Abstract
We studied the morphology of the chondrocranial orbitotemporal region in the snakelike gymnophthalmid lizard Bachia bicolor and its relation to other structures such as the ophthalmic division of the trigeminal nerve and eye muscles, to show its particular morphology, and discuss the homology of some skeletal structures relative to other squamates. We used three-dimensional computer reconstructions from serial histological sections; additionally we studied the embryonic and postembryonic development of the orbitosphenoid bone using cleared and double-stained whole-mount skeletal material. The chondrocranial orbitotemporal morphology in B. bicolor was found to be severely reduced as described for other miniaturized snakelike squamates, but it was accompanied by extensive orbitosphenoid ossifications. Within squamates, only amphisbaenians showed an expanded orbitosphenoid, which originates from fused endochondral and membranous ossifications. In B. bicolor the orbitosphenoid was also found to be formed by endochondral and membranous ossifications, but contrary to the amphisbaenian condition the membranous ossifications were found to arise as membrane bone outgrowths from the perichondral ossification of the chondral core. Despite its derived morphologies, we argue that the orbitosphenoids in amphisbaenians and B. bicolor are homologous to the orbitosphenoids of other squamates. Thus, the expanded orbitosphenoid morphology is found to be achieved by different ontogenetic processes in amphisbaenians and B. bicolor, representing a case of independent evolution by convergence.
Keywords: chondrocranium, eye muscles, orbitosphenoid, snakelike lizard, Squamata
Introduction
The evolution of snakelike forms has occurred independently several times within Squamata (Lee, 1998; Wiens et al. 2006), obscuring morphology based phylogenetic analyses due to the convergent evolution of numerous features, such as limb reduction and overall skull morphology, often correlated to burrowing life styles and by underlying miniaturization (Lee, 1998). Furthermore, the possible instances of mosaic evolution between the cranial and postcranial skeleton has shown us the impressive variation of the limbs within Squamata (Kearney & Stuart, 2004; Apesteguia & Zaher, 2006), ranging from limb reduced to limbless lizards without following any trend from limbed to limbless forms, and even the occurrence of possible cases of limb re-evolution (Apesteguia & Zaher, 2006; Kohlsdorf & Wagner, 2006). Thus regarding the limbs by themselves, the overall similarities of the postcranial skeleton among snakelike lizards dubiously represent reliable evidence about its phylogenetic position, as derived or basal lizards, considering solely the degree of limb reduction, hence directing our attention to a detailed study of skull morphology.
Besides the overall similarity, snakelike lizards bear particular morphological features other than those convergent traits assigned to a generalized limbless morphotype, which are probably informative for future comparative anatomical studies, as morphology-based phylogenetic analyses, and for the understanding of character evolution and homology in such highly derived lizards. On the other hand, the repeated evolution of numerous traits in snakelike lizards might shed light on the possible phenotypic plasticity or constraint (Hodin, 2000) of the phenotypic evolution within Squamata, taking into account the ontogenetic mechanism underlying those traits.
As independently evolved in other genera of the family Gymnophthalmidae (Pellegrino et al. 2001; Castoe et al. 2004), the genus Bachia represent a severe case of limb reduction (Dixon, 1973), ranging from fully limbed to almost limbless forms, in which the hindlimbs show a more extreme degree of limb reduction than the forelimbs (Presch, 1975; Kizirian & McDiarmid, 1998). Limb reduction in Bachia has apparently evolved without following any particular trend or morphocline, including possible cases of limb re-evolution (Kohlsdorf & Wagner, 2006). However, with the exception of some recent accounts (Tarazona et al. 2008) there is no detailed information about skull morphology in the genus Bachia, which is needed for future studies of morphological evolution focusing on the cranial vs. the postcranial skeleton.
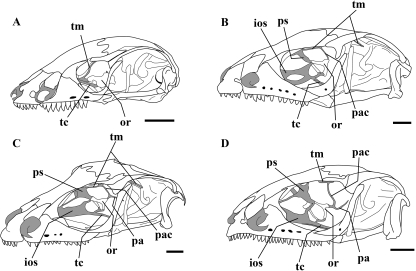
Although detailed information about skull morphology in the genus Bachia is very scarce, the orbitosphenoid bone has been recognized by a few authors as derived, due to its particular morphology relative to other squamate lizards (Bellairs & Gans, 1983; Tarazona et al. 2008). Recently it was discovered that the orbitosphenoid in Bachia bicolor has a compound nature by virtue of its chondral and membranous components (Tarazona et al. 2008), in contrast to the entirely chondral replacement bone usually present in other lizards (Bellairs & Kamal, 1981). In B. bicolor the orbitosphenoid bone is paired, broad and located on the braincase floor, unlike the same bone in other squamate lizards (Fig. 1). Despite its unusual morphology the orbitosphenoid in B. bicolor resembles the orbitosphenoids of some amphisbaenians (Tarazona et al. 2008), which usually present an unpaired and very broad orbitosphenoid bone located on the braincase floor.
Fig. 1.
Reduced orbitotemporal scaffolding and extended orbitosphenoid ossification in B. bicolor, compared to the more complete orbitotemporal region and small bar-like orbitosphenoid ossification in other lizards within Gymnophthalmidae, observed in cleared and double-stained specimens. (A) B. bicolor (UIS-R-1463). (B) Ptychoglosus bicolor (UIS-R-1535). (C) Tretioscincus bifasciatus (UIS-R-1533). (D) Leposoma sp. (UIS-R-1534). ios, interorbital septum; or, orbitosphenoid; pa, pila antotica; pac, pila accesoria; ps, planum supraseptale; tc, trabecula communis; tm, taenia marginalis; Scale bars = 1 mm.
