Abstract
The symmetry of, and physical characteristics influencing, the thickness of the lateral abdominal muscles at rest and during abdominal exercises were examined in 57 healthy subjects (20 men, 37 women; aged 22–62 years). M-mode ultrasound images were recorded from the abdominal muscles at rest and during abdominal hollowing exercises in hook-lying. The fascial lines bordering the transvs. abdominis, obliquus internus and obliquus externus were digitized and the absolute thickness, relative thickness (% of total lateral thickness) and contraction ratio (thickness during hollowing/thickness at rest), as well as the asymmetry (difference between sides expressed as a percent of the smallest value for the two sides) for each of these parameters were determined for each muscle. Both at rest and during hollowing, obliquus internus was the thickest and transvs. abdominis the thinnest muscle. There were no significant differences between left and right sides for group mean thicknesses of any muscle; however, individual asymmetries were evident, with mean values for the different muscles ranging from 11% to 26%; asymmetry was much less for the contraction ratios (mean % side differences, 5–14% depending on muscle). Body mass was the most significant positive predictor of absolute muscle thickness, for all muscles at rest and during hollowing, accounting for 30–44% variance. Body mass index explained 20–30% variance in transvs. abdominis contraction ratio (negative relationship). The influence of these confounders must be considered in comparative studies of healthy controls and back pain patients, unless groups are very carefully matched. Asymmetries observed in patients should be interpreted with caution, as they are also common in healthy subjects.
Keywords: abdominal muscles, age, anthropometry, gender, healthy controls, side differences, ultrasound
Introduction
The osteoligamentous spine is inherently unstable (Crisco & Panjabi, 1992). Stabilization of the passive elements is achieved by the active system, which is made up of the muscles surrounding and spanning the spinal column (i.e. the global muscle system) and those acting directly on it (i.e. the local muscle system), directed and controlled by the neural system (nerves and central nervous system) (Panjabi, 1992). Stability can also be achieved by the co-contraction of the abdominal muscles, with the specific recruitment pattern being dependent on the given task and posture (McGill et al. 2003; Kavcic et al. 2004; Vera-Garcia et al. 2006). In connection with this, much emphasis has been placed on the function of the deep-lying trunk muscle, transvs. abdominis (TrA), which has been shown in mathematical models (Gardner Morse & Stokes, 1998), cadaveric studies (Barker et al. 2006) and in vivo studies (Hodges et al. 2003a) to make a notable contribution to spinal stability by its tensioning of the thoracolumbar fascia (Urquhart et al. 2005). Indeed, on the basis of this, exercise programmes designed to specifically train these muscles have been implemented as a treatment for low back pain (Richardson & Jull, 1995; Richardson et al. 1999; Ferreira et al. 2006; Rackwitz et al. 2006).
In determining the need for, or the effects of, such exercise programmes, the size and function of the TrA and of its neighbouring muscles, obliquus internus (OI) and externus (OE) abdominus, are typically assessed using ultrasound measures of muscle thickness change (Hides et al. 1998; Critchley & Coutts, 2002; Henry & Westervelt, 2005; Hodges, 2005; Henry & Teyhen, 2007; Teyhen, 2007; Teyhen et al. 2007). This represents the method of choice because neither the cross-sectional area (CSA) nor the strength of these skeletal muscles is easily measurable by the usual means: they are too large for measurement of their CSA, and their mechanical output can not be isolated to allow recording of an external force/moment. Ultrasound measures of abdominal muscle thickness correlate well with those made using magnetic resonance imaging (Hides et al. 2006), and thickness changes in the muscle during activation correlate well with the electromyographic (EMG) activity of the muscle (at least for TrA and OI) (McMeeken et al. 2002; Hodges et al. 2003b), suggesting that such measures can be used as a surrogate index of muscle activation.
Rankin et al. (2006) used real-time ultrasound to examine the size and symmetry of the abdominal muscles of a large group of healthy individuals with no back pain, to provide normative data that would assist in the subsequent identification of abnormalities or asymmetries in clinical groups. Although they provided a valuable reference base and improved our understanding of the factors influencing abdominal muscle thickness, e.g. age, gender and anthropometry, their assessments were limited to resting muscle. However, the deficiency commonly identified in connection with low back pain is concerned less with the size of the resting TrA than with the ability to activate the muscle (i.e. increase its thickness) during exercises such as the ‘abdominal hollowing manoeuvre’, a test used in the assessment and training of TrA function (Critchley & Coutts, 2002; Henry & Westervelt, 2005; Henry & Teyhen, 2007; Teyhen et al. 2007). Hence, we considered it of interest to quantify the normal symmetry of contraction during this exercise task, hypothesizing that, as for resting muscle (Rankin et al. 2006), there would be minimal difference between body sides. In addition, we sought to examine further the factors influencing muscle thicknesses and their changes during hollowing, hypothesizing that the indices recently introduced by Teyhen et al. (2005) would be less susceptible to confounding factors such as age, gender and anthropometry than are absolute thickness measures (Rankin et al. 2006; Springer et al. 2006). If confirmed, this would render the contraction indices of greater value in future clinical studies in which controls and patients were not identically matched in terms of these variables.
Methods
Subjects
Fifty-seven healthy volunteers, 20 men and 37 women, participated in the study. Their physical characteristics are shown in Table 1. All were colleagues from the authors’ institutions or were recruited via flyers placed in the local universities. They had to have been free from low back pain (LBP) for the last year, and have no history of LBP requiring medical attention or time off work in the last 10 years. They were excluded if they were pregnant or had been pregnant within the last 2 years (Ferreira et al. 2004).
Table 1.
Physical characteristics of the subjects [mean ± SD (95% confidence interval)]
| Men (N = 20) | Women (N = 37) | |
|---|---|---|
| Age (years) | 40.5 ± 14.0 | 42.1 ± 13.1 |
| (34.0–47.1) | (37.7–46.5) | |
| Height (m) | 1.80 ± 0.08* | 1.66 ± 0.06 |
| (1.77–1.84) | (1.64–1.68) | |
| Body mass (kg) | 76.8 ± 11.9* | 65.2 ± 12.7 |
| (71.2–82.3) | (61.0–69.5) | |
| BMI (kg m−2) | 23.6 ± 3.4 | 23.7 ± 4.6 |
| (22.1–25.2) | (22.2–25.2) | |
| Handedness# | 16 (80%) right | 34 (92%) right |
| 3 (15%) left | 1 (3%) left | |
| 1 (5%) ambidextrous | 2 (5%) ambidextrous |
BMI, body mass index.
