Abstract
In the last decade, there has been an increasing interest in cognitive alterations during the early course of schizophrenia. From a clinical perspective, a better understanding of cognitive functioning in putative at-risk states for schizophrenia is essential for developing optimal early intervention models. Two approaches have more recently been combined to assess the entire course of the initial schizophrenia prodrome: the predictive “basic symptom at-risk” (BS) and the ultra high-risk (UHR) criteria. Basic symptoms are considered to be present during the entire disease progression, including the initial prodrome, while the onset of symptoms captured by the UHR criteria expresses further disease progression toward frank psychosis. The present study investigated the cognitive functioning in 93 subjects who met either BS or UHR criteria and thus were assumed to be at different points on the putative trajectory to psychosis. We compared them with 43 patients with a first episode of psychosis and to 49 help-seeking patient controls. All groups performed significantly below normative values. Both at-risk groups performed at intermediate levels between the first-episode (FE) group and normative values. The UHR group demonstrated intermediate performance between the FE and BS groups. Overall, auditory working memory, verbal fluency/processing speed, and declarative verbal memory were impaired the most. Our results suggest that cognitive impairments may still be modest in the early stages of the initial schizophrenia prodrome and thus support current efforts to intervene in the early course of impending schizophrenia because early intervention may prevent or delay the onset of frank psychosis and thus prevent further cognitive damage.
Keywords: prodrome, at-risk state, basic symptoms, ultra high risk, cognition
Introduction
Over recent years, the goal to intervene during the initial prodrome of schizophrenia has drawn great interest both in clinical psychiatry and in research. However, for early intervention to succeed, an accurate definition of the initial prodrome and its various stages are pivotal. Various approaches to define the schizophrenia prodrome have been described over the last decade (see review1). Two approaches have received significant attention: the “basic symptom at-risk” (BS) and the “ultra high-risk” (UHR) approach.
Basic symptoms are thought to describe subtle, subclinical, self-experienced, and self–reported disturbances of thought processes, perception, drive, and affect which are similarly reported in early stages preceding a manifest psychosis, during the acute episode, and after remission. Basic symptoms were originally considered the underlying “roots” of frank psychotic symptoms and were regarded as the direct expression of the “organic” basis of psychosis, hence the term “basic.”2 Traditionally, basic symptoms have been assessed with the “Bonn Scale for the Assessment of Basic Symptoms” (BSABS).3 The prospective Cologne Early Recognition study was able to demonstrate in a help-seeking population that a subset of basic symptoms demonstrated a good predictive value for developing schizophrenia in the next 9–10 years (see table 1). The best predictive accuracy was associated with cognitive and perceptive basic symptoms.4 (In the BSABS, the term “cognitive” denotes subjective complaints of thought interference, thought blockages, or thought pressure and not objectively measurable neurocognitive deficits.)
Table 1.
Inclusion Criteria for Study Groups
| FE |
| At least 1 SOPS positive symptom score of 6 for at least 1 week, irrespective of basic symptoms |
| UHR |
| Any SOPS positive symptom score between 3 and 5, at least once per week over the past month, with beginning in past year or scoring 1 scale point higher compared with 12 months ago (=Attenuated Positive Prodromal Syndrome) |
| Any SOPS positive symptom score of 6 for less than 1 week (=Brief Intermittent Psychotic Syndrome) |
| First-degree relative with any psychotic disorder and/or patient meeting DSM-IV Schizotypal Personality Disorder criteria and a 30% or greater drop in the GAF score during the last month compared with 12 months ago (=Genetic Risk and Deterioration Syndrome) |
| All UHR criteria irrespective of basic symptoms |
| BS |
| Presenting at least 1 predictive basic symptom4 with an SPI-A score of at least 3, not meeting any UHR or FE criteria (predictive basic symptoms: thought interference; thought perseveration; thought pressure; thought blockages; disturbances of receptive language; decreased ability to discriminate between ideas and perception, fantasy, and true memory; unstable ideas of reference; derealization; visual and acoustic perception disturbances) |
| PCo |
| Patients meeting none of the criteria for BS, UHR, or FE groups |
Note: FE, first-episode group; SOPS, Scale of Prodromal Symptoms (SOPS positive symptoms: unusual thought content/delusional ideas, suspiciousness/persecutory ideas, grandiose ideas, perceptual abnormalities/hallucinations, disorganized communication); UHR, ultra high-risk group; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; GAF, Global Assessment of Functioning29; BS, basic symptom at-risk group; SPI-A, Schizophrenia Prediction Instrument—Adult Version; PCo, patient control group.
In contrast to BS, the UHR criteria are thought to capture the late prodrome of schizophrenia (see table 1), ie, the interval just prior to the emergence of a full-blown psychosis. They include intermittent or attenuated positive symptoms and a genetic risk/deterioration syndrome (Yung et al5 and T. H. McGlashan, T. J. Miller, S. W. Woods, J. L. Rosen, R. E. Hoffman, L. Davidson, unpublished data, 2001). Two manuals have been developed to assess UHR criteria: the “Structured Interview for Prodromal Syndromes” (SIPS; T. H. McGlashan, T. J. Miller, S. W. Woods, J. L. Rosen, R. E. Hoffman, L. Davidson, unpublished data, 2001) and the “Comprehensive Assessment for At-Risk Mental States” (CAARMS).6
In a number of studies such as the German Research Network on Schizophrenia7 and the European Prediction of Psychosis Study,8 these 2 approaches have recently been combined. The combination of these 2 approaches is based on the conceptualization of the prodrome as an extended phase that starts with subtle symptoms captured by the basic symptoms and ends with the emergence of intermittent or attenuated psychotic symptoms that are captured by the UHR criteria and express a further disease progression beyond the underlying basic syndrome. While intuitively appealing this conceptualization of the prodrome as a quasilinear process, it is not based yet on prospective data clearly demonstrating that subjects going through a prodrome really show such a course. Furthermore, this conceptualization focuses primarily on psychotic symptoms. However, not all patients with schizophrenia present with psychotic or psychosis-like symptoms during their disease progression but are primarily characterized by functional and cognitive decline9—an observation that Eugen Bleuler tried to capture in his diagnostic category of schizophrenia simplex. Moreover, this functional and cognitive decline may not follow the same trajectory as positive symptoms—an important consideration for interventional models given the strong evidence that cognitive impairment is an important aspect of established chronic and first-episode schizophrenia10–13 and one of the major determinants for successful rehabilitation in established schizophrenia.14,15 A clear understanding of these processes may help to better define risk states at different stages of the prodrome.