In lizards the orbitotemporal region of the chondrocranium is generally formed by paired and complex cartilaginous scaffolding which outlines membrane-filled fenestrae (Bellairs & Kamal, 1981). The paired cartilaginous bars are continuous anteriorly and medially with the trough-like planum supraseptale and the interorbital septum, and ventrally with the trabecula communis. Those cartilaginous bars and membrane-filled fenestrae serve as eye muscle attachments and are pierced by various cranial nerves (Underwood, 1970). The orbitosphenoid bone ossifies from some of those cartilaginous bars, posterodorsal to the emergence of the optic nerve. As a result the orbitosphenoid is a chondral replacement bone which shows a roughly arched shape and conserves characteristic topological relationships relative to other orbitotemporal structures of the chondrocranium (Fig. 1).
Snakelike squamates, however, frequently show considerable reduction on the orbitotemporal region of the chondrocranium. For instance, in amphisbaenians, dibamids and snakes the complex cartilaginous scaffolding is absent and, excluding amphisbaenians, they also lack the orbitosphenoid bone. In B. bicolor the orbitosphenoid bone is evident as already mentioned, but there is no information about the chondrocranial orbitotemporal morphology.
We describe the morphology of the orbitotemporal region and the embryonic development of the orbitosphenoid bone in B. bicolor. We focus on the skeletal elements to establish the topological relationships of the orbitotemporal skeletal structures. Their relationship to other structures such as cranial nerves and eye muscles is also mentioned. Given the role of the cartilaginous scaffolding and the orbitosphenoid bone in eye muscle insertion, we describe the eye muscle configuration and its relation to the orbitotemporal skeletal structures. Furthermore, we discuss the homology of the orbitosphenoid bone and other skeletal structures relative to other squamates and also the possible parallelism or convergence involved in the attainment of its unusual morphology in B. bicolor and amphisbaenians, as a possible case of independent evolution within an ontogenetic perspective.
Material and methods
All the specimens used were obtained from the Colección Herpetológica of the Museo de Historia Natural, Universidad Industrial de Santander (UIS-R), including adults, juveniles and neonatal specimens (see Appendix). Whole-mount skeletal material was cleared and double-stained for bone and cartilage according to Wassersug (1976) and examined through an Olympus stereomicroscope. Additionally, the head of three adult specimens were removed and processed for histological standard techniques after tissue decalcification with formic and hydrochloric acids (Luna, 1969). Serial sections of 7–10 µm were made and stained with hematoxylin-eosin.
Tridimensional computer reconstructions were made with Reconstruct v. 1.1.0.0 (Fiala, 2005) using one of the sectioned specimens. Tridimensional images were generated, illustrating the topological relationships of bone, cartilage, muscles and nerves, focusing on the chondrocranial orbitotemporal region. Structures with bilateral symmetry such as the orbitosphenoid, eye muscles and cranial nerves were traced only for the right side, and then mirror traces were created for subsequent bilateral reconstructions.
The anatomical terminology used herein follows Bellairs & Kamal (1981) for chondrocranial structures, Underwood (1970) for eye muscles and Oelrich (1956) for cranial nerves.
Embryonic and postembryonic development
We studied the orbitosphenoid embryonic and postembryonic development using whole-mount specimens stained for bone and cartilage. We arranged the specimens for postnatal development in size series according the snout–vent length.
Embryonic material
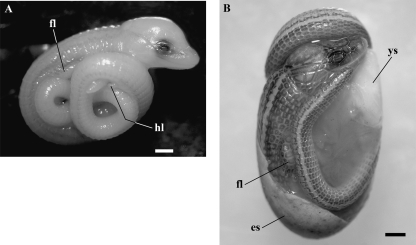
We had the opportunity to analyse two embryos of B. bicolor at late developmental stages regarding its external morphological features (Fig. 2). The embryos were cleared and double-stained for bone and cartilage (Wassersug, 1976). To our knowledge, this is the first report dealing with embryonic material of this rare fossorial lizard. We briefly describe the external morphological features of the embryos.
Fig. 2.
Late embryos of B. bicolor. (A) Embryo I. (B) Embryo II. es, eggshell; fl, forelimb; hl, hindlimb; ys, yolk sac. Scale bars = 1 mm.
Embryo I [Snout–vent length (SVL), 18. 51 mm; Tail length (TL), 20. 71 mm]. The embryo resembles a neonate in overall development and body proportions but is almost unpigmented, showing only two scattered pigmented dorsolateral stripes on each side along its body and tail. There is also scattered pigmentation on the posterodorsal face of the forelimbs (Fig. 2A). The embryo shows hindlimb vestiges and fully developed forelimbs with four separated digits with claws. It shows fully formed postcephalic scales and faint cephalic scales. Dorsally the paired endolymphatic sacs can be observed.
Embryo II (SVL, 24.62 mm; TL, 27.30 mm). The embryo is indistinguishable from a neonate; however, it has a slightly shorter body (SVL) and TL than the neonate. It shows the pigmentation pattern characteristic of hatchlings and adults (Fig. 2B). Despite its advanced developmental stage, in this embryo the yolk sac was still evident.
Results
Adult skeletal morphology
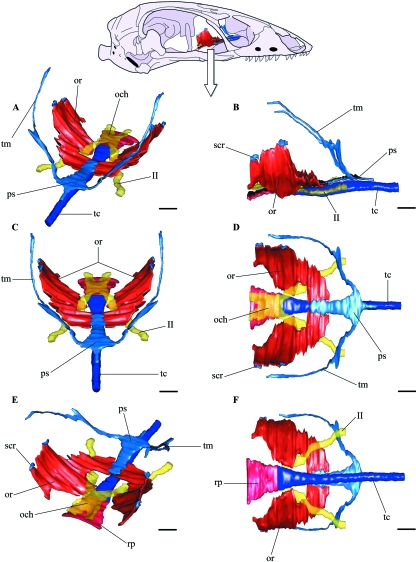
The cartilaginous orbitotemporal scaffolding of the chondrocranium in B. bicolor is notably reduced. There is an extremely simplified cartilaginous orbitotemporal configuration above the trabecula communis, without a recognizable interorbital septum (Fig. 3). The orbitosphenoids are broad ossifications, placed posterior to the planum supraseptale and flanking the trabecula communis. Besides the cartilaginous taenia marginalis we were not able to identify other cartilaginous bars in the adult, homologous with those present in other lizards. Thus, the orbitotemporal region lacks the membrane-filled fenestrae supported by cartilaginous bars usually present in other lizards.