Enquired about with a single question in a questionnaire: ‘Are you: a) right-handed, b) left-handed, c) ambidextrous’.
Significantly different from the women.
The study conformed to the standards set by the Declaration of Helsinki and was approved by the local medical ethics committee. All suitable participants received verbal and written information about the test procedure and gave their signed informed consent to participate.
Test protocol
The test procedure and equipment used were identical to those described by Mannion et al. (2008), in which the intra-examiner between-day reliability of measures was also reported in detail. Briefly, for all muscle thickness measures, the median standard error of measurement (SEM) was 0.71 mm, or 10.9% when expressed as a percent of the corresponding mean value.
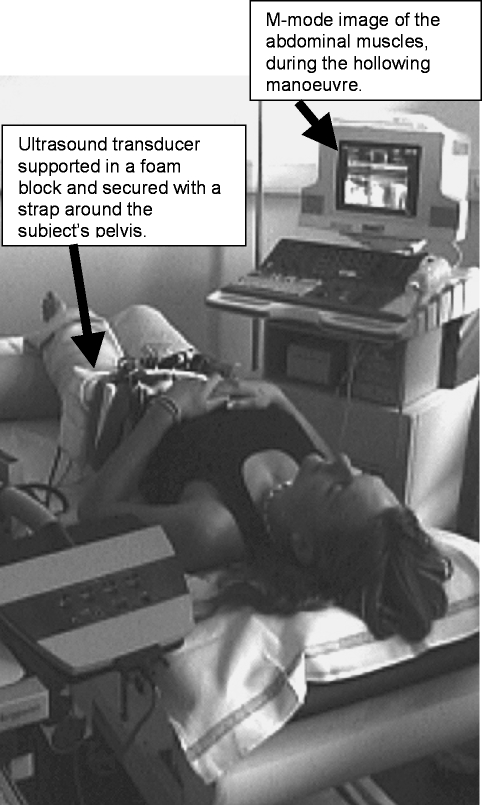
Abdominal hollowing exercises were performed in the supine hook-lying position (hips in ∼30° flexion; Fig. 1), by slowly contracting the abdominals to draw in the abdomen, and holding for 5 s. The subjects received a practice session (5–15 min), using ultrasound as a biofeedback tool (Hides et al. 1998; Henry & Westervelt, 2005; Henry & Teyhen, 2007). Ten repeated abdominal hollowing exercises were then performed, with a 1–2 min rest period between each: five were performed with the transducer over the right abdominal muscles and five, with it over the left abdominals, with the starting side being randomized amongst the subjects to limit any potential sequence effect. During the measurement the subjects were not able to see the ultrasound imagines and they received no verbal feedback on their performance.
Fig. 1.
Test set-up for the abdominal hollowing exercise showing the hook-lying position of the subject, the ultrasound transducer head secured in a foam supporting block and strapped over the subject's left lateral abdominal muscles, and the TDI/M-mode image in the background.
Ultrasound recordings
Ultrasound images were recorded at 333 Hz using a Philips HDI-5000 (Philips Medical Systems, Bothell, WA, USA) with a linear-array transducer (5–12 MHz); the images were superimposed with tissue Doppler image (TDI) data. Using B (brightness)-mode ultrasound, the transducer was positioned 2.5 cm anteromedial to the mid-point between the iliac crest and the costal margin on the mid-axillary line, where the fascial boundaries between TrA, OI and OE and the superior edge of the TrA fascia lie parallel (Misuri et al. 1997). A 130 × 120 × 10 mm gel stand-off pad (Sonar-Aid, Alloga AG, Burgdorf, Switzerland) and transmission gel were placed between the transducer head and the skin. To ensure constant pressure and minimize relative movement between the transducer and abdomen during the tests, the transducer was housed in a high-density foam block, which was secured with Velcro straps around the pelvis. Recordings were made approximately 2–3 s prior to and throughout the 5 s abdominal hollowing manoeuvre.
The grey scale and TDI tissue velocity data from the M-mode ultrasound files, and the event-marker data previously fed into the ECG channel of the ultrasound machine to indicate when the instruction to begin contraction was given, were exported in digital form using the ResearchLink option of the HDI-5000 system, and stored on computer.
Data processing
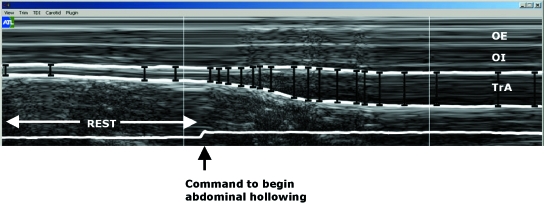
The leading edge points (i.e. the upper border) of the fascia of the muscle of interest were marked as manually selected control points at regular intervals throughout the M-mode image (Fig. 2). A custom-written plug-in of the HDI-Lab software (version 1.9 ATL/Philips Medical Systems) was then used to track the borders automatically between adjacent control points, relying on the TDI velocity information to derive the displacement of a given point between two adjacent M-mode columns. Displacement was equal to tissue velocity (measured with TDI) multiplied by the time difference between adjacent M-mode columns (3 ms). In other words, knowing the tissue depth of the first marked point, and the corresponding tissue velocity at that point, calculations could be made to indicate where that point would be in 3 ms’ time (i.e. in the next column), and in this manner the fascial border could be ‘tracked’. Marking multiple points enabled this to be done with greater accuracy, using both forward and backward iterations between each pair of marked points. Once the depth of each of the fascial lines was digitized in this way, the vertical distance between the top and bottom fascial lines (depths) for each M-mode column could be calculated to give a measure of the thickness of the muscle over time. (TDI was only utilised to facilitate this particular fascial edge digitization process; in principle, any custom-written image analysis programme could be used to trace manually the fascial borders of the M-mode grey-scale image and determine the difference in depth between them.) The data were exported as text data, into a custom-written LabView (National Instruments Corporation, Austin, TX, USA) software programme to determine: 1) the resting thicknesses of TrA, OI and OE, given by the 1-s value during quiet rest, just before the contraction began; 2) the maximal thickness of TrA over any given 3-s period during the contraction; and 3) the thicknesses of OI and OE at the point of maximum TrA thickness.
Fig. 2.
M-mode ultrasound image of the external oblique (OE), internal oblique (OI), and transvs. abdominis (TrA) muscles. White lines indicate the fascial borders between the muscles. The line at the bottom of the image is a switch trace, indicating when the instruction was given to start with the contraction in expiration.