Recent studies of neuropsychological functioning in UHR subjects demonstrated cognitive impairments that were intermediate between healthy controls and patients with first-episode schizophrenia.16–18 These abnormalities included deficits in working memory,19,20 sustained attention,21 and verbal memory.22,23 Previous studies in genetic high-risk subjects found significant deficits in measures of attention, verbal memory, and executive functions (see review24). While these findings suggest that cognitive deficits may precede prodromal stages by years, no actual data exist on the cognitive status of subjects at risk as defined by predictive BS criteria.4 Thus, it is not known if and to what extent such patients already show cognitive deficits and, importantly, if such deficits follow a similar trajectory as assumed for positive symptoms. From both a theoretical and clinical perspective, a better understanding of cognitive functioning and its course in putative at-risk states of psychosis seem to be pivotal for developing optimal early intervention models.
The present study is a first attempt at answering these important questions by comparing—in a cross-sectional manner—the cognitive functioning between these 2 at-risk groups, a patient control (PCo) group and first-episode (FE) patients. Based on the evidence reviewed above, we hypothesized that (1) patients with predictive basic symptoms show cognitive impairment when compared with normative values and patient controls and (2) that these deficits are comparable to those observed in patients meeting the UHR criteria.
MATERIALS AND METHODS
Study Subjects
A total of 185 patients (124 males/61 females) were recruited from the Bruderholz Early Psychosis Service, a clinical research facility in Northwestern Switzerland established in 2002 which functions as an outpatient clinic to assess at-risk states for psychosis and serves a semiurban population of 300 000. It was designed as collaboration between the departments of youth and adult psychiatry and included a large sensitization of primary care groups such as general practitioners.25 It is part of a longitudinal study of symptoms and social as well as neuropsychological functioning in patients defined as being at risk for psychosis. Referred subjects had experienced potential early warning signs of psychosis such as attenuated or brief intermittent positive psychotic symptoms, sustained decline in social functioning in a still relatively asymptomatic state, or “odd” behavior. Patients are primarily adolescents and young adults. Consenting patients are enrolled in a prospective study to assess the course of at-risk states for psychosis. All subjects participating in this study have signed a written informed consent, and additional written informed consent was obtained from the parents if subjects were under 18. The study was approved by the ethics committee of Basel cantons (Ethikkommission beider Basel). Exclusion criteria were present first episode of psychosis or a history of a past psychotic episode; traumatic brain injury, epilepsy, or other known neurological disorder; other significant medical condition considered to affect cognitive performance and self-perception; IQ below 70; or age below 14 years. We chose the 14-year criterion as there are concerns in the field that there are differences between childhood-onset schizophrenia (onset by age 13) and onset of schizophrenia in adolescents (onset by age 14 and later) in etiology, prognosis, and natural course of the disease.26
Symptom Assessment
Patients were assessed using the following instruments: potential early prodromes were assessed using the Schizophrenia Prediction Instrument—Adult Version (SPI-A)27 and potential late prodromes were assessed using the Scale of Prodromal Symptoms (SOPS) with its companion interview manual (SIPS) (T. H. McGlashan, T. J. Miller, S. W. Woods, J. L. Rosen, R. E. Hoffman, L. Davidson, unpublished data, 2001, and Miller et al28). Interrater reliability was established by extensive training by one of the authors of the SPI-A and by repeated training session involving all raters. However, formal assessment of interrater reliability was not conducted.
The SPI-A has been developed from a hierarchical cluster analysis of the BSABS.3 It features 58 items that can be rated between a score of 0 (absent) and 6 (continuously present and subjectively very distressing) and includes those 10 basic symptoms that were recently reported by Klosterkötter et al4 to have good predictive power for later schizophrenia (see table 1).
The high validity and reliability of the SOPS have been reported elsewhere (T. H. McGlashan, T. J. Miller, S. W. Woods, J. L. Rosen, R. E. Hoffman, L. Davidson, unpublished data, 2001, and Miller et al28). The SOPS covers 19 items divided into 4 subscales (positive, negative, disorganized, and general symptom subscales). Symptoms are rated between scores of 0 (absent) and 6 (severe and psychotic for positive symptoms, extreme for all other symptoms, respectively). Furthermore, the SIPS includes a schizotypal rating scale, a section for the assessment of psychotic disorders in first-degree relatives and a research version of the Global Assessment of Functioning.29
All structured interviews were administered by an experienced consultant research psychiatrist (A.E.S.) or by trained masters-level psychologists (D.A., D.N.D.). Additionally, lifetime Axis I diagnoses were made for all patients and were based on interviews using the Structured Clinical Interview for DSM-IV29 and on information from patients and parents.
Group Assignment
Depending on symptom severity, patients were assigned to 1 of the 4 following groups: first-episode, ultra high-risk, basic symptom at-risk, and patient control groups. For exact group assignment criteria, see table 1. The UHR and BS groups are the at-risk groups. Patient controls were help-seeking patients who were referred to the prodromal clinic for risk assessment but who did not meet FE, UHR, or BS criteria.
Neuropsychological Measures
All subjects in the 4 subgroups received a comprehensive neuropsychological assessment (see table 2). Neuropsychological functions were assessed within 3 days after assessment of symptoms for each subject. The measures assessed distinct dimensions of cognitive impairment that have been identified in patients with schizophrenia30 and have extensively published normative data. These domains comprise verbal learning and memory, executive functions, working memory, sustained attention, and speed of processing. The total time for test administration for the neurocognitive test battery was approximately 2 hours. (In 20 subjects, the test battery could not be completed due to patients' refusal).
Table 2.
Neuropsychological Battery and Variables Selected for Study Entry Assessments
| Cognitive Domain (a priori)a | Test | Variable Employed | Normative Population (N; age range) |
| Verbal IQ | Vocabulary test (Mehrfach-Wortschatz-Test)31 | Raw score correct | 1952; 2064 y31 |
| Working memory | Letter-Number Span35 | Raw score correct | 30; 17–60 y35 |
| Speed of processing | Trail-Making Tests (B, B/A)32 | Time to completion | 84; >15 y32 |
| Verbal fluency (letters/categories)33 | Sum of correct responses | S-words: 567; 8–15 y, 18–65 y33 | |
| p-words: 633; 8–15 y, 18–65 y33 | |||
| Animals: 634; 8–15 y, 18–65 y33 | |||
| Professions: 633; 8–15 y, 18–65 y33 | |||
| Reasoning and problem solving | Wisconsin Card Sorting Test (64-card deck)37 | Perseverative errors | 897; 7–89 y37 |
| Categories completed | |||
| Verbal learning and memory | Rey Auditory Verbal Learning Test34 | Total recall, trials 1–5 | 515; 6–79 y34 |
| Delayed recall total raw scores | |||
| Sustained Attention | Testbatterie zur Aufmerksamkeitsprüfung36 | Commission/omission errors | 200; 7–90 y36 |
| Alertness | Testbatterie zur Aufmerksamkeitsprüfung36 | Reaction time | 533; 6–19 y/599; 20–89 y36 |
Functional domains according to National Institute of Mental Health—Measurement and Treatment Research to Improve Cognition in Schizophrenia.