Fig. 3.
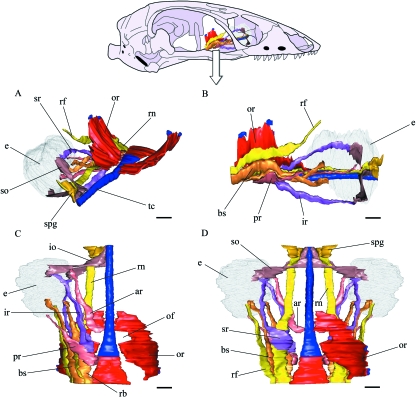
Three-dimensional computer reconstruction from serial histological sections of the orbitotemporal skeletal structures in B. bicolor. (A) Perspective view, a roughly laterodorsal view. (B) Lateral view. (C) Anterodorsal view. (D) Dorsal view. (E) Perspective view, a roughly posterodorsal view. (F) Ventral view. och, optic chiasm; or, orbitosphenoid; ps, planum supraseptale; rp, rostral process; scr, small cartilaginous remnants; tc, trabecula communis; tm, taenia marginalis; II, optic nerve. Scale bars = 200 µm.
The cartilaginous trabecula communis lies midventrally, connecting the cartilaginous nasal capsule and the ossified otico-occipital region. Anteriorly, the trabecula communis is continuous with the nasal septum; posteriorly it is ossified and is connected synostotically with the basisphenoid bone, forming the rostral process of the basisphenoid. Anterior to the rostral process the trabecula communis is flattened in cross-section (Fig. 4B), but it becomes rounded anteriorly as it approaches the level of the planum supraseptale(Fig. 4C). Further anteriorly beneath the planum supraseptale the trabecula communis is pear-shaped in cross-section, which can be regarded as an incipient interorbital septum (Fig. 4D,E). Anterior to the planum supraseptale the trabecula communis becomes rounded in cross-section again.
Fig. 4.
Histological transverse sections at different anteroposterior levels. (A) Orbitosphenoid bone extension and relative position to other structures at the level of the optic chiasm and basisphenoid rostral process, scale bar = 200 µm. (B) Orbitosphenoid attachment through connective tissue to the plane-shaped trabecula communis, scale bar = 200 µm. (C) Round-shaped trabecula communis, below to the site of attachment between the orbitosphenoid and the planum supraseptale, scale bar = 50 µm. (D) Pear-shaped trabecula communis. Note the connective tissue dividing it from the planum supraseptale, scale bar = 50 µm. (E) Planum supraseptale at its widest level, above the pear-shaped trabecula communis, scale bar = 100 µm. ct, connective tissue; che, cerebral hemisphere; Hg, Harderian gland; ir, inferior rectus; oc, oral cavity; och, optic chiasm; or, orbitosphenoid; pl, palatine bone; ps, planum supraseptale; rb, retractor bulbi; rf, ramus frontalis; rn, ramus nasalis; rp, rostral process; tc, trabecula communis; II, optic nerve.
The planum supraseptale is completely cartilaginous and is not connected by cartilage to the trabecula communis (Figs 3, 4C–E); instead, it is joined to it along its ventral surface by connective tissue. The planum supraseptale is narrow and extended anteroposteriorly, lying horizontally and parallel to the trabecula communis. Its anterior end is sharp and widens posteriorly reaching its maximum midlateral extension. At that point on each side of the planum supraseptale the taenia marginalis rises dorsolaterally, reaching the skull roof posteriorly. The planum supraseptale is flat in cross-section along its entire anteroposterior extension (Fig. 4C–E). The orbitosphenoids attach at the posterolateral borders of the planum supraseptale. There is no connection between the nasal capsule and the planum supraseptale, and thus the sphenethmoidal commissures are absent.
The taenia marginalis projects dorsally from the planum supraseptale (Fig. 3). They are paired cartilaginous bars which reach the skull roof up to the frontoparietal suture. There is no trace of the taenia marginalis further posteriorly, and thus a posterior connection with the otico-occipital region is absent.
The orbitosphenoids are broad, paired and laminar ossifications, forming a ventrolateral braincase wall (Figs 3, 4A). The paired orbitosphenoids attach posteriorly to the planum supraseptale, each one showing two to three small cartilaginous bars attached on its dorsal border. The orbitosphenoid has an unusual shape; medially it shows a large notch where the optic nerve passes to innervate the retina laterally. The optic nerves emerge from the optic chiasm, placed above the basisphenoid rostral process. The orbitosphenoid has a slightly concave dorsal surface, the medial region lying ventral and horizontal. Laterally the bone bends towards the dorsolateral border, and thus the dorsolateral region is almost vertical. Posterior to the optic notch the medial border of the orbitosphenoid is firmly joined to the trabecula communis and the basisphenoid rostral process by connective tissue (Fig. 4A,B). As the orbitosphenoid diverges posteriorly from its contralateral twin, it moves away from the rostral process, but it continues to be joined to the basisphenoid rostral process by connective tissue.
The orbitosphenoid bone is not connected to fully formed cartilaginous bars; as described above it bears two to three small bars dorsally. However, these cartilages have a free end and thus are not connected to the trabecula communis, taenia marginalis or the otico-occipital region. Due to its lack of connectivity and the unusual shape of the orbitosphenoid bone, the identification of those small bars with homologous cartilaginous bars usually present in other lizards is dubious.