From the above data, the following indices were determined (Teyhen et al. 2005):
TrA contraction ratio = TrA thickness contracted/TrA thickness at rest
OE + OI contraction ratio = OE + OI thickness contracted/OE + OI thickness at rest
-
TrA preferential activation ratio (difference in the TrA proportion of the total lateral abdominal muscle thickness in going from the relaxed to the contracted state) = (TrA contracted/TrA + OI + OE contracted) – (TrA at rest/TrA + OI + OE at rest)
The utility of two further indices relating to the individual thickening of OI and of OE were also investigated (Mannion et al. 2008), because co-activation of OI, but not OE, is sometimes considered acceptable during hollowing and hence these might best be examined separately:
OI contraction ratio = OI thickness contracted/OI thickness at rest
OE contraction ratio = OE thickness contracted/OE thickness at rest
The asymmetry across body sides of each thickness measure and contraction index was determined using the method described by Rankin et al. (2006), in which the absolute difference in values between right and left sides was expressed as a percent of the smallest value recorded on either of the two sides. For reference, the data were also given in their absolute form [e.g. the absolute difference between left and right side thicknesses (in mm), or ratio values, etc.].
Data analysis/statistics
The mean values from the five trials for a given person on a given side were used for further analysis. Where ratio values were determined, these were also firstly determined for each of the five trials before averaging and further analyses. Descriptive data (mean ± SD and minimum and maximum values) are given for left and right sides for the men and women separately.
Gender and side-differences in each of the muscle thicknesses and contraction indices were examined using a mixed model repeated measures analysis of variance (anova), with one between-group factor (gender) and one within-group factor (body side). The main effect of gender indicated differences between men and women regardless of body side; the main effect of body side indicated right/left differences regardless of gender; the interaction between these two indicated whether differences between the sexes were dependent on the side under investigation (and post-hoc t-tests were used to locate any such differences).
Forward conditional multiple regression analyses were used to identify the unique factors predicting each of the muscle thickness measures/indices. The variables entered as possible predictors for selection into the model were in each case age, gender, body mass, height and body mass index.
Statview 5.0 (SAS Institute, Cary, NC, USA) was used for the statistical analyses. Significance was accepted at the 5% level. No corrections were made for multiple testing, as previously recommended (Perneger 1998), but caution was exercised in the interpretation of the data when P-values were borderline significant, or where inconsistent (incidental) group differences arose.
Results
Muscle thicknesses and contraction indices
Table 2 shows the mean muscle thicknesses and contraction indices for the left and right abdominal muscles, for men and women separately. OI was the thickest muscle and TrA the thinnest in both sexes, at both rest and during hollowing. The main effect of body side (anova) was not significant for any of the thickness measures or contraction indices (Table 2), i.e. there were no statistically significant differences in the mean values between left and right sides for any of the parameters (P > 0.05).
Table 2.
Mean ± SD (95% confidence interval) values for abdominal muscle thicknesses and index values in men and women
| Men (n = 20) | Women (n = 36) | P value from ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Main effect gender | Main effect body side | Interaction | |||||
| Left | Right | Left | Right | ||||
| TrA | |||||||
| Thickness at rest (cm) | 0.40 ± 0.10 | 0.39 ± 0.09 | 0.36 ± 0.10 | 0.37 ± 0.10 | 0.26 | 0.69 | 0.38 |
| (0.36–0.45) | (0.35–0.43) | (0.33–0.40) | (0.34–0.40) | ||||
| Relative thickness rest (% of total TrA, OI, OE) | 21.0 ± 3.4 | 21.1 ± 5.3 | 22.6 ± 3.6 | 22.6 ± 5.4 | 0.16 | 0.92 | 0.93 |
| (19.5–22.6) | (18.7–23.6) | (21.4–23.8) | (20.8–24.4) | ||||
| Thickness during hollowing (cm) | 0.58 ± 0.10 | 0.55 ± 0.11 | 0.51 ± 0.95 | 0.51 ± 0.12 | 0.05 | 0.17 | 0.31 |
| (0.53–0.63) | (0.50–0.60) | (0.48–0.54) | (0.47–0.55) | ||||
| Relative thickness hollowing (% of total TrA, OI, OE) | 26.9 ± 3.3 | 26.7 ± 5.5 | 29.1 ± 5.4 | 28.3 ± 6.7 | 0.18 | 0.47 | 0.66 |
| (25.4–28.5) | (24.2–29.3) | (27.3–30.9) | (26.1–30.5) | ||||
| Difference, rest to hollowing (cm) | 0.18 ± 0.07 | 0.16 ± 0.08 | 0.15 ± 0.07 | 0.14 ± 0.07 | 0.15 | 0.18 | 0.75 |
| (0.15–0.21) | (0.12–0.20) | (0.13–0.17) | (0.11–0.16) | ||||
| TrA contraction ratio | 1.48 ± 0.21 | 1.45 ± 0.30 | 1.47 ± 0.27 | 1.40 ± 0.21 | 0.61 | 0.13 | 0.52 |
| (1.38–1.56) | (1.31–1.59) | (1.37–1.55) | (1.33–1.46) | ||||
| TrA preferential activation ratio | 0.059 ± 0.023 | 0.056 ± 0.035 | 0.065 ± 0.037 | 0.057 ± 0.030 | 0.65 | 0.14 | 0.51 |
| (0.048–0.070) | (0.040–0.073) | (0.053–0.078) | (0.047–0.067) | ||||
| OI | |||||||