Verbal IQ was measured by a vocabulary test (Mehrfach-Wortschatz-Test),31 which consists of 37 series of 4 nonwords and 1 word that have to be identified. In addition to the intelligence scale, the test battery included (in order of administration) visuomotor sequencing (Trail-Making Tests A and B, TMT A&B)32; letter and category fluency (Regensburger Wortflüssigkeits-Test)33; verbal learning and memory (Rey Auditory Verbal Learning Test, RAVLT)34; verbal working memory (Letter-Number Span, LNS)35; alertness and sustained attention (Testbatterie zur Aufmerksamkeitsprüfung)36; and Wisconsin Card Sorting Test (WCST).37
Statistical Analyses
For all patients, clinical and neuropsychological characteristics at study entry were analyzed. Statistical analyses were performed with the Statistical Package for Social Science (SPSS 12) for Windows. Comparative analyses of sociodemographic data were performed with the help of 1-way analysis of variance (ANOVA) for continuous variables and with chi-square tests for categorical variables. Raw scores of neuropsychological tests were transformed into standard z scores using the normative data which were obtained for each test. When applicable, tests were reverse scored so that higher scores always reflected better performance. The only exception to this approach was the analysis of the verbal IQ measures. Because the normative data only covered subjects with age of 20 and older, normalized verbal IQ values were compared with zero only for patients aged 20 years and older. Secondly, the raw IQ values were compared between the 4 groups including all subjects. Because this latter analysis did not reveal significant differences, verbal IQ was not entered in further statistical equations as covariate. One-sample t tests were used to assess deviations of cognitive measures against normative values. Between-group differences were assessed with ANOVAs (all analyses were also run with family history of psychotic disorders, presence of schizotypal personality disorder, age, and level of education as covariates, but the result did not differ). If ANOVAs revealed significant differences between groups, least significant difference (LSD) tests were used for post hoc comparisons. Due to the exploratory nature of our analyses, Bonferroni corrections were not applied to post hoc tests. Additionally, an approximation of the global cognitive functioning within every patient group was established by averaging the mean z scores of the main cognitive domains.
Results
Demographic and Clinical Characteristics
Of 185 patients, 43 met the FE, 69 the UHR, 24 the BS, and 49 the PCo criteria. Thus, 93 patients met the at-risk criteria. Demographic and clinical characteristics of the 4 patient groups are displayed in table 3. The 4 groups did not significantly differ in age, gender distribution, or level of education. A total of 16 FE patients (37%) received low-dose treatment of atypical antipsychotics (7 patients received risperidone 2 mg/d, 6 patients olanzapine 10 mg/d, 2 patients quetiapine 300 mg/d, 1 patient aripiprazole 5 mg/d). Treatment duration had not extended over 2 weeks in any patient. All other patients were free of medication.
Table 3.
Sociodemographic and Clinical Characteristics of the Study Sample (N = 185)
| FE (n = 43) | UHR (n = 69) | BS (n = 24) | PCo (n = 49) | Test Statistic | P | Significant Post hoc Tests | |
| Mean age (±SD; range) | 22.2 (±6.1; 14–38) | 20.5 (±5.2; 14–40) | 21.7 (±4.3; 15–30) | 21.8 (±4.9; 14–36) | F = 1.103 | .349 | |
| Gender (male/female) | 30/13 | 40/29 | 15/9 | 39/10 | χ2 = 6.429 | .092 | |
| Education (school)a | χ2 = 5.770 | .927 | |||||
| Continuing | 6 | 15 | 3 | 6 | |||
| Successfully completed | 34 | 50 | 19 | 38 | |||
| Interrupted | 3 | 2 | 1 | 4 | |||
| DSM-IV Axis I diagnosis (n)b | |||||||
| Psychotic disorders (n = 50) | 43 | 7 | |||||
| Paranoid schizophrenia | 35 | ||||||
| Disorganized schizophrenia | 2 | ||||||
| Catatonic schizophrenia | 3 | ||||||
| Undifferentiated schizophrenia | 1 | ||||||
| Delusional disorder | 2 | ||||||
| Brief psychotic disorder | 7 | ||||||
| Mood disorders (n = 61) | 25 | 14 | 22 | ||||
| Anxiety disorders (n = 16) | 10 | 3 | 3 | ||||
| Adjustment disorders (n = 16) | 7 | 1 | 8 | ||||
| Dissociative disorders (n = 4) | 2 | 2 | |||||
| Eating disorder (n = 1) | 1 | ||||||
| SOPSc [mean (±SD)] | |||||||
| Positive scale | 11.1 (5.9) | 7.4 (3.9) | 2.2 (2.4) | 1.5 (2.7) | F = 51.215 | <.001 | FE > UHR > BS = PCo |
| Negative scale | 16.3 (7.4) | 14.1 (6.3) | 12.1 (5.6) | 8.3 (6.0) | F = 13.206 | <.001 | FE = UHR > BS > PCo |
| Disorganized item scale | 4.4 (3.4) | 3.8 (3.0) | 2.2 (2.1) | 2.0 (3.0) | F = 6.631 | <.001 | FE = UHR > BS = PCo |
| General item scale | 8.5 (4.3) | 8.2 (4.1) | 7.8 (4.0) | 5.5 (3.5) | F = 5.800 | .001 | FE = UHR = BS > PCo |
| SPI-Ad [mean (±SD)] | |||||||
| Thought interference | 1.9 (2.3) | 0.9 (1.6) | 0.4 (1.1) | 0.2 (0.6) | |||
| Thought perseveration | 2.0 (2.2) | 1.8 (2.0) | 1.7 (2.0) | 0.1 (0.5) | |||
| Thought pressure | 1.1 (2.0) | 0.3 (0.9) | 1.5 (2.2) | 0.1 (0.6) | |||
| Thought blockages | 2.0 (2.1) | 1.6 (2.0) | 1.4 (1.8) | 0.3 (0.7) | |||
| Disturbed receptive language | 1.7 (2.3) | 1.0 (1.9) | 0.7 (1.6) | 0.1 (0.2) | |||
| Disability to discriminate between perception and fantasy | 0.9 (1.8) | 0.2 (0.7) | 0.1 (0.6) | 0.0 (0.0) | |||
| Unstable ideas of reference | 1.2 (2.1) | 0.6 (1.4) | 0.4 (1.2) | 0.1 (0.1) | |||
| Derealization | 1.7 (2.3) | 1.5 (2.0) | 1.3 (1.9) | 0.1 (0.5) | |||
| Visual perception disturbances | 1.3 (1.9) | 1.2 (1.8) | 0.8 (1.5) | 0.1 (0.4) | |||
| Acoustic perception disturbances | 1.2 (1.8) | 0.9 (1.7) | 0.3 (0.8) | 0.1 (0.5) |
Note: Abbreviations are explained in the footnote to table 1. SD, standard deviation.