The orbitosphenoids: embryonic and postnatal development
In the embryo the orbitotemporal cartilages of the chondrocranium are completely formed and have the adult configuration. However, the orbitosphenoid bone is not a broad ossification as in the adult (Fig. 5A).
Fig. 5.
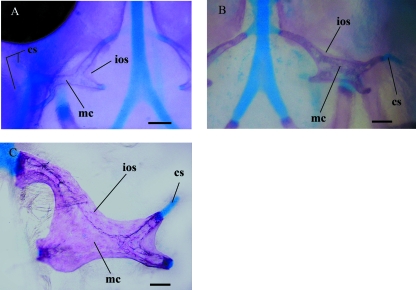
Early orbitosphenoid development showing the compound nature of the bone from cleared and double-stained material. (A) Left orbitosphenoid of embryo I in dorsal view. (B) Right orbitosphenoid of embryo II in dorsal view. (C) Right orbitosphenoid of a neonate (UIS-R-1452) in dorsal view. cs, cartilaginous scaffolding; ios, intramembranous ossification; mc, medullar cavity. Scale bars = 100 µm.
The orbitosphenoid is formed by a roughly X-shaped orbital cartilage, which shows an extended tubular perichondral ossification. The X-shaped scaffolding has two medial and two to three lateral arms, its anteromedial arm is larger and it is connected to the planum supraseptale, the posteromedial arm is slightly ventral relative to the anteromedial one and lies near the trabecula communis. The lateral arms are larger than the posteromedial arm but smaller than the anteromedial arm and have slightly curved ends projecting posterodorsally. Excluding the anteromedial arm, the other arms are free-ended, as they are not continuous with any other orbitotemporal cartilages, such as the pila antotica or the pila accesoria (Fig. 5A).
Between the arms of the orbitosphenoid scaffolding, we observe the emergence of faint membranous outgrowths from the tubular perichondral ossification (Figs 5A, 6A). These membranous outgrowths arising from the embryonic orbitosphenoid scaffolding resemble tiny bone sheets, which expand, and in the neonate completely surround, the orbitosphenoid scaffolding, forming a wider and almost laminar bone, which still outlines the shape of the embryonic chondral scaffolding (Figs 5C, 6C). As a result, the orbitosphenoid bone of B. bicolor is formed by chondral and membranous contributions. Nonetheless, the membranous contributions arise as appositional bone growth from the ossified perichondrium of the orbitosphenoid scaffolding and not from a separate membranous ossification center (Fig. 6).
Fig. 6.
Orbitosphenoid development, from late embryonic development to adult morphology. Orbitosphenoids and planum supraseptale in dorsal view. (A) Embryo I. (B) Embryo II. (C) Neonate (UIS-R-1452). (D) Juvenile (UIS-R-1451). (E) Adult (UIS-R-1442). (F) Adult (UIS-R-1453). Cartilage in black, intramembranous ossifications in light gray and cartilages undergoing endochondral ossification in dark gray, white spaces denote the medullar cavity formed by cartilaginous scaffolding which underwent endochondral ossification. eo, cartilaginous scaffolding undergoing endochondral ossification; ios, intramembranous ossification; mc, medullar cavity; of, optic foramen; ps, planum supraseptale; scr, small cartilaginous remnants; tm, taenia marginalis. Scale bars = 200 µm.
The orbitosphenoid broadens during postnatal development by appositional bone growth, narrowing the medial optic notch and forming an extended ventrolateral braincase wall, achieving the adult shape. As the orbitosphenoid grows, the medullar cavity is lost early in postnatal development, and the orbitosphenoid develops into a completely compact laminar bone (Fig. 6).
The orbitosphenoid cartilaginous scaffolding is not completely ossified perichondrally; the lateral arms of the orbital cartilage remain as small cartilaginous bars, although some of them may ossify postnatally. Those small bars, which remain cartilaginous, are attached to the orbitosphenoid dorsal border during postnatal development as the orbitosphenoid bone expands.
Additionally, we were able to observe endochondral ossification during postnatal development. Although the orbitosphenoid is entirely ossified in the neonate, there are zones of endochondral ossification at the site where the orbitosphenoid attaches to the planum supraseptale and at the small cartilaginous bars placed in the orbitosphenoid dorsal border. In turn, during postnatal development the orbitosphenoid shows both endochondral and appositional bone growth.
Eye muscles
Bachia bicolor, as many other fossorial lizards, shows a clear case of eye reduction. Nevertheless, the case of B. bicolor is not as severe as the cases of microphthalmia known in fossorial lizards such as the members of Dibamidae or Amphisbaenidae.
A detailed eyeball description of B. bicolor is beyond the scope of the present work, but we observed some gross morphological features which are comparable to a structurally complete eyeball of other lizards, such as the presence of the lens, organized retinal layers, scleral ossicles and scleral cartilages. There are six oculorotatory muscles in B. bicolor, four rectus muscles (anterior, posterior, inferior and superior) and two oblique muscles, superior and inferior. We also identified the Bursalis, retractor bulbi and levator bulbi muscles (Fig. 7).
Fig. 7.
Three-dimensional computer reconstruction from serial histological sections of the eye, eye muscles and the ophthalmic division of the trigeminal nerve in B. bicolor, showing its relation to the orbitosphenoid and trabecula communis, the levator bulbi muscle, optic nerves, planum supraseptale and taenia marginalis are not shown. In (A,B,C) only the right side is shown, and the orbitosphenoid is the only structure shown on the left side. In (D) the two sides are shown but the orbitosphenoid has been removed from the left side. (A) Perspective view, a roughly laterodorsal view. (B) Lateral view. (C) Ventral view. (D) dorsal view. ar, anterior rectus; bs, Bursalis; e, eye; io, inferior oblique; ir, inferior rectus; of, optic foramen; or, orbitosphenoid; pr, posterior rectus; rb, retractor bulbi; rf, ramus frontalis; rn, ramus nasalis; so, superior oblique; spg, sphenethmoidal ganglion; sr, superior rectus; tc, trabecula communis. Scale bars = 200 µm.