| Thickness at rest (cm) | 0.86 ± 0.24 | 0.83 ± 0.24 | 0.67 ± 0.21 | 0.73 ± 0.24 | 0.02 | 0.59 | 0.06 |
| (0.75–0.97) | (0.72–0.94) | (0.60–0.74) | (0.65–0.81) | ||||
| Relative thickness rest (% of total TrA, OI, OE) | 44.2 ± 4.9 | 43.0 ± 5.6 | 41.2 ± 5.9 | 43.0 ± 5.9 | 0.26 | 0.74 | 0.09 |
| (41.9–46.5) | (40.4–45.6) | (39.2–43.2) | (41.0–45.0) | ||||
| Thickness during hollowing (cm) | 0.98 ± 0.26 | 0.91 ± 0.29 | 0.70 ± 0.21* | 0.77 ± 0.26 | 0.002 | 0.99 | 0.01* |
| (0.86–1.10) | (0.77–1.05) | (0.63–0.77) | (0.68–0.86) | ||||
| Relative thickness hollowing (% of total TrA, OI, OE) | 44.5 ± 5.1 | 42.3 ± 6.5 | 38.7 ± 4.9* | 41.4 ± 6.6 | 0.02 | 0.81 | 0.004 |
| (42.1–46.9) | (39.2–45.5) | (27.0–40.3) | (39.2–43.6) | ||||
| Difference, rest to hollowing (cm) | 0.12 ± 0.10 | 0.08 ± 0.09 | 0.03 ± 0.05* | 0.05 ± 0.06 | 0.001 | 0.287 | 0.03 |
| (0.07–0.16) | (0.04–0.12) | (0.01–0.05) | (0.02–0.07) | ||||
| OI contraction ratio | 1.15 ± 0.14 | 1.10 ± 0.11 | 1.06 ± 0.09 | 1.07 ± 0.10 | 0.02 | 0.15 | 0.06 |
| (1.08–1.21) | (1.04–1.15) | (1.03–1.09) | (1.03–1.10) | ||||
| OE | |||||||
| Thickness at rest (cm) | 0.67 ± 0.21 | 0.71 ± 0.34 | 0.61 ± 0.33 | 0.59 ± 0.26 | 0.25 | 0.84 | 0.27 |
| (0.58–0.77) | (0.55–0.87) | (0.50–0.72) | (0.50–0.68) | ||||
| Relative thickness rest (% of total TrA, OI, OE) | 34.8 ± 6.1 | 35.8 ± 7.8 | 36.2 ± 6.7 | 34.4 ± 6.2 | 0.99 | 0.67 | 0.10 |
| (31.9–37.6) | (32.2–39.5) | (34.0–38.4) | (32.3–36.5) | ||||
| Thickness during hollowing (cm) | 0.63 ± 0.18 | 0.68 ± 0.32 | 0.61 ± 0.33 | 0.57 ± 0.25 | 0.42 | 0.79 | 0.07 |
| (0.54–0.71) | (0.53–0.83) | (0.50–0.72) | (0.49–0.65) | ||||
| Relative thickness hollowing (% of total TrA, OI, OE) | 28.6 ± 4.8 | 31.0 ± 7.1 | 32.2 ± 6.6* | 30.4 ± 6.6 | 0.37 | 0.69 | 0.007 |
| (26.3–30.8) | (27.7–34.4) | (30.0–34.4) | (28.2–32.6) | ||||
| Difference, rest to hollowing (cm) | −0.05 ± 0.07 | −0.03 ± 0.06 | 0.00 ± 0.04* | −0.02 ± 0.04 | 0.02 | 0.84 | 0.01 |
| (−0.08–−0.02) | (−0.06–0.00) | (−0.02–0.01) | (−0.03–−0.01) | ||||
| OE contraction ratio | 0.94 ± 0.09 | 0.97 ± 0.10 | 1.00 ± 0.08* | 0.97 ± 0.07 | 0.13 | 0.98 | 0.02 |
| (0.89–0.98) | (0.92–1.01) | (0.97–1.02) | (0.95–0.99) | ||||
| OE + OI contraction ratio | 1.05 ± 0.08 | 1.04 ± 0.09 | 1.03 ± 0.06 | 1.03 ± 0.06 | 0.22 | 0.41 | 0.54 |
| (1.01–1.09) | (1.00–1.07) | (1.00–1.05) | (1.00–1.04) | ||||
| Sum TrA, OI and OE at rest (cm) | 1.94 ± 0.45 | 1.93 ± 0.56 | 1.65 ± 0.56 | 1.69 ± 0.50 | 0.06 | 0.73 | 0.51 |
| (1.73–2.15) | (1.67–2.19) | (1.46–1.84) | (1.52–1.85) | ||||
| Sum TrA, OI and OE during hollowing (cm) | 2.18 ± 0.45 | 2.14 ± 0.60 | 1.83 ± 0.55 | 1.85 ± 0.50 | 0.03 | 0.78 | 0.41 |
| (1.97–2.40) | (1.86–2.42) | (1.65–2.01) | (1.69–2.02) | ||||
TrA, transvs. abdominis; OI, internal oblique abdominis; OE, external oblique abdominis; OE + OI, OE and OI considered together.
Relative thickness is expressed as a percentage of total thickness of all three muscles together.
Contraction ratios = muscle thickness during hollowing/muscle thickness at rest.
TrA preferential activation ratio (difference in the TrA proportion of the total lateral abdominal muscle thickness from rest to hollowing) = (TrA hollowing/TrA + OE + OI hollowing) – (TrA rest/TrA + OE + OI rest).
Bold P value for main effect of gender indicates significant difference between men and women for both body sides considered together.
Bold P value for main effect of body side indicates significant difference between body sides for both men and women considered together.
Bold P value for interaction indicates that the gender effect is different for the two sides;
then indicates the side for which a significant difference between genders was obtained using posthoc tests.
Asymmetry of muscle thicknesses and contraction indices
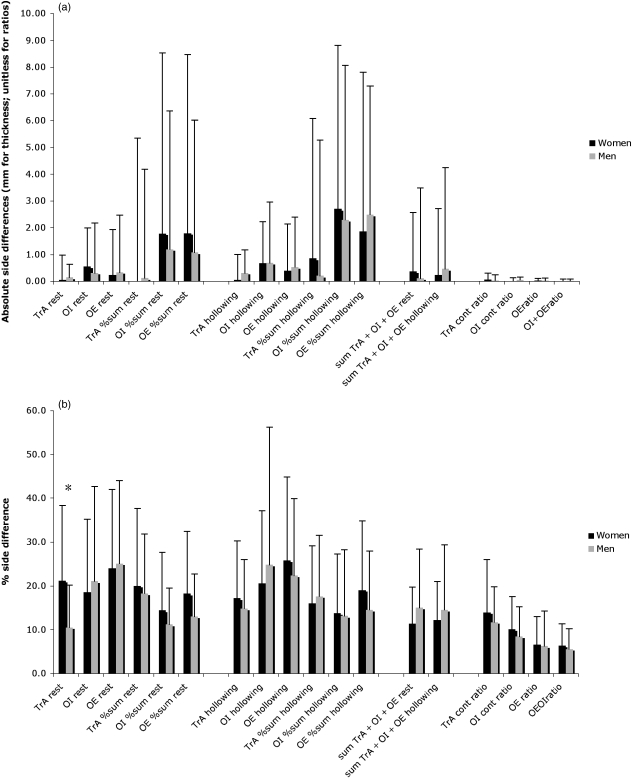
The mean values for the individual side differences for the various muscle thicknesses and contraction ratios are shown in Fig. 3a(absolute side differences) and Fig. 3b (% side differences; i.e. difference as a percent of the smallest value on either left or right sides). Mean values for individual % asymmetry of muscle thicknesses at rest ranged from 11% to 25% (11–20% if expressed as a proportion of the whole lateral muscle thickness) (Fig. 3b); the corresponding range for % asymmetry of thickness during hollowing was 14–26% (13–19% if expressed as proportional thicknesses). Symmetry was generally much better (5–14%) for the various contraction ratios (Fig. 3a,b). For all thicknesses and indices there was large inter-individual variability, with coefficients of variation for the group data (SD/mean) ranging from 66% to 126% depending on the muscle in question. There was no difference between the degree of asymmetry in men and women for any of the muscle thicknesses or indices, except for TrA at rest, which was more asymmetric in the women than the men when expressed as % side differences (21.1% vs. 10.5%, respectively; P = 0.01; see Fig. 3b). The mean % side differences were considerably higher and more variable for the TrA preferential activation ratio (90%) and for the absolute changes in thickness from hollowing to rest (−32% to 137%, depending on the muscle in question) than they were for any of the other muscle thicknesses or indices (data not displayed graphically).