Level of education was not assessed in 4 patients.
Only primary Axis I diagnosis considered; not all patients met criteria for Axis I diagnosis.
SOPS (T. H. McGlashan, T. J. Miller, S. W. Woods, J. L. Rosen, R. E. Hoffman, L. Davidson, unpublished data, 2001).
SPI-A29 (only predictive basic symptoms shown here).
Significant differences were observed between the groups with regard to the total scores of the SOPS subscales (see table 3). A similar pattern was observed on all subscores with FE and UHR subjects showing similar scores that were significantly higher than those of BS subjects (because the SOPS positive symptom score was part of the group assignment, the differences on this subscale were expected). Highest SOPS scores were found on the negative symptom subscale, whereby social withdrawal (SOPS N1) and functional disability (SOPS N6) showed highest scores in all 4 groups (data not shown).
We only retained the primary Axis I diagnosis if patients presented more than 1 Axis I diagnoses (see table 3).
The UHR group was constituted by the following subgroups: Attenuated Positive Prodromal Syndrome = 60, Brief Intermittent Psychotic Syndrome = 7, Genetic Risk and Deterioration Syndrome = 2 (for criteria see table 1).
Cognitive Functioning (Overall Profile Analysis)
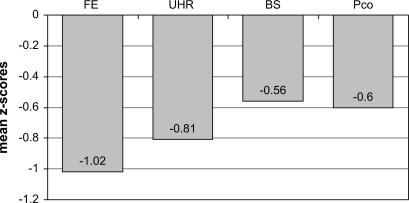
To obtain an approximation of an overall cognitive score for each subject, we averaged the mean z scores of all cognitive domains (see figure 1). ANOVA demonstrated a significant group effect (F3,168 = 3.216, P < .05). FE patients were significantly more impaired than BS and PCo subjects (LSD test P < .05). There was an intermediate position of the UHR group in overall cognitive impairment between the BS at-risk and the FE groups. In subjects aged 20 years and older, normalized verbal IQ scores of each group did not differ from normative data. In addition, verbal IQ scores did not differ between the 4 patient groups (ANOVA F3,168 = 1.440, df = 3, P = .235) and subsequently were not entered in further statistical equations as covariate.
Fig. 1.
Overall Group Mean Cognitive Functioning.
Cognitive Functioning (Specific Domain and Individual Test Analyses)
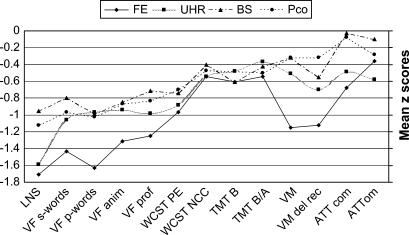
Mean raw scores, F values of ANOVAs, and P values for t test comparisons against normative data for all cognitive measures are shown in table 4. Figure 2 shows the z scores for all cognitive measures by groups.
Table 4.
Cognitive Measures in FE, UHR, BS, and PCo Subgroups and Between-Group Comparisons (N = 185)
| FE (n = 43) | UHR (n = 69) | BS (n = 24) | PCo (n = 49) | F | |||||||
| Measure | Mean (SD) | t | Mean (SD) | t | Mean (SD) | t | Mean (SD) | t | |||
| Verbal IQ | 98.6 (9.9) | 0.033 | 99.8 (11.3) | <0.001 | 101.9 (10.9) | NS | 101.9 (10.9) | NS | NS | ||
| Letter-Number Span | 12.5 (4.0) | <0.001 | 12.8 (3.7) | <0.001 | 14.8 (2.8) | <0.001 | 14.3 (3.3) | <0.001 | 0.013a | ||
| TMT B | 71.6 (37.3) | 0.004 | 67.7 (32.5) | 0.001 | 71.5 (27.7) | 0.006 | 67.9 (32.9) | 0.009 | NS | ||
| TMT B/A | 42.1 (26.7) | 0.003 | 38.0 (26.1) | 0.008 | 39.1 (26.3) | 0.072 | 41.1 (28.9) | 0.01 | NS | ||
| Letter fluency (S) | 10.5 (4.7) | <0.001 | 12.2 (4.8) | <0.001 | 13.4 (4.6) | 0.001 | 12.6 (4.5) | <0.001 | NS | ||
| Letter fluency (P) | 7.1 (4.0) | <0.001 | 9.5 (3.9) | <0.001 | 9.4 (2.4) | <0.001 | 9.3 (3.6) | <0.001 | 0.013b | ||
| Category fluency (animals) | 16.4 (5.7) | <0.001 | 18.9 (5.3) | <0.001 | 19.5 (4.1) | <0.001 | 19.3 (6.5) | <0.001 | NS | ||
| Category fluency (professional groups) | 12.1 (4.9) | <0.001 | 13.4 (4.6) | <0.001 | 14.7 (4.8) | 0.005 | 14.1 (4.7) | <0.001 | NS | ||
| WCST perseverative errors | 5.8 (2.4) | <0.001 | 5.7 (2.3) | <0.001 | 5.4 (1.4) | <0.001 | 5.4 (2.0) | <0.001 | NS | ||
| WCST categories completed | 4.1 (2.6) | 0.039 | 4.1 (1.1) | <0.001 | 4.4 (0.8) | 0.001 | 4.2 (1.1) | <0.001 | NS | ||
| Verbal memory immediate recall | 45.6 (18.5) | <0.001 | 51.8 (14.1) | 0.005 | 53.8 (10.2) | NS | 53.7 (11.7) | NS | 0.040c | ||
| Verbal memory delayed recall | 9.4 (4.7) | <0.001 | 10.8 (3.4) | <0.001 | 11.3 (3.6) | 0.026 | 12.0 (3.3) | NS | 0.015d | ||
| Sustained attention commission errors | 3.4 (8.6) | 0.047 | 2.6 (7.2) | 0.027 | 0.6 (1.0) | NS | 0.8 (2.0) | NS | NS | ||
| Sustained attention omission errors | 1.2 (1.7) | 0.021 | 1.6 (2.0) | <0.001 | 0.7 (0.9) | NS | 1.0 (1.6) | NS | NS | ||
| Alertness (median) | 248.6 (111.0) | NS | 239.7 (137.5) | NS | 236.5 (30.3) | NS | 211.1 (43.7) | NS | NS | ||
Note: Abbreviations are explained in the footnote to table 1. t: 1-sample t tests against normative values, only levels of significance are indicated in this table; F: analysis of variance over all study groups, only levels of significance are indicated in this table; SD, standard deviation; TMT, Trail-Making Test; WCST, Wisconsin Card Sorting Test. The following footnotes are post hoc comparisons.