The oblique muscles arise anteriorly, immediately posterior to the nasal capsule and posteromedially to the sphenethmoidal ganglion. They arise above the trabecula communis, from dense connective tissue lying among the paired frontal ventral process and the trabecula communis. The origin of the inferior oblique lies anterior to the superior oblique origin. Posteriorly between the inferior and superior oblique muscles lies the ramus nasalis of the trigeminal ophthalmic division, dividing the superior oblique projecting posterodorsally, and the inferior oblique projecting posteroventrally. The superior oblique inserts on the dorsal surface of the eyeball and the inferior oblique on the ventral surface.
The Bursalis and retractor bulbi muscles arise posteriorly from the internal surface of the basisphenoid bone, posterior and lateral to the hypophysis. The Bursalis lies lateral to the retractor bulbi; they project anteriorly almost parallel to the trabecula communis, ventrolateral to the orbitosphenoid and the ramus nasalis and ramus frontalis of the trigeminal ophthalmic division. Medial to the Bursalis and retractor bulbi is the origin of the rectus muscles. Further anteriorly the Bursalis and retractor bulbi diverge from the trabecula communis, immersing into the Harderian gland. The Bursalis muscle inserts on the posteromedial surface of the eyeball, but the retractor bulbi travels further anteriorly together with the optic nerve and inserts on the anteromedial surface of the eyeball, anterior to eyeball innervation by the optic nerve.
The posterior rectus muscle arises from the orbitosphenoid ventral border and the lateral border of the basisphenoid rostral process, at the level of the anterior end of the optic chiasm, ventromedial to the retractor bulbi. The posterior rectus projects anterolaterally, crossing the Bursalis and retractor bulbi muscles ventrally, and inserting on the posterior surface of the eyeball.
The inferior rectus arises from the orbitosphenoid ventral border, anterior to the posterior rectus origin. The inferior rectus projects ventrally and anterolaterally, immersing into the Harderian gland and inserting on the ventral surface of the eyeball.
The superior rectus arises from the orbitosphenoid ventral border, dorsal and slightly anterior to the inferior rectus origin and posterior to the optic foramen. The superior rectus projects dorsally and anterolaterally, between the ramus nasalis ventrolaterally and the orbitosphenoid dorsomedially, inserting on the dorsal surface of the eyeball.
The anterior rectus muscle arises from the lateral surface of the trabecula communis and the orbitosphenoid ventral border, immediately anterior to the optic foramen and medial to the optic nerve and the ramus nasalis. The anterior rectus projects anterolaterally, crossing the ramus nasalis ventrally, but traveling in close proximity to the latter and inserting on the anterior surface of the eyeball.
Discussion
Chondrocranial orbitotemporal morphology
The orbitotemporal region of the chondrocranium in B. bicolor is very reduced, resulting in the loss of several structures compared to the ‘generalized’ pattern observed in other squamates, including other gymnophthalmid lizards. In B. bicolor there is no complex cartilaginous scaffolding, the orbitotemporal region is composed of a narrow planum supraseptale, in close proximity to the trabecula communis, sigmoidal-shaped taenia marginalis projects on each side in posterodorsal direction from the planum supraseptale, and broad orbitosphenoids attach to the posterolateral corners of the planum supraseptale. The broad orbitosphenoid ossifications are located in an almost horizontal position, thus forming the anterior braincase floor. Despite its modified chondrocranium, the identities of most of the orbitotemporal structures in B. bicolor can be clearly established by the topological relationships with surrounded cranial structures and nerves. However, we were not able to determine the identities of the small cartilages attached to the orbitosphenoid dorsolateral border, compared to orbitotemporal cartilages present in other squamates.
Loss or extreme reduction of the complex orbitotemporal cartilaginous scaffolding has been described for various serpentiform squamates (Bellairs & Kamal, 1981) such as snakes, amphisbaenians, dibamids, the scincid Acontias meleagris, the pygopodid Aprasia repens, and the anguids Anniela pulchra and Anguis fragilis. However, only adult amphisbaenians show no trace of orbital cartilages together with a broad orbitosphenoid; the latter were previously believed to represent an endochondral replacement bone, formed by ossification of the orbitotemporal cartilage planum supraseptale during embryonic development (Bellairs & Kamal, 1981).
Snakes and several snakelike burrowing lizards show different degrees of orbitotemporal cartilage reduction. Snakes, amphisbaenians and dibamids show the most severe cases of reduction in which only the trabecular cartilages are present. The trabecular cartilages are conserved in all snakes and serpentiform squamates, as paired trabecular cartilages in snakes (platytrabic condition) and as the trabecula communis in non-ophidian squamates (tropitrabic condition). The interorbital septum in snakelike burrowing lizards is very low or absent as in B. bicolor, the planum supraseptale is very narrow and can be connected to the nasal capsules through the sphenethmoidal commissures, as in Anguis fragilis and Anniela pulchra, or unconnected as in B. bicolor and Acontias meleagris. The orbital scaffolding posterior to planum supraseptale is much more reduced in various snakelike burrowing lizards showing chondrocranial orbitotemporal reductions, but it can be present as diverse cartilaginous remnants; however, an orbitosphenoid ossification is completely absent, except in the case of amphisbaenians and B. bicolor.