Fig. 3.
Asymmetry of abdominal muscle thicknesses/indices in men and women, expressed a) in absolute units and b) as the percentage difference between sides (side difference as a percent of the smallest mean value on either side). In each case, values are mean ± SD. For explanations of abbreviations, see text or Table 2. *P < 0.05, men vs. women.
Gender differences in muscle thickness parameters
Overall, there was a tendency for the men to have greater muscle thicknesses than the women, and the main effect of gender was significant for all the OI parameters (except OI relative thickness at rest), OE change in thickness from rest to hollowing, and the sum of all lateral abdominal muscles during hollowing (Table 2). Significant interactions (between gender and body side) were observed for most of the OI and OE hollowing parameters (absolute thickness, relative thickness, and change in thickness); post-hoc analyses revealed that these interactions arose due to significant gender differences for the muscles on the left side only (Table 2).
Factors influencing muscle thickness parameters
As no significant side differences were observed for the mean values of any of the thickness variables, the multiple regression analyses were conducted using the average of right and left sides for each thickness measure/contraction ratio as the dependent variable. The regression models showed that the physical characteristics were able uniquely and significantly to explain between 9% (for the TrA change in thickness from rest to hollowing) and 44% [for the sum of the resting thickness (TrA + OI + OE)] of the variance in the various abdominal muscle thickness/contraction ratio measures (Table 3). Body mass uniquely accounted for 29–44% variance in the absolute muscle thicknesses of each of the three muscles, TrA, OI and OE, and in their summed thickness, both at rest and during hollowing, with heavier individuals showing higher values for all these variables (Table 3). BMI significantly explained 9% variance in the TrA change in thickness from rest to hollowing and 20–28% variance in both the TrA contraction ratio and the TrA preferential activation ratio (with greater BMI being associated with lower values for each of these parameters) (Table 3). Age uniquely explained 6–9% variance in the TrA and OI contraction ratios (greater age, higher values). Gender was a significant predictor of OI thickness change from rest to hollowing and of OI contraction ratio (R2 = 10–18%), with men having greater values than women. Gender also accounted for 10% variance in the OE contraction ratio, with women having higher values than men (Table 3).
Table 3.
Results of the multiple regression analysis showing the variance in muscle thickness/ratios explained by physical characteristics (gender, age, height, body mass, BMI) of the subjects. Only the significant predictors in the multivariate model are listed
| Dependent variable | Significant predictors | Final standardized beta coefficient (predictor) | Significance of predictor, P-value | Change in R2 for addition of predictor |
|---|---|---|---|---|
| TrA | ||||
| Resting thickness (cm) | Body mass | 0.636 | < 0.0001 | 0.41 |
| Thickness during hollowing (cm) | Body mass | 0.559 | < 0.0001 | 0.31 |
| Difference, rest to hollowing (cm) | BMI | −0.295 | 0.026 | 0.09 |
| TrA contraction ratio | BMI | −0.524 | < 0.0001 | 0.20 |
| Age | 0.251 | 0.047 | 0.06 | |
| TrA preferential activation ratio | BMI | −0.531 | < 0.0001 | 0.28 |
| OI | ||||
| Resting thickness (cm) | Body mass | 0.563 | < 0.0001 | 0.29 |
| Age | −0.264 | 0.020 | 0.07 | |
| Thickness during hollowing (cm) | Body mass | 0.563 | < 0.0001 | 0.32 |
| Difference, rest to hollowing (cm) | Gender | 0.422 | 0.001 | 0.18 |
| OI contraction ratio | Gender | 0.333 | 0.009 | 0.10 |
| Age | 0.299 | 0.018 | 0.09 | |
| OE | ||||
| Resting thickness (cm) | Body mass | 0.547 | < 0.0001 | 0.30 |
| Thickness during hollowing (cm) | Body mass | 0.553 | < 0.0001 | 0.31 |
| Difference, rest to hollowing (cm) | Gender | −0.314 | 0.017 | 0.10 |
| OE contraction ratio | − | − | − | − |
| OE + OI contraction ratio | − | − | − | − |
| Sum TrA + OI + OE | ||||
| Resting thickness (cm) | Body mass | 0.650 | < 0.0001 | 0.42 |
| Thickness during hollowing (cm) | Body mass | 0.663 | < 0.0001 | 0.44 |
TrA, transvs. abdominis; OI, internal oblique abdominis; OE, external oblique abdominis; OE + OI, OE and OI together (see text for details) ‘−’ indicates that none of the physical characteristics assessed was a significant predictor.
Discussion
The present study examined the factors influencing the size and symmetry of the abdominal muscles at rest and during the abdominal hollowing manoeuvre – a clinical test commonly used to assess trunk muscle function in association with low back pain (Critchley & Coutts, 2002; Henry & Westervelt, 2005). It was considered important to examine the extent to which the muscle thickness measures were influenced by these potential confounders, as this may influence the interpretation of studies in which low back pain patients are compared with healthy controls.
