles and provide the basis for future quantitative studies of muscle function and architecture in chelonians.
Concluding remarks
Geochelone and Testudo exhibit a strongly derived muscular bauplan. Terrapene also has a terrestrial bauplan but not as extreme. Whereas an intermediate morphology can be observed in Glyptemys and Rhinoclemmys. Phrynops, Podocnemis, Trachemys and all geoemydid turtles exhibit a more generalized pattern. Based on the data collected in the present study, we suggest that the muscular structure of the forelimb is different between terrestrial and aquatic turtles, and that those differences can be ascribed to functional constraints imposed by the different locomotor environments in emydids and testudinids. However, this clear ecomorphological signal is not present in the geoemydids.
Acknowledgments
We are grateful to Pablo Aceñolaza, Cristian Abdala, Marissa Alcaide and Susana Mangione for their valuable help. We also thank Juan Daza, Tony Gamble, Boaz Sacham, Yehuda Werner, Walter Joyce and Gregory Watkins-Colwell for their generous help in obtaining many of turtle specimens. We greatly appreciate Walter Joyce and an anonymous reviewer for suggested improvement to the manuscript. This study was supported by PIP-CONICET 6347 to V.A., PICT – Agencia 12418, PI-UADER, and project 05/06 FWO Belgium – SECYT Argentina.
Appendix I: material analysed
Acronyms: AH, personal collection of Anthony Herrel; DIAMR, herpetological collection of Diamante-CONICET, Argentina; FML, herpetological collection of Fundación Miguel Lillo, Argentina; HUJ, herpetological collection of the Hebrew University of Jerusalem, Israel; RT, private collection of Richard Thomas, University of Puerto Rico, USA; YPM, Peabody Museum of Natural History, Yale University.
Ph. hilarii: DIAMR-044 (one specimen); DIAMR-042 (one specimen); DIAMR-041 (one specimen); DIAMR-043 (one specimen); DIAMR-037 (one specimen); DIAMR-005 (one specimen); DIAMR-006 (one specimen); DIAMR-007 (one specimen). Ge. chilensis: DIAMR-038 (two specimens, male and female); DIAMR-039 (two specimens, male and female); DIAMR-040 (one specimen, juvenile); DIAMR-077 (one specimen); FML 16564 (one specimen); FML16878 (one specimen); FML 16879 (one specimen); FML 16880 (one specimen); FML16595 (one specimen) FML 00005 (one specimen); FML 16978 (one specimen). Podocnemys unifilis: DIAMR-078 (six specimens, juveniles). Tr. scripta RT uncatalogued (two specimens). Tes. graeca HUJ-R 22843; HUJ-R 22845 (two specimens). R. pulcherrima AH uncatalogued (one specimen). Gl. insculpta YPM R 5952 (one specimen). C. galbinifrons YPM R 12735 (one specimen). C. amboinensis YPM R 14443 (one specimen). Ter. carolina YPM R 13624 (1 specimen). Ter. carolina YPM R 13622 (one specimen). S. bealei YPM R 14670-71 (two specimens). M. caspica rivulata YPM R 16233-36 (two specimens).