Bachia bicolor therefore has a reduced orbitotemporal region of the chondrocranium comparable to that described for other snakelike fossorial lizards, but the region is not as reduced as in snakes, amphisbaenians and dibamids. Accompanying these orbitotemporal reductions, there is an expanded orbitosphenoid bone, arising from the reduced orbital cartilaginous scaffolding connected to the planum supraseptale observed during late embryonic development. The latter are cartilages without a clear connectivity or topological relationship, and cannot be easily compared to the generalized situation in Squamata. Although the anteromedial and posteromedial cartilages connected to the planum supraseptale can be homologized with the taenia medialis and pila metoptica, the lateral processes are hardly comparable to equivalent cartilaginous bars in other squamates. As such, we were not able to identify either the pila antotica or the pila accesoria.
The orbitosphenoid in Bachia and the amphisbaenian tabulosphenoid
Among squamates, only amphisbaenians have a broad orbitosphenoid ossification forming the braincase floor of the skull (although its homology is currently debated, and it has been called the Tabulosphenoid, see Montero & Gans, 1999; Montero et al. 1999). Nevertheless, it is an unpaired element and much more extended relative to the orbitosphenoids in B. bicolor. Contrary to now living amphisbaenians, the fossilized amphisbaenid Rhineura hatcherii shows a paired and less extended orbitosphenoid ossification (Kearney et al. 2005), comparable to that observed in B. bicolor (Tarazona et al. 2008). Besides the orbitosphenoid similarities in B. bicolor, living amphisbaenians and the fossilized Rhineura hatcherii, the skull morphology of amphisbaenians is extremely derived compared to other squamate lizards, including B. bicolor (Tarazona et al. 2008). There is thus little doubt about the independent evolution of that feature in Amphisbaenidae and the genus Bachia (Bellairs & Gans, 1983).
Considered homologous with the orbitosphenoids of other squamate lizards, the element forming the braincase floor in amphisbaenids was regarded in the absence of any embryonic material as an endochondral ossification of the chondrocranium, possibly the planum supraseptale (Bellairs & Kamal, 1981). However, Bellairs & Gans (1983; Montero et al. 1999) more recently revealed that the orbitosphenoid in amphisbaenians has a paired origin and was formed by intramembranous and endochondral ossifications. The broad morphology of the bone is attained mainly by the extended intramembranous ossification around the chondral core, and not from the endochondral replacement of the supposedly extended embryonic planum supraseptale. Furthermore, the reduced orbitotemporal region of the amphisbaenian embryonic chondrocranium makes very difficult to establish the identity of the small chondral components of the amphisbaenian orbitosphenoid: ‘Based upon their topology, these cartilages might be homologous to the planum supraseptale, or perhaps to the saurian pila antotica, the pila metoptica or the taenia marginalis.’ (Montero et al. 1999, p. 22).
Bellairs & Gans (1983) also describe paired and broad orbitosphenoid ossifications in Bachia trisanale, forming the braincase floor and attached to the posterior edge of the planum supraseptale on each side, very similar to the condition observed in B. bicolor. According to Bellairs & Gans (1983, p. 244) ‘To some extent the bone [the orbitosphenoid of B. trisanale] resembles the paired orbitosphenoid of embryonic Leposternon’ and also they believed ‘possibly it [the orbitosphenoid of B. trisanale] arises as an intramembranous extension from the planum’. In contrast to previous assumptions about the developmental origin of the orbitosphenoids in the genus Bachia, here we found that the orbitosphenoid of B. bicolor does not arise as an intramembranous ossification from the planum supraseptale, but rather as intramembranous bone sheets from the perichondral ossification of the reduced orbitotemporal scaffolding connected to the planum supraseptale. These cartilages in B. bicolor conserve a topology comparable to the orbitotemporal cartilage precursors of the orbitosphenoid in other squamate lizards. Therefore, despite the reduced orbitotemporal cartilages of the B. bicolor chondrocranium, the homology of the orbitosphenoid bones in B. bicolor relative to the small paired orbitosphenoids in other squamates is clear.
Assuming a possible homology of the chondral core of the amphisbaenian orbitosphenoid with orbitotemporal cartilaginous precursors of the orbitosphenoid bone in other squamate lizards, it is tempting to regard the membranous bone sheets in amphisbaenians and B. bicolor as independent apomorphic features of homologous bones, regardless of their derived ontogeny. Nonetheless, the compound nature (chondral and membranous) of the orbitosphenoids in amphisbaenians and B. bicolor seems to be subjected to different ontogenetic processes. In B. bicolor the membranous contributions arise as membrane bone outgrowths from the ossified perichondrium of the reduced cartilaginous scaffolding, but in amphisbaenians the membranous contributions of the embryonic orbitosphenoid ‘do not resemble perichondral ossifications’ (Bellairs & Gans, 1983, p. 243). Instead they are deep ossifications within the mesenchyme in Leposternon microcephalum (Bellairs & Gans, 1983) or, as described for Amphisbaena darwini, they arise as paired membranous centers of ossifications posterodorsal to the paired cartilaginous contributions, further enveloping the latter during embryonic development (Montero et al. 1999).
Thus, in contrast to the amphisbaenian orbitosphenoid development, B. bicolor shows a more common evolutionary modification of a cartilage replacement bone within vertebrates (Patterson, 1977). Hence the orbitosphenoid of B. bicolor shows membrane outgrowths similar to other intramembranous extensions of chondral bones frequently observed in other skull bones within Squamata, such as the laterosphenoid or the prootic alar crest, both intramembranous extensions from the otico-occipital region of the chondrocranium. The former, observed in snakes and the skink A. meleagris, and the latter are a widespread feature present in several squamate lizards (Bellairs & Kamal, 1981).
Considering the differences between the orbitosphenoid development in B. bicolor and amphisbaenians, the independent evolution of that feature should be regarded as a convergence and not as an ontogenetic parallelism.