In previous studies on limb muscles (Maughan et al. 1983) and trunk muscles (Mannion et al. 2000) it has been shown that fat-free mass (Maughan et al. 1983; Mannion et al. 2000) and whole body mass (unpublished data from Mannion et al. 2000) are significant predictors of muscle cross-sectional area (CSA), accounting for 47% to 57% variance (for body mass and lean body mass, respectively) (Mannion et al. 2000). Given that muscle thickness is strongly correlated with the CSA or overall volume of the muscle (Miyatani et al. 2002, 2004; Sanada et al. 2006), it was hypothesized that body mass would have a significant influence also on measures of abdominal muscle thickness. In the present study, this influence was indeed evident: men generally had greater muscle thicknesses than women (by 10–20%), and multivariate analyses revealed that body mass was the most consistent unique predictor of muscle thicknesses both at rest and during hollowing, accounting for 30–40% variance. The gender differences in thickness and the dependence of thicknesses on body mass concur with the findings for resting muscle reported by Rankin et al. (2006), although in contrast to these authors we found no additional influence of gender on OI and OE thickness, once body mass had been accounted for. This mirrored our previous findings in relation to the prediction of erector spinae muscle cross-sectional area (Mannion et al. 2000).
Similar to the findings of Rankin et al. (2006), where a correlation between age and muscle thickness was found at all, it was generally low and negative, reaching significance as a unique predictor in multivariate analyses only for OI thickness at rest. In other skeletal muscles, age has been shown to have a similarly low negative correlation with muscle CSA (Edwards et al. 1977; Mannion et al. 2000).
It was hypothesized that use of the contraction ratios developed by Teyhen et al. (2005) may remove some of the potential influence of anthropometric factors on the absolute thicknesses, and may also be less sensitive to differences in measurement site and measurement method [e.g. the use of the instantaneous maximum, as typically recorded with B-mode ultrasound (Critchley, 2002; Teyhen et al. 2005; Hides et al. 2006), or the sustained maximum, as in the present study], allowing better comparability across studies. Interestingly, when the present data are compared with those of Hides et al. (2006) it can be seen that, although our mean values for absolute muscle thicknesses were approximately half those of their elite cricketers (present study: TrA, ∼0.4 cm and OI, ∼0.8 cm; Hides et al. (Hides et al. 2006): TrA, ∼0.7 cm and OI, ∼1.6 cm), the contraction indices in each study were almost identical (∼1.5 for TrA and ∼1.2 for OI) and were also very similar to those reported previously (Critchley, 2002). However, in two further studies, much higher mean TrA contraction ratios were reported [∼1.8 for non-LBP subjects (Springer et al. 2006) and ∼2.2 to 3.0 for LBP-sufferers, depending on the training/practice given (Teyhen et al. 2005)], although in the latter study the OI + OE contraction ratio was similar to that reported here (∼1.03) (Teyhen et al. 2005). Overall, these findings suggest that further work is required to locate the identity of the between-study differences and hence determine the utility of the TrA contraction ratio as a ‘normalized’ index of TrA function during hollowing.
In the present study, in multivariate analysis body mass had no significant influence on the TrA contraction ratio, but BMI showed a significant negative relationship, accounting for ∼20% variance in this index. One previous study suggested an influence of BMI on absolute TrA thickness during hollowing, but the relationship was positive (i.e., higher BMI, greater thickness) (Springer et al. 2006). A positive relationship is difficult to explain because BMI is typically an indicator of body fatness (Baecke et al. 1982; Rookus et al. 1985; Deurenberg et al. 1991; Welborn et al. 2000), and there is no plausible reason to explain why fatter people should have thicker muscles. However, the subjects in the Springer et al. (2006) study were all Department of Defence beneficiaries for whom the usual interpretation of BMI as an indicator of body fatness may not have applied if they were of a more muscular build than normal. Alternatively, as the men in that study had significantly higher BMI than the women (27.8 vs. 22.3 kg m−2, respectively) (Springer et al. 2006), and men typically have larger muscles than women, BMI may simply have been acting as a surrogate measure/marker for maleness. Unfortunately, multiple regression analyses including both gender and the anthropometric variables were not carried out to unravel these complicated interrelationships. Our own tentative suggestion for the negative relationship between BMI and TrA contraction ratio observed in the present study is that it may reflect the typically ‘less active lifestyle’ of those with a higher BMI and a correspondingly less well developed ability to activate the muscle. However, this hypothesis needs to be investigated in a large sample of individuals with widely varying activity levels and BMIs, and including accurate measures of % body fat.
In keeping with previous studies (Springer et al. 2006) we also found that the TrA tended to represent a greater proportion of the lateral abdominal muscles in women, although the trend did not reach significance. However, dispelling the notion that this may be an indicator that women are better able preferentially to contract the TrA, signifying gender differences in neuromuscular control (Springer et al. 2006), we found no significant differences or even trends for a gender difference in the TrA contraction ratio. Indeed, closer examination of the data of the authors who proposed this phenomenon (their Table 4;Springer et al. 2006) also reveals no such sex differences in the ability to contract TrA.
It was interesting to note that, in some publications, it is suggested that the abdominal hollowing exercise is designed to activate the TrA in relative isolation and that a change in thickness of the more superficial abdominal muscles indicates incorrect test performance (Richardson et al. 1999; Jull & Richardson, 2000). However, in the present study and also in two previous studies (Richardson et al. 2002; Hides et al. 2006) an approximate 10–20% increase in thickness of OI accompanied the hollowing manoeuvre. Only OE showed minimal mean change in thickness or even a reduction, perhaps due to stretching (thinning) induced by thickening of the apposing muscles. The (in part) co-activation of TrA and OI during this clinical muscle test may be a reflection of the overlap in function between these two muscles – particularly marked in their respective mid-regions (Urquhart et al. 2005) – in their roles as contributors to spine stabilization.
Consistent with previous studies (Rankin et al. 2006; Springer et al. 2006), there were no significant side differences for any of the mean values of thickness or thickness change during contraction. Such findings have previously been taken to imply that any asymmetries in lateral abdominal muscle thickness, observed in individuals with LBP, may be interpreted as pathological (Springer et al. 2006), in the same way as side differences in the size of the multifidus have previously been interpreted (Hides et al. 1994). However, in the present study, and also in a previous study of healthy subjects (resting muscle only) (Rankin et al. 2006), individual % side differences in the thickness of the lateral abdominal muscles were at times large, with group mean values ranging from around 11% to 26%, with high standard deviations (Fig. 3b). Although these % differences reflect relatively small absolute differences (< 1 mm), the situation is still quite different from the < 5% side differences reported for the multifidus (Hides et al. 1994). Even when the thicknesses on each side were normalized, i.e. expressed relative to the whole lateral abdominal muscle thickness, side differences were still evident (10–20%), albeit less marked. Hence, we maintain that, in clinical practice, caution should be exercised in over-interpreting any asymmetries observed in the lateral abdominal muscles in individuals with LBP. Interestingly, side differences were lowest for all the contraction ratios (5–15%); this provides the impetus for examining the utility of these measures as a means of improved standardization in future cross-sectional studies of LBP patients vs. controls. Prospective interventional studies might also be carried out to examine whether these contraction ratio indices, with their lower within-subject variability, are more sensitive to change than absolute measures of muscle thickness/thickness change.