FE: vs BS 0.010, vs PCo 0.019; UHR: vs BS 0.020, vs PCo 0.038.
FE: vs UHR 0.002, vs BS 0.020, vs PCo 0.009.
FE: vs UHR 0.031, vs BS 0.028, vs PCo 0.010.
FE: vs BS 0.050, vs PCo 0.002.
Fig. 2.
Measures of Cognitive Functioning in First-Episode (FE), Ultra High-Risk (UHR), Basic Symptom At-Risk (BS), and Patient Control (PCo) Groups.
Most cognitive measures were significantly below normative data in all groups, with the exception of alertness in the FE and UHR groups and the exception of verbal memory and sustained attention in the BS and PCo groups.
ANOVAs revealed significant differences between groups in LNS working memory task, letter fluency p-words, and for both measures of verbal learning memory (see table 4).
In all groups, results of the LNS showed the strongest impairments (FE: z = −1.71; UHR: z = 1.59; BS: z = −0.95; PCo: z = −1.12). Both the FE and UHR groups were significantly more impaired than the BS and PCo groups in the LNS (effect of group: F3,168 = 3.716, P < .05; for further details, see table 4). The average z score of all 4 measures of verbal fluency was −1.41 for the FE, −0.99 for the UHR, −0.84 for the BS, and −0.92 for the PCo groups (F3,168 = 3.082, P < .05). Letter fluency p-words were significantly more impaired in the FE group compared with all other groups.
Both RAVLT measures showed a steady increase in impairment from the BS at-risk to the FE group (immediate—PCo: z = −0.32; BS: z = −0.32; UHR: z = −0.51; FE: z = −1.12/delayed—PCo: z = −0.32; BS: z = −0.55; UHR: z = −0.70; FE: z = −1.15). While immediate recall in the FE group differed significantly from all other groups (ANOVA: F3,168 = 2.835, P < .05), delayed recall showed no significant difference between the FE and UHR groups (ANOVA: F3,166 = 3.588, P = .058).
A steady though nonsignificant progression of impairment on WCST perseverative errors (PEs) was demonstrated from PCo to FE groups (PCo: z = −0.70; BS: z = −0.74; UHR: z = −0.88; FE: z = −0.97). In contrast, WCST number of completed categories showed impairment around the −0.5 standard deviation (SD) level for all groups (PCo: z = −0.47; BS: z = −0.40; UHR: z = −0.54; FE: z = −0.54).
Impairments on the TMT B were as follows—PCo: z = −0.48; BS: z = −0.61; UHR: z = −0.48; FE: z = −0.61; and for the TMT B-A measure—PCo: z = −0.50; BS: z = −0.42; UHR: z = −0.37; FE: z = −0.54. No significant between-group differences were found in the Trail-Making measures.
Sustained attention showed very discrete impairment in both measures (commission and omission errors) in the UHR and FE groups, while the BS and PCo groups showed quasinormal values (commission errors—PCo: z = −0.08; BS: z = −0.03; UHR: z = −0.49; FE: z = −0.68/omission errors—PCo: z = −0.28; BS: z = −0.10; UHR: z = −0.58; FE: z = −0.36). No significant between-group differences were found in any measures of sustained attention.
All analyses were also run with family history of psychotic disorders, presence of schizotypal personality disorder, age, and level of education as covariates. The results did not change, and none of these covariates had a significant effect.
Discussion
To the best of our knowledge, this is the first study to compare the level of cognitive functions between 2 groups of individuals at risk for developing psychosis—as identified by the 2 most commonly employed sets of at-risk criteria, predictive BS4 and UHR criteria (T. H. McGlashan, T. J. Miller, S. W. Woods, J. L. Rosen, R. E. Hoffman, L. Davidson, unpublished data, 2001)—patients suffering from a first psychotic episode and patient controls. All patients but 16 with a first psychotic episode were free of medication. In these patients, treatment duration had not extended over 2 weeks.
The present results refute our initial hypotheses: (1) While patients with predictive basic symptoms show impaired cognition in comparison with normative data, their neurocognitive deficits were not worse than those observed in patient controls and (2) these deficits were smaller than the deficits in patients meeting the UHR criteria.
The clinical criteria used to assign patients to the 4 groups (PCo, BS, UHR, FE) reflect an increasing severity of symptoms. This classification is based on the assumption that symptom severity increases more or less linearly as the subject progresses through the prodromal phase toward full-blown psychosis. Whether neuropsychological deficits develop along a similar trajectory is an open question. In patients with established schizophrenia, neuropsychological deficits are independent of severity of positive symptoms.14 This could suggest that the development of these 2 domains may not be parallel. Indeed, in our study, the increasing severity of symptoms was not exactly paralleled by an increasing impairment in cognitive functions. Instead the BS group performed at the same level as the PCo group, whereas the UHR patients showed impairment between these former groups and the FE patients. Thus, to the extent that our data inform us about the development of neuropsychological deficits during the prodrome, they suggest that basic symptoms precede the development of significant neuropsychological deficits and that the latter develop while subjects move into an UHR stage.
These data support the view that the prodrome is characterized by increasing levels not only of symptoms but also of cognitive impairment even though the trajectories of these 2 domains are not parallel.