References
- Abdala V, Moro S. Comparative myology of the forelimb of Liolaemussand lizards. Acta Zologica (Stockholm) 2006;87:1–12. [Google Scholar]
- Auffenberg W, Iverson JB. Demography of terrestrial turtles. In: Harless M, Morlock H, editors. Turtles: Perspectives and Research. New York: Wiley; 1979. pp. 541–569. [Google Scholar]
- Biewener AA, Gillis GB. Dynamics of muscle function during locomotion: accommodating variable conditions. J Exp Biol. 1999;202:3387–3396. doi: 10.1242/jeb.202.23.3387. [DOI] [PubMed] [Google Scholar]
- Bojanus LH. Anatome testudinis europaeae. Facsimile Reprints in Herpetology. 1819;26:178. [Google Scholar]
- Cabrera M. Las tortugas continentales de Sudamérica austral. Córdoba, Argentina, BR: Copias; 1998. [Google Scholar]
- Carrillo de Espinoza N, Rothenstein D, Salas A, Werner Y. Radiation and convergence among desert geckos: Phyllodactylusspecies resembling both Ptyodactylus and Stenodactylus. Amphibia-Reptilia. 1990;15:275–284. [Google Scholar]
- Collette BB. Correlation between ecology and morphology in anolinae lizards from Havana, Cuba and southern Florida. Bull Mus Comp Zool. 1961;125:137–162. [Google Scholar]
- De Braga M, Rieppel O. Reptile phylogeny and the interrelationships of turtles. Zool J Linn Soc. 1997;120:281–354. [Google Scholar]
- George JC, Patel V. The myology of the chelonian limb. I. The forelimb of Lissemys punctata. J Animal Morph Physiol. 1957;4:81–94. [Google Scholar]
- Gillis GB. Environmental effects on undulatory locomotion in the American Eel Anguilla rostrata: kinematics in water and on land. J Exp Biol. 1998;201:949–961. [Google Scholar]
- Gillis GB, Blob RW. How muscles accommodate movement in different physical environments: aquatic vs. terrestrial locomotion in vertebrates. Comp Biochem Physiol A. 2001;131:61–75. doi: 10.1016/s1095-6433(01)00466-4. [DOI] [PubMed] [Google Scholar]
- Haines RW. A revision of the extensor muscles of the forearm in tetrapods. J Anat. 1939;73:211–233. [PMC free article] [PubMed] [Google Scholar]
- Haines RW. The flexor muscle of the forearm and hand in lizards and mammals. J Anat. 1950;84:13–29. [PMC free article] [PubMed] [Google Scholar]
- Hamrick MW. Articular size and curvature as determinants of carpal joint mobility and stability in strepsirhine primates. J Morphol. 1996;230:113–127. doi: 10.1002/(SICI)1097-4687(199611)230:2<113::AID-JMOR1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Joyce WG, Gauthier JA. Palaeoecology of Triassic stem turtles sheds new light on turtle origins. Proc R Soc Lond B. 2004;271:1–5. doi: 10.1098/rspb.2003.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kley NJ, Kearney M. Adaptations for digging and burrowing. In: Hall B, editor. Fins into Limbs. Evolution, Development, and Transformation. Chicago: The University of Chicago Press; 2007. pp. 284–309. [Google Scholar]
- Landsmeer JMF. Morphology of the anterior limb in relation to sprawling gait in Varanus. Symposia Zool Soc Lond. 1984;52:27–45. [Google Scholar]
- McMurrich JP. The phylogeny of the crural flexors. J Anat. 1905;4:36–75. [Google Scholar]
- Moro S, Abdala V. Análisis descriptivo de la miología flexora y extensora del miembro anterior de Polychrus acutirostris(Squamata, Polychrotidae) Papeis Av Zool. 2004;44:81–89. [Google Scholar]
- Odendaal FJ. Notes on the adaptive ecology and behaviour of four species of Rhoptropus(Gekkonidae) from the Namib desert with special reference to a thermorregulatory mechanism employed by Rhoptropus afer. Madoqua. 1979;11:255–260. [Google Scholar]
- Pace CM, Blob RW, Westneat MW. Comparative kinematics of the forelimb during swimming in red-eared slider (Trachemys scripta) and spiny shoftshell (Apalone spinifera) turtles. J Exp Biol. 2001;204:3261–3271. doi: 10.1242/jeb.204.19.3261. [DOI] [PubMed] [Google Scholar]