Concluding remarks
There were no significant differences between left and right sides for group mean thicknesses of any of the abdominal muscles studied, but individual asymmetries were common. Similar asymmetries observed in patients with low back pain should hence be interpreted with caution. Body mass predicted absolute muscle thickness (positive relationship), and BMI predicted TrA contraction ratio (negative relationship). The influence of these confounders should be considered in comparative studies of healthy controls and back pain patients, unless groups are carefully matched.
Acknowledgments
Supported by a grant from the Swiss National Research Program NRP 53 ‘Musculoskeletal Health – Chronic Pain’ of the Swiss National Science Foundation (Project 405340-104787/2) and the Schulthess Klinik Research Funds.
Thanks to Mark Gorelick, David O’Riordan, Hans Gerber, Deborah Gubler, Daniel Helbling, Marlies Hug De los Santos, Mahmud Kiani-Fard and Judith Reutimann for their assistance with the data collection and analysis. We are grateful to Gordon Adam for his help in producing the Figures. Thanks to Prof. Beat Michel for providing the infrastructure to carry out this work within the Department of Rheumatology and Institute of Physical Medicine, University Hospital Zürich, Switzerland.
References
- Baecke JA, Burema J, Deurenberg P. Body fatness, relative weight and frame size in young adults. Br J Nutr. 1982;48:1–6. doi: 10.1079/bjn19820081. [DOI] [PubMed] [Google Scholar]
- Barker PJ, Guggenheimer KT, Grkovic I, et al. Effects of tensioning the lumbar fasciae on segmental stiffness during flexion and extension: Young Investigator Award winner. Spine. 2006;31:397–405. doi: 10.1097/01.brs.0000195869.18844.56. [DOI] [PubMed] [Google Scholar]
- Crisco JJ, Panjabi MM. Euler stability of the human ligamentous lumbar spine. Part 1, Theory and Part 2, Experiment. Clin Biomech. 1992;7:19–32. doi: 10.1016/0268-0033(92)90003-M. [DOI] [PubMed] [Google Scholar]
- Critchley D. Instructing pelvic floor contraction facilitates transvs. abdominis thickness increase during low-abdominal hollowing. Physiother Res Int. 2002;7:65–75. doi: 10.1002/pri.243. [DOI] [PubMed] [Google Scholar]
- Critchley DJ, Coutts FJ. Abdominal muscle function in chronic low back pain patients. Physiotherapy. 2002;88:322–332. [Google Scholar]
- Deurenberg P, Weststrate JA, Seidell JC. Body mass index as a measure of body fatness: age- and sex-specific prediction formulas. Br J Nutr. 1991;65:105–114. doi: 10.1079/bjn19910073. [DOI] [PubMed] [Google Scholar]
- Edwards RH, Young A, Hosking GP, Jones DA. Human skeletal muscle function: description of tests and normal values. Clin Sci Mol Med. 1977;52:283–290. doi: 10.1042/cs0520283. [DOI] [PubMed] [Google Scholar]
- Ferreira PH, Ferreira ML, Hodges PW. Changes in recruitment of the abdominal muscles in people with low back pain: ultrasound measurement of muscle activity. Spine. 2004;29:2560–2566. doi: 10.1097/01.brs.0000144410.89182.f9. [DOI] [PubMed] [Google Scholar]
- Ferreira PH, Ferreira ML, Maher CG, Herbert RD, Refshauge K. Specific stabilisation exercise for spinal and pelvic pain: a systematic review. Aust J Physiother. 2006;52:79–88. doi: 10.1016/s0004-9514(06)70043-5. [DOI] [PubMed] [Google Scholar]
- Gardner Morse MG, Stokes IAF. The effects of abdominal muscle coactivation on lumbar spine stability. Spine. 1998;23:86–92. doi: 10.1097/00007632-199801010-00019. [DOI] [PubMed] [Google Scholar]
- Henry SM, Teyhen DS. Ultrasound imaging as a feedback tool in the rehabilitation of trunk muscle dysfunction for people with low back pain. J Orthop Sports Phys Ther. 2007;37:627–634. doi: 10.2519/jospt.2007.2555. [DOI] [PubMed] [Google Scholar]
- Henry SM, Westervelt KC. The use of real-time ultrasound feedback in teaching abdominal hollowing exercises to healthy subjects. J Orthop Sports Phys Ther. 2005;35:338–345. doi: 10.2519/jospt.2005.35.6.338. [DOI] [PubMed] [Google Scholar]
- Hides JA, Stokes MJ, Saide M, Jull GA, Cooper DH. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine. 1994;19:165–172. doi: 10.1097/00007632-199401001-00009. [DOI] [PubMed] [Google Scholar]
- Hides JA, Jull GA, Richardson CA. Use of real-time ultrasound imaging for feedback in rehabilitation. Man Ther. 1998;3:125–131. [Google Scholar]
- Hides J, Wilson S, Stanton W, et al. An MRI investigation into the function of the transvs. abdominis muscle during ‘drawing-in’ of the abdominal wall. Spine. 2006;31:E175–178. doi: 10.1097/01.brs.0000202740.86338.df. [DOI] [PubMed] [Google Scholar]
- Hodges PW. Ultrasound imaging in rehabilitation: just a fad? J Orthop Sports Phys Ther. 2005;35:333–337. doi: 10.2519/jospt.2005.0106. [DOI] [PubMed] [Google Scholar]
- Hodges P, Kaigle Holm A, Holm S, et al. Intervertebral stiffness of the spine is increased by evoked contraction of transvs. abdominis and the diaphragm: in vivo porcine studies. Spine. 2003a;28:2594–2601. doi: 10.1097/01.BRS.0000096676.14323.25. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Pengel LH, Herbert RD, Gandevia SC. Measurement of muscle contraction with ultrasound imaging. Muscle Nerve. 2003b;27:682–692. doi: 10.1002/mus.10375. [DOI] [PubMed] [Google Scholar]
- Jull GA, Richardson CA. Motor control problems in patients with spinal pain: a new direction for therapeutic exercise. J Manipulative Physiol Ther. 2000;23:115–117. [PubMed] [Google Scholar]
- Kavcic N, Grenier S, McGill SM. Determining the stabilizing role of individual torso muscles during rehabilitation exercises. Spine. 2004;29:1254–1265. doi: 10.1097/00007632-200406010-00016. [DOI] [PubMed] [Google Scholar]