Chondrocranial orbitotemporal modification and eye muscle insertion
Orbitotemporal simplification of the chondrocranium has been correlated with snakelike burrowing forms showing severe cases of microphthalmia (Bellairs & Kamal, 1981). In amphisbaenians and dibamids, eye muscles are completely absent, and snakes at least lack the retractor muscle group (Bursalis and retractor bulbi muscles) (Underwood, 1970; Carprette et al. 2004). Despite its significant chondrocranial orbitotemporal reduction, B. bicolor conserves the main eye muscle groups usually present in other lizard (Underwood, 1970), such as: four rectus, two oblique and two retractor muscles. Furthermore, compared to other lizards those muscles arise from similar positions of the orbitotemporal region, although some from slightly different structures (oblique muscles) owing to the modified orbitotemporal region, but inserting on the eyeball as they do in other lizards.
Similar to the eye muscle attachments described in other lizards (Underwood, 1970), in B. bicolor the retractor muscles arise far posterior from the basisphenoid bone. The posterior muscle arises from the trabecula communis and the superior, inferior and anterior rectus arise mainly from the orbitosphenoid bone. However, in other lizards the oblique muscles usually arise from the interorbital septum, which is absent in B. bicolor. Therefore, in B. bicolor the oblique muscles arise at an ‘equivalent position’, far anterior to and above the trabecula communis, but from dense connective tissue between the trabecula communis and the frontal bone.
Additionally, the topology of the eye muscles and the ophthalmic division of the trigeminal nerve relative to the orbitosphenoid bone and the remaining orbitotemporal structures of the chondrocranium, reinforce our conclusions about the homology of the orbitosphenoid bone in B. bicolor compared with other squamate lizards.
Conclusion
Bachia bicolor shows severe chondrocranial orbitotemporal reductions comparable to other snakelike burrowing lizards, but accompanied by unusual orbitosphenoid bones. In addition to the shared convergent features with other snakelike lizards, the combination of features such as its chondrocranial orbitotemporal morphology, orbitosphenoid development and eye muscle arrangement is distinctive in B. bicolor, providing important information for future comparative anatomical studies. However, we have no information about the chondrocranial orbitotemporal morphology in other species of the genus Bachia, which would enable us to establish whether those features are shared derived characteristics or how variable the orbitotemporal morphology within the genus is. The relation of chondrocranial orbitotemporal simplification and eye reduction is not surprising, taking into account the repeated evolution of those traits within miniaturized snakelike squamates (Bellairs & Kamal, 1981). The nervous and sensory components of the head (eyes, nasal and otic capsules) play a critical role in skull morphogenesis, particularly for the chondrocranial morphology (Hanken, 1983; Rieppel, 1984, 1996). The combined effect of miniaturization (see Rieppel, 1984, 1996) and eye reduction therefore probably underlie the convergent evolution of several cranial morphological similarities of snakelike lizards, such as the increase of the otico-occipital region and the orbitotemporal reductions. However, the potential role of eye reduction and miniaturization in the cranial structural design has to be analysed rigorously in future studies, with a broad taxonomic scope within Squamata, including derived and non-derived forms.
Acknowledgments
We thank M. Sánchez-Villagra, B. K. Hall, C. Holliday and one anonymous reviewer for helpful comments on the manuscript. We thank R. Montero for previous discussions about the homology and development of the amphisbaenian orbitosphenoid/tabulosphenoid. O.T. thanks F. Leal for providing technical advice and support.
Appendix 1
Specimens of Bachia bicolor examined. Specimen number is followed by snout–vent length and sex (where known). Embryos: UIS-R-1536, 18.51 mm; UIS-R-15337, 24.62 mm. Neonates: UIS-R-1465, 25.48 mm; UIS-R-1452, 27.52 mm. Juveniles: UIS-R-1252, 28.18 mm; UIS-R-1451, 30.58 mm; UIS-R-1450, 31.62 mm; UIS-R-1253, 32.50 mm; UIS-R-1456, 36.96 mm (female); UIS-R-1245, 40.24 mm; UIS-R-1251, 40.32 mm; UIS-R-1462, 42.84 mm; UIS-R-1438, 45.00 mm; UIS-R-1461, 45.72 mm; UIS-R-1455, 47.69 mm; UIS-R-1443, 48.04 mm. Adults: UIS-R-1458, 49.31 mm (male); UIS-R-1447, 50.46 mm (female); UIS-R-1246, 51.28 mm; UIS-R-1247, 52.35 mm; UIS-R-1464, 54.97 mm (female); UIS-R-1445, 56.73 mm (female); UIS-R-1460, 57.24 mm (male), UIS-R-1248, 58.14 mm; UIS-R-1448, 59.00 mm (male); UIS-R-1437, 61.02 mm; UIS-R-1249, 61.42 mm (female); UIS-R-1250, 62.05 mm (male); UIS-R-1439, 62.21 mm; UIS-R-1444, 62.33 mm (female); UIS-R-1449, 62.40 mm (male); UIS-R-1441, 63.51 mm; UIS-R-1454, 64.16 mm (female); UIS-R-1442, 64.30 mm (male); UIS-R-1440, 64.32 mm; UIS-R-1446, 65.84 mm (male); UIS-R-1453, 66.27 mm (male); UIS-R-1459, 67.69 mm (male); UIS-R-1457, 69.60 (male); UIS-R-1463, 71.39 mm (female). Additional material: UIS-R-1535, Ptychoglosus bicolor; UIS-R-1533, Tretioscincus bifasciatus; UIS-R-1534, Leposoma sp.