- Payne RC, Crompton RH, Isler K, et al. Morphological analysis of the hindlimb in apes and humans. I. Muscle architecture. J Anat. 2006;208:709–724. doi: 10.1111/j.1469-7580.2006.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RC, Hutchinson JR, Robillard JJ, Smith NC, Wilson AM. Functional specialization of pelvic limb anatomy in horses (Equus caballus. J Anat. 2005;206:557–574. doi: 10.1111/j.1469-7580.2005.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RC, Veenman P, Wilson AM. The role of the extrinsic thoracic muscles in equine locomotion. J Anat. 2004;205:479–490. doi: 10.1111/j.0021-8782.2004.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renous S, Bels VL. Comparison between aquatic and terrestrial locomotions of the leatherback sea turtle (Dermochelys coriacea. J Zool Lond. 1993;230:357–378. [Google Scholar]
- Richard E. Tortugas de las regiones áridas de Argentina. Buenos Aires, Argentina: Literature of Latin America; 1999. Monografía 10, LOLA. [Google Scholar]
- Rieppel O. Turtles as diapside reptiles. Zoo Scripta. 2000;29:199–212. [Google Scholar]
- Romer AS. The Osteology of Reptiles. Chicago: The University of Chicago Press; 1956. [Google Scholar]
- Russell AP. Limb muscles in relation to lizard systematics: a reappraisal. In: Estes R, Pregill G, editors. Phylogenetic Relationships of Lizard Families: Essays Commemorating Charles L. Camp. Stanford: Stanford University Press; 1988. pp. 119–281. [Google Scholar]
- Russell AP, Bauer A. The morphology of the digits of the golden gecko Calodactylodes aureus and its implication for the occupation of rupicolous habitats. Amphibia-Reptilia. 1989;10:125–140. [Google Scholar]
- Sheil CA. Skeletal development in turtles: Patterns of ossifications through ontogeny in Apalone spinifera, Chelydra serpentina, Macrochelys temminchii and Eretmochelys imbricata (Reptilia: Chelonii). PhD Dissertation, University of Kansas.
- Smith NC, Wilson AM, Jespers KJ, Payne RC. Muscle architecture and functional anatomy of the pelvic limb of the ostrich (Struthio camelus. J Anat. 2006;209:765–779. doi: 10.1111/j.1469-7580.2006.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rolph FJ. Biometry. 3. New York: W. H. Freeman and Company; 1995. [Google Scholar]
- Spinks PQ, Bradley Shaffer H, Iverson JB, McCord WP. Phylogenetic hypothesis for the turtle family Geoemydidae. Mol Phylogenet Evol. 2004;32:164–182. doi: 10.1016/j.ympev.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Walker FW. A structural and functional analysis of walking in the turtle, Chrysemys picta marginata. J Morphol. 1971;134:195–214. doi: 10.1002/jmor.1051340205. [DOI] [PubMed] [Google Scholar]
- Walker FW. The locomotor apparatus of Testudines. In: Gans C, Parsons P, editors. Biology of the Reptilia vol. 4 Morphology D. London: Academic Press; 1973. pp. 1–99. [Google Scholar]
- Walther WG. Die Neu-Guinea-Schildkröte Carettolcheys insculpta Ramsay. Brill-Leiden: 1922. [Google Scholar]
- Whidden HP. Comparative myology of moles and the phylogeny of the Talpidae (Mammalia, Lypotyphla) Amer Mus Novit. 2000;3294:1–53. [Google Scholar]
- Wilbur HM, Morin PJ. Life history evolution in turtles. In: Gans C, Huey H, editors. Biology of the Reptilia vol. 16, Ecology B. London: Academic Press; 1994. pp. 388–439. [Google Scholar]
- Williams SB, Wilson AM, Payne RC. Functional specialization of the thoracic limb of the hare (Lepus europeus. J Anat. 2007;210:491–505. doi: 10.1111/j.1469-7580.2007.00703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren K, Claussen DL, Kunz M. The effects of body size and extrinsic mass on the locomotion of the ornate box turtle, Terrapene ornata. J Herpetol. 1998;32:144–150. [Google Scholar]
- Wyneken J. The Anatomy of the Sea Turtle. 2001:1–172. In: U.S. Department of Commerce NOAA. Technical Memorando NMFS-SEFSC-470, pp. [Google Scholar]
- Yasukawa Y, Hikida T. Musculus biceps in Testudines, with special reference to its variations in the family Bataguridae. J Herpetol. 1999;33:487–490. [Google Scholar]
- Zani PA, Claussen DL. Effects of extrinsic load on locomotion in painted turtles (Chrysemys picta. Copeia. 1995;1995:735–738. [Google Scholar]
- Zani PA, Gottschall JS, Kram R. Giant Galapagos tortoises walk without inverted pendulum mechanical-energy exchange. J Exp Biol. 2005;208:1489–1494. doi: 10.1242/jeb.01554. [DOI] [PubMed] [Google Scholar]