A recent neuropsychological study of prodromal patients demonstrated a degree of impairment between schizophrenic patients and healthy controls with regard to neuropsychological deficits.18 In our investigation, the same constellation was observed; however, the differences between at-risk groups and patient controls were more subtle, most likely because patient controls—the majority diagnosed with mood disorder—showed some cognitive impairment as well. We intentionally chose to use patient controls with a variety of Axis I diagnoses because one of the major challenges in clinical practice is to identify patients at risk for psychosis in a help-seeking population. Comparing patients at risk for psychosis with help-seeking patients suffering from other psychiatric disorders may have greater validity. The results concerning cognitive impairment of patient controls are in agreement with previous research demonstrating cognitive impairment in a variety of psychiatric disorders.38
There is some debate on defining the normalized cutoff values for relevant cognitive impairment because higher cutoff values increase specificity but reduce sensitivity.39 An impairment of 1 SD is usually considered moderate. Both our at-risk groups showed impairment around and below the 1 SD in working memory and verbal fluency and were significantly impaired compared with normative data.
The pattern of impairment on the various tasks employed is quite similar to results obtained in previous studies in prodromal and FE patients.
Working memory has consistently been found to be impaired in patients with established schizophrenia40 and more recently also in patients meeting criteria for the initial schizophrenia prodrome.19,20 Interestingly, a recent study did not find impaired working memory in an at-risk sample41 using the Digit Span Backwards task. However, this task is comparatively less demanding than the LNS35 applied in the present study and thus may not have yielded enough discriminatory power.
According to the National Institute of Mental Health—Measurement and Treatment Research to Improve Cognition in Schizophrenia (NIMH-MATRICS30), we chose the WCST to measure reasoning and problem solving. In our at-risk subjects, the impairment on the WCST PEs was moderate and was more pronounced than the WCST number of categories completed. This particular pattern has also been observed in patients with a first episode of schizophrenia12,42 and a clinical at-risk sample.21
Similarly, impaired verbal fluency, a measure of speed of processing according to the NIMH-MATRICS,30 has been reported in a sample with mixed prodromal criteria.16 Finally, verbal memory showed discrete impairment in our at-risk groups. Previous studies have reported highly discrepant findings, ranging from similarly discrete41 to considerable impairment23 in verbal memory that almost reached the magnitude reported in FE studies.13
Interestingly, we did not find sustained attention to be impaired in either of our at-risk groups. Despite the consistent pattern of impairments in sustained attention in studies of adults with schizophrenia,43 studies of at-risk patients have yielded conflicting results which may be caused by a significant variance across applied tasks. While Cornblatt et al21 reported sustained attention to be impaired along the entire prodrome and thus defined attention deficits to constitute a core feature of schizophrenia vulnerability, other studies have not been able to show such deficits in clinical at-risk groups44–46 or in genetic high-risk samples.24 There is recent indication from studies among patients with first-episode schizophrenia that the attentional deficit is task specific rather than a global cognitive deficit of attentional mechanisms.47,48 In our study, we employed a task which is a measure of pure sustained attention,36 while Cornblatt et al21 use the Continuous Performance Test-identical pairs version, which may not be considered a pure sustained attention task because it additionally includes a working memory component.43 Thus, the working memory impairment which—as pointed out above—was the most robust finding in both our at-risk groups may reconcile this apparent discrepancy. Alternatively, it has been discussed that some computerized attentional tasks tap a now relatively overlearned and well-developed skill set in the current generation of adolescents and young adults, thus obscuring subtle deficits.24
Despite some inconsistencies of results across studies in at-risk patients which may be due to the heterogeneity of both patients and tasks, a certain theme of findings emerges: our results as well as those of other groups suggest that the most pronounced deficits affect executive functions and working memory. Executive functions include the ability to organize and plan a behavioral response to solve a complex problem, activation of remote memories, shifting and maintaining behavioral sets appropriately, and using verbal skills to guide behavior.49 Functionally, the dorsolateral prefrontal cortex (DLPFC) circuit subserves executive function and working memory. Both verbal fluency and the WCST are related to cognitive functions associated with DLPFC. The prefrontal cortex is also part of the neural network that is involved in attention, and prefrontal-hippocampal circuits are implicated in verbal memory tasks.50 These networks are of similar importance in the WCST because it is well established that the WCST taps numerous cognitive subprocesses and may also challenge hippocampal function.51
The current findings add to the growing body of research on discrete frontal lobe dysfunction in at-risk groups.19 Frontal cortical regions become more metabolically active in late adolescence and early adulthood, the typical life period for the emergence of prodromes and first episodes of schizophrenia. As these reflect higher cognitive functions, our findings may further suggest that impairments in impending psychosis become more apparent as task difficulty increases. In contrast, functions that normally come “online” early in life such as sensory, motor, and basic memory functions, when the brain is more adaptable, show fewer deficits at illness onset.52
Many researchers have emphasized the potential of cognitive dysfunction as a possible endophenotype of genetic risk for schizophrenia. Our results only indicate that such deficits are a manifestation of aberrant brain maturational processes that occur prior to the onset of the full clinical symptoms. However, they cannot answer the question at which period in the brain development these aberrant processes occur. Similarly, Cornblatt et al21 have proposed that subjects at risk and people who already have schizophrenia share a core deficit that includes cognitive impairment. However, as mentioned before, the current study reports cross-sectional findings and does not permit accurate determination when cognitive deficits exactly emerge in at-risk patients. Nonetheless, our finding of impaired immediate verbal memory in UHR but not BS patients are consistent with 1 model of schizophrenia that proposes verbal memory impairment to be partly genetic,53 partly a specific state marker of emerging psychosis, possibly associated with reduction in hippocampal volumes as patients convert to full psychosis.54
In summary, our results support previous findings of an association between UHR states for psychosis with neuropsychological deficits, particularly with such affecting frontal cortical functions. With the exception of a measure of working memory, levels of impairment are still well below those observed in patients with first-episode schizophrenia, where general impairments of 1.5 SDs and above have regularly been described.10,12,13 This provides further support for efforts to intervene during the early course of schizophrenia in order to prevent or delay the onset of frank psychosis and thus potentially stop further cognitive damage. The finding that cognitive deficits in the BS group are still lower than in the UHR group may suggest a “stage model.” Alternatively, the BS group may not be specific enough and may contain a proportion of “false positives” that will never convert to full-blown psychosis, thus leading to less cognitive impairment in the overall group. If, however, neuropsychological deficits indeed worsen markedly when patients move from a BS to a UHR stage, then interventions in patients showing only basic symptoms may potentially delay or even abort further cognitive decline—an effect with far-reaching consequences.