- Mannion AF, Kaser L, Weber E, et al. Influence of age and duration of symptoms on fibre type distribution and size of the back muscles in chronic low back pain patients. Eur Spine J. 2000;9:273–281. doi: 10.1007/s005860000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannion AF, Pulkovski N, Gubler D, et al. Muscle thickness changes during abdominal hollowing: an assessment of between-day measurement error in controls and patients with chronic low back pain. Eur Spine J. 2008;17:494–501. doi: 10.1007/s00586-008-0589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan RJ, Watson JS, Weir J. Relationships between muscle strength and muscle cross-sectional area in male sprinters and endurance runners. Eur J Appl Physiol Occup Physiol. 1983;50:309–318. doi: 10.1007/BF00423237. [DOI] [PubMed] [Google Scholar]
- McGill SM, Grenier S, Kavcic N, Cholewicki J. Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol. 2003;13:353–359. doi: 10.1016/s1050-6411(03)00043-9. [DOI] [PubMed] [Google Scholar]
- McMeeken JM, Beith ID, Critchley D, Milligan P, Newham DJ. Measurements of thickness and electrical activity in transvs. abdominis in healthy subjects. Sydney, Australia: International Conference in Physiotherapy; 2002. [Google Scholar]
- Misuri G, Colagrande S, Gorini M, et al. In vivo ultrasound assessment of respiratory function of abdominal muscles in normal subjects. Eur Respir J. 1997;10:2861–2867. doi: 10.1183/09031936.97.10122861. [DOI] [PubMed] [Google Scholar]
- Miyatani M, Kanehisa H, Kuno S, Nishijima T, Fukunaga T. Validity of ultrasonograph muscle thickness measurements for estimating muscle volume of knee extensors in humans. Eur J Appl Physiol. 2002;86:203–208. doi: 10.1007/s00421-001-0533-9. [DOI] [PubMed] [Google Scholar]
- Miyatani M, Kanehisa H, Ito M, Kawakami M, Fukunaga T. The accuracy of volume estimates using muscle thickness measurements in different muscle groups. Eur J Appl Physiol. 2004;91:264–272. doi: 10.1007/s00421-003-0974-4. [DOI] [PubMed] [Google Scholar]
- Panjabi MM. The stabilizing system of the spine. Part 1. Function, dysfunction, adaptation and enhancement. J Spinal Disorders. 1992;5:383–389. doi: 10.1097/00002517-199212000-00001. [DOI] [PubMed] [Google Scholar]
- Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackwitz B, de Bie R, Limm H, et al. Segmental stabilizing exercises and low back pain. What is the evidence? A systematic review of randomized controlled trials. Clin Rehabil. 2006;20:553–567. doi: 10.1191/0269215506cr977oa. [DOI] [PubMed] [Google Scholar]
- Rankin G, Stokes M, Newham DJ. Abdominal muscle size and symmetry in normal subjects. Muscle Nerve. 2006;34:320–326. doi: 10.1002/mus.20589. [DOI] [PubMed] [Google Scholar]
- Richardson CA, Jull GA. Muscle control – pain control. What exercises would you prescribe? Man Ther. 1995;1:2–10. doi: 10.1054/math.1995.0243. [DOI] [PubMed] [Google Scholar]
- Richardson C, Jull G, Hodges P, Hides J. Therapeutic Exercise for Spinal Stabilisation: Scientific Basis and Practical Techniques. Edinburgh: Churchill Livingstone; 1999. [Google Scholar]
- Richardson CA, Snijders CJ, Hides JA, et al. The relation between the transvs. abdominis muscles, sacroiliac joint mechanics and low back pain. Spine. 2002;27:399–405. doi: 10.1097/00007632-200202150-00015. [DOI] [PubMed] [Google Scholar]
- Rookus MA, Burema J, Deurenberg P, Van der Wiel-Wetzels WA. The impact of adjustment of a weight-height index (W/H2) for frame size on the prediction of body fatness. Br J Nutr. 1985;54:335–342. doi: 10.1079/bjn19850118. [DOI] [PubMed] [Google Scholar]
- Sanada K, Kearns CF, Midorikawa T, Abe T. Prediction and validation of total and regional skeletal muscle mass by ultrasound in Japanese adults. Eur J Appl Physiol. 2006;96:24–31. doi: 10.1007/s00421-005-0061-0. [DOI] [PubMed] [Google Scholar]
- Springer BA, Mielcarek BJ, Nesfield TK, Teyhen DS. Relationships among lateral abdominal muscles, gender, body mass index, and hand dominance. J Orthop Sports Phys Ther. 2006;36:289–297. doi: 10.2519/jospt.2006.2217. [DOI] [PubMed] [Google Scholar]
- Teyhen DS. Rehabilitative ultrasound imaging: the roadmap ahead. J Orthop Sports Phys Ther. 2007;37:431–433. doi: 10.2519/jospt.2007.0107. [DOI] [PubMed] [Google Scholar]
- Teyhen DS, Miltenberger CE, Deiters HM, et al. The use of ultrasound imaging of the abdominal drawing-in maneuver in subjects with low back pain. J Orthop Sports Phys Ther. 2005;35:346–355. doi: 10.2519/jospt.2005.35.6.346. [DOI] [PubMed] [Google Scholar]
- Teyhen DS, Gill NW, Whittaker JL, et al. Rehabilitative ultrasound imaging of the abdominal muscles. J Orthop Sports Phys Ther. 2007;37:450–466. doi: 10.2519/jospt.2007.2558. [DOI] [PubMed] [Google Scholar]
- Urquhart DM, Hodges PW, Story IH. Postural activity of the abdominal muscles varies between regions of these muscles and between body positions. Gait Posture. 2005;22:295–301. doi: 10.1016/j.gaitpost.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Vera-Garcia FJ, Brown SH, Gray JR, McGill SM. Effects of different levels of torso coactivation on trunk muscular and kinematic responses to posteriorly applied sudden loads. Clin Biomech (Bristol, Avon) 2006;21:443–455. doi: 10.1016/j.clinbiomech.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Welborn TA, Knuiman MW, Vu HT. Body mass index and alternative indices of obesity in relation to height, triceps skinfold and subsequent mortality: the Busselton health study. Int J Obes Relat Metab Disord. 2000;24:108–115. doi: 10.1038/sj.ijo.0801093. [DOI] [PubMed] [Google Scholar]