References
- Apesteguia S, Zaher H. A Cretaceous terrestrial snake with robust hindlimbs and a sacrum. Nature. 2006;440:1037–1040. doi: 10.1038/nature04413. [DOI] [PubMed] [Google Scholar]
- Bellairs Ad’A, Gans C. A reinterpretation of the amphisbaenian orbitosphenoid. Nature. 1983;302:243–244. [Google Scholar]
- Bellairs Ad’A, Kamal AM. The chondrocranium and the development of the skull in recent reptiles. In: Gans C, Parsons T, editors. Biology of the Reptilia, vol. 11. Morphology F. New York: Academic Press; 1981. pp. 1–263. [Google Scholar]
- Carprette CL, Lee MSY, Shine R, Mokany A, Downhower JR. The origin of snakes (Serpentes) as seen through eye anatomy. Biol J Linnean Soc. 2004;81:469–482. [Google Scholar]
- Castoe TA, Doan TM, Parkinson CL. Data partitions and complex models in Bayesian analysis: the phylogeny of gymnophthalmid lizards. Syst Biol. 2004;53:448–469. doi: 10.1080/10635150490445797. [DOI] [PubMed] [Google Scholar]
- Dixon JR. Systematic review of the teiid lizard genus Bachia with remarks on Heterodactylus and Anotosaura. Univ Kansas Museum Nat Hist, Miscellaneous Publ. 1973;57:1–47. [Google Scholar]
- Fiala JC. Reconstruct: a free editor for serial section microscopy. J Microsc. 2005;218:52–61. doi: 10.1111/j.1365-2818.2005.01466.x. [DOI] [PubMed] [Google Scholar]
- Hanken J. Miniaturization and its effects on cranial morphology in plethodontid salamanders, genus Thorius (Amphibia: Plethodontidae). II. The fate of the brain and sense organs and their role in skull morphogenesis and evolution. J Morphol. 1983;177:255–268. doi: 10.1002/jmor.1051770304. [DOI] [PubMed] [Google Scholar]
- Hodin J. Plasticity and constraints in evolution and development. J Exp Zool. 2000;288:1–20. [PubMed] [Google Scholar]
- Kearney M, Stuart BL. Repeated evolution of limblessness and digging heads in worm lizards revealed by DNA from old bones. Proc R Soc Lond B. 2004;271:1677–1683. doi: 10.1098/rspb.2004.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney M, Maisano JA, Rowe T. Cranial anatomy of the extinct amphisbaenian Rhineura hatcherii(Squamata, Amphisbaenia) based on high-resolution X-ray computed tomography. J Morphol. 2005;264:1–33. doi: 10.1002/jmor.10210. [DOI] [PubMed] [Google Scholar]
- Kizirian DA, McDiarmid RW. A new species of Bachia(Squamata: Gymnopthalmidae) with plesiomorphic limb morphology. Herpetologica. 1998;54:245–253. [Google Scholar]
- Kohlsdorf T, Wagner GP. Evidence for the reversibility of digit loss: a phylogenetic study of limb evolution in the genus Bachia(Gymnophthalmidae: Squamata) Evolution. 2006;60:1896–1912. [PubMed] [Google Scholar]
- Lee MYS. Convergent evolution and character correlation in burrowing reptiles: towards a resolution of squamate relationships. Biol J Linnean Soc. 1998;63:369–453. [Google Scholar]
- Luna LG. Manual of Histologic Staining Methods of the Armed Forces Institute of the Pathology. 3. New York: McGraw-Hill.; 1969. pp. 32–100. [Google Scholar]
- Montero R, Gans C. The head skeleton of Amphisbaena alba Linnaeus. Ann Carnegie Museum. 1999;68:15–80. [Google Scholar]
- Montero R, Gans C, Lions ML. Embryonic development of the skeleton of Amphisbaena darwini heterozonata(Squamata: Amphisbaenidae) J Morphol. 1999;239:1–25. doi: 10.1002/(SICI)1097-4687(199901)239:1<1::AID-JMOR1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Oelrich TM. The anatomy of the head of Ctenosaura pectinata(Iguanidae) Miscellaneous Publ Mus Zoo Univ Mich. 1956;94:1–122. [Google Scholar]
- Patterson C. Cartilage bones, dermal bones, and membrane bones, or the exoskeleton versus the endoskeleton. In: Andrews S, Miles M, Walker AD, editors. Problems in Vertebrate Evolution. London: Academic Press; 1977. pp. 77–121. [Google Scholar]
- Pellegrino KCM, Rodrigues MT, Yonenaga-Yassuda Y, Sites JW. A molecular perspective on the evolution of microteiid lizards (Squamata, Gymnophthalmidae), and a new classification for the family. Biol J Linnean Soc. 2001;74:315–338. [Google Scholar]
- Presch W. The evolution of limb reduction in the teiid lizard genus Bachia. Bull South Calif Acad Sci. 1975;74:113–121. [Google Scholar]
- Rieppel O. Miniaturization of the lizard skull: its functional and evolutionary implications. Symp Zool Soc Lond. 1984;52:503–520. [Google Scholar]
- Rieppel O. Miniaturization in tetrapods: consequences for skull morphology. Symp Zool Soc Lond. 1996;69:47–61. [Google Scholar]
- Tarazona OA, Fabrezi M, Ramírez-Pinilla MP. Cranial morphology of Bachia bicolor(Squamata: Gymnophthalmidae) and its postnatal development. Zool J Linnean Soc. 2008;152:775–792. [Google Scholar]
- Underwood G. The eye. In: Gans C, Parsons TS, editors. Biology of the Reptilia. Vol. 2. London: Academic Press; 1970. pp. 1–97. [Google Scholar]
- Wassersug R. A procedure for differential staining of cartilage and bone in whole formalin fixed vertebrates. Stain Technol. 1976;51:131–134. doi: 10.3109/10520297609116684. [DOI] [PubMed] [Google Scholar]
- Wiens JJ, Brandley MC, Reeder TW. Why does a trait evolve multiple times within a clade? Repeated evolution of snake-like body form in squamate reptiles. Evolution. 2006;60:123–141. [PubMed] [Google Scholar]