Limitations
A number of methodological limitations of the present investigation need to be mentioned. First, our study reports cross-sectional data, and we thus do not know how many at-risk patients will go on to develop manifest psychosis. This limitation may have contributed to the inclusion of some false positives who could have elevated the overall neuropsychological functioning of both at-risk groups and may not reveal the actual level of deficiency of truly prodromal cases. Second, developmental issues of normal development would need to be measured alongside putatively abnormal changes associated with the prodrome and would thus require appropriately matched healthy controls. A third limitation may be the lack of adequate matching on parental socioeconomic status, gender, and age. However, the 2 at-risk groups did not differ in terms of school education, gender, and age. Further, our study did not assess parental education or premorbid reading ability, both potential confounding factors. Fourth, it must be kept in mind that our FE group was defined on the basis of the 1-week duration criterion of at least 1 positive symptom. This may have caused the inclusion of subsyndromal diagnostic entities and—particularly in adolescents—may not reliably predict a syndromal psychotic disorder55 but rather describe various thresholds of positive symptoms. Fifth, because the exact duration of illness was not consistently available in FE subjects, we cannot provide descriptives of this parameter. Finally, some of the employed neuropsychological tests are not age scaled (eg, LNS35) or were not normalized across the entire adolescent age range assessed in the current study (eg, verbal IQ,31 TMT A/B,32 verbal fluency,33 LNS35), potentially contributing to falsely impaired cognitive values in adolescents.
Acknowledgments
We gratefully acknowledge the advice and contribution of Dr Pietro Ballinari, statistician at the Institute of Psychology, University of Berne, Switzerland, as well as Frauke Schultze-Lutter, University of Cologne, Germany, for training in SPI-A and SIPS manuals. The study was supported by an educational grant from the Freiwillige Akademische Gesellschaft Basel, Switzerland. The pilot phase of this study was supported by an unrestricted grant from Astra Zeneca, Switzerland.
References
- 1.Olsen KA, Rosenbaum B. Prospective investigations of the prodromal state of schizophrenia: assessment instruments. Acta Psychiatr Scand. 2006;113:273–282. doi: 10.1111/j.1600-0447.2005.00698.x. [DOI] [PubMed] [Google Scholar]
- 2.Huber G. Reine Defektsyndrome und Basisstadien endogener Psychosen. Fortschr Neurol Psychiatr. 1966;34:409–426. [Google Scholar]
- 3.Gross G, Huber G, Klosterkötter J, Linz M. Bonn Scale for the Assessment of Basic Symptoms—BSABS. Berlin, Germany: Springer; 1987. [Google Scholar]
- 4.Klosterkötter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- 5.Yung AR, McGorry PD, McFarlane CA, Jackson HJ, Patton GC, Rakkar A. Monitoring and care of young people at incipient risk of psychosis. Schizophr Bull. 1996;22:283–303. doi: 10.1093/schbul/22.2.283. [DOI] [PubMed] [Google Scholar]
- 6.Yung A, Phillips L, McGorry P, Ward J, Donovan K, Thompson K. Comprehensive Assessment of At Risk Mental States (CAARMS) Melbourne, Australia: PACE Clinic, Department of Psychiatry, University of Melbourne; 2002. [Google Scholar]
- 7.Häfner H, Maurer K, Ruhrmann S, et al. Early detection and secondary prevention of psychosis: facts and visions. Eur Arch Psychiatry Clin Neurosci. 2004;254:117–128. doi: 10.1007/s00406-004-0508-z. [DOI] [PubMed] [Google Scholar]
- 8.Klosterkötter J, Ruhrmann S, Schultze-Lutter F, Salokangas RKR, Linszen D. The European Prediction of Psychosis Study (EPOS): integrating early recognition and intervention in Europe. World Psychiatry. 2005;4:161–167. [PMC free article] [PubMed] [Google Scholar]
- 9.Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT., Jr A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58:165–171. doi: 10.1001/archpsyc.58.2.165. [DOI] [PubMed] [Google Scholar]
- 10.Saykin AJ, Shtasel DL, Gur RE, et al. Neuropsychological deficits in neuroleptic naïve patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- 11.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 12.Mohamed S, Paulsen JS, O'Leary D, Arndt S, Andreasen N. Generalized cognitive deficits in schizophrenia. Arch Gen Psychiatry. 1999;56:749–754. doi: 10.1001/archpsyc.56.8.749. [DOI] [PubMed] [Google Scholar]
- 13.Bilder RM, Goldman RS, Robinson D, et al. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry. 2000;157:549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- 14.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 15.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the right stuff? Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 16.Hambrecht M, Lammertink M, Klosterkötter J, Matuschek E, Pukrop R. Subjective and objective neuropsychological abnormalities in a psychosis prodrome clinic. Br J Psychiatry. 2002;181(suppl 43):s30–s37. doi: 10.1192/bjp.181.43.s30. [DOI] [PubMed] [Google Scholar]
- 17.Brewer WJ, Wood SJ, McGorry PD, et al. Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. Am J Psychiatry. 2003;160:1790–1794. doi: 10.1176/appi.ajp.160.10.1790. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins KA, Addington J, Keefe SF, et al. Neuropsychological status of subjects at high risk for a first episode of psychosis. Schizophr Res. 2004;67:115–122. doi: 10.1016/j.schres.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Wood SJ, Pantelis C, Proffitt T, et al. Spatial working memory ability is a marker of risk-for-psychosis. Psychol Med. 2003;33:1239–1247. doi: 10.1017/s0033291703008067. [DOI] [PubMed] [Google Scholar]
- 20.Smith C, Park S, Cornblatt B. Spatial working memory deficits in adolescents at clinical high risk for schizophrenia. Schizophr Res. 2006;81:211–215. doi: 10.1016/j.schres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 21.Cornblatt B, Lencz T, Smith CW, Correll CU, Auther A, Nakayama E. The schizophrenia prodrome revisited: a neurodevelopmental perspective. Schizophr Bull. 2003;29:633–651. doi: 10.1093/oxfordjournals.schbul.a007036. [DOI] [PubMed] [Google Scholar]
- 22.Brewer WJ, Francey SM, Wood SJ, et al. Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. Am J Psychiatry. 2005;162:71–78. doi: 10.1176/appi.ajp.162.1.71. [DOI] [PubMed] [Google Scholar]
- 23.Lencz T, Smith CW, McLaughlin D, et al. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry. 2005;59:863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Seidman LJ, Giuliano AJ, Smith CW, et al. Neuropsychological functioning in adolescents and young adults at genetic risk for schizophrenia and affective psychoses: results from the Harvard and Hillside Adolescent High Risk Studies. Schizophr Bull. 2006;32:507–524. doi: 10.1093/schbul/sbj078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon AE, Roth B, Zmilacher S, Isler E, Umbricht D. Developing services for the early detection of psychosis: a critical consideration of the current state of the art. Eur Child Adolesc Psychiatry. 2006 doi: 10.1007/s00787-006-0579-7. In press. [DOI] [PubMed] [Google Scholar]
- 26.Rapoport JL, Giedd JN, Blumenthal J, et al. Progressive cortical change during adolescence in childhood-onset schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:649–654. doi: 10.1001/archpsyc.56.7.649. [DOI] [PubMed] [Google Scholar]
- 27.Klosterkötter J, Schultze-Lutter F, Wieneke A, Picker H, Steinmeyer EM. Introduction and reliability of the first version of the Schizophrenia Prediction Instrument (SPI-A) Schizophr Res. 2001;49:4. [Google Scholar]
- 28.Miller TJ, McGlashan TH, Woods SW, et al. Symptom assessment in schizophrenic prodromal states. Psychiatr Q. 1999;70:273–287. doi: 10.1023/a:1022034115078. [DOI] [PubMed] [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. American Psychiatric Association, Washington, DC. 1994 [Google Scholar]
- 30.Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Lehrl S. Mehrfachwortschatz-Test (MWT-B) Balingen, Germany: Spitta Verlag; 1999. [Google Scholar]
- 32.Reitan RM. TMT, Trail Making Test A & B. Reitan Neuropsychology Laboratory, South Tucson, AR, 1992. [Google Scholar]
- 33.Aschenbrenner S, Tucha O, Lange KW. Regensburger Wortflüssigkeits-Test (RWT) Göttingen, Germany: Hogrefe Verlag; 2000. [Google Scholar]
- 34.Helmstaedter C, Lendt M, Lux S. Verbaler Lern- und Merkfähigkeitstest, VLMT. Göttingen, Germany: Beltz Test GmbH; 2001. [Google Scholar]
- 35.Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997;54:159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- 36.Zimmermann P, Fimm B. Testbatterie zur Aufmerksamkeitsprüfung Version 1.7. Herzogenrath, Germany: Psytest; 2000. [Google Scholar]
- 37.Grant DA. Computer version of the Wisconsin Card Sorting Test, WCST. Odessa, Fla: Psychological Assessment Resources; 2000. [Google Scholar]
- 38.Weiser M, Reichenberg A, Rabinowitz J, et al. Cognitive performance of male adolescents is lower than controls across psychiatric disorders: a population-based study. Acta Psychiatr Scand. 2004;110:471–475. doi: 10.1111/j.1600-0447.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 39.Doyle AE, Biederman J, Seidman LJ, Weber W, Faraone SV. Diagnostic efficiency of neuropsychological test scores for discriminating boys with and without attention deficit-hyperactivity disorder. J Consult Clin Psychol. 2000;68:477–488. doi: 10.1037/0022-006X.68.3.477. [DOI] [PubMed] [Google Scholar]
- 40.Pantelis C, Stuart GW, Nelson HE, Robbins TW, Barnes TRE. Spatial working memory deficits in schizophrenia: relationship with tardive dyskinesia and negative symptoms. Am J Psychiatry. 2001;158:1276–1285. doi: 10.1176/appi.ajp.158.8.1276. [DOI] [PubMed] [Google Scholar]
- 41.Niendam TA, Bearden CE, Johnson JK, et al. Neurocognitive performance and functional disability in the psychosis prodrome. Schizophr Res. 2006;84:100–111. doi: 10.1016/j.schres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Hutton SB, Puri BK, Duncan LJ, Robbins TW, Barnes TRE, Joyce EM. Executive function in first-episode schizophrenia. Psychol Med. 1998;28:463–473. doi: 10.1017/s0033291797006041. [DOI] [PubMed] [Google Scholar]
- 43.Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr Bull. 1994;20:31–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- 44.Oie M, Sunde K, Rund BR. Contrasts in memory functions between adolescents with schizophrenia or ADHD. Neuropsychologia. 1999;37:1351–1358. doi: 10.1016/s0028-3932(99)00043-3. [DOI] [PubMed] [Google Scholar]
- 45.Cosway R, Byrne M, Clafferty R, et al. Sustained attention in young people at high risk for schizophrenia. Psychol Med. 2002;32:277–286. doi: 10.1017/s0033291701005050. [DOI] [PubMed] [Google Scholar]
- 46.Kravariti E, Morris RG, Rabe-Hesketh S, Murray RM, Frangou S. The Maudsley Early-Onset Schizophrenia Study: cognitive function in adolescent-onset schizophrenia. Schizophr Res. 2003;65:95–103. doi: 10.1016/s0920-9964(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 47.Cattapan-Ludewig K, Hilti CC, Ludewig S, Vollenweider FX, Feldon J. Rapid visual information processing in schizophrenic patients: the impact of cognitive load and duration of stimulus presentation. A pilot study. Neuropsychobiology. 2005;52:130–134. doi: 10.1159/000087558. [DOI] [PubMed] [Google Scholar]
- 48.Gal G, Mendlovic S, Bloch Y, et al. Learned irrelevance is disrupted in first-episode but not chronic schizophrenia patients. Behav Brain Res. 2005;159:267–275. doi: 10.1016/j.bbr.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 49.Simon AE, Giacomini V, Ferrero F, Mohr S. Dysexecutive syndrome and social adjustment in schizophrenia. Aust N Z J Psychiatry. 2003;37:340–346. doi: 10.1046/j.1440-1614.2003.01186.x. [DOI] [PubMed] [Google Scholar]
- 50.Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychol Rev. 2003;13:43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- 51.Keefe RS. The contribution of neuropsychology to psychiatry. Am J Psychiatry. 1995;152:6–15. doi: 10.1176/ajp.152.1.6. [DOI] [PubMed] [Google Scholar]
- 52.Pantelis C, Yücel M, Wood S, McGorry PD, Velakoulis D. The timing and functional consequences of structural brain abnormalities in schizophrenia. Neurosci News. 2001;4:36–46. [Google Scholar]
- 53.Toulopoulou T, Morris RG, Rabe-Hesketh S, Murray RM. Selectivity of verbal memory deficit in schizophrenic patients and their relatives. Am J Med Genet B Neuropsychiatr Genet. 2003;116:1–7. doi: 10.1002/ajmg.b.10027. [DOI] [PubMed] [Google Scholar]
- 54.Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalitites before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 55.Correll CU, Lencz T, Smith CW, et al. Prospective study of adolescents with subsyndromal psychosis: characteristics and outcome. J Child Adolesc Psychopharmacol. 2005;15:418–433. doi: 10.1089/cap.2005.15.418. [DOI] [PubMed] [Google Scholar]