Abstract
Catechol-O-methyl transferase (COMT) is a catabolic enzyme involved in the degradation of a number of bioactive molecules; of principal interest to psychiatry, these include dopamine. The enzyme is encoded by the COMT gene. COMT is located (along with 47 other genes) in a fragment of chromosome 22q11 which when deleted results in a complex syndrome, the psychiatric manifestations of which include schizophrenia and other psychoses. These 2 observations have placed COMT near the top of a rather long list of plausible candidate genes for schizophrenia. The ability to test the hypothesis that COMT might be a susceptibility gene for schizophrenia has been simplified in principle by the existence of a valine-to-methionine (Val/Met) polymorphism which results respectively in high and low activity forms of the enzyme. Given the unequivocal effect of this polymorphism on the function of COMT, and the evidence for a critical role for dopamine in the pathophysiology and treatment of psychosis, there are strong prior expectations that Val/Met influences susceptibility to schizophrenia as well as other psychiatric phenotypes. Indeed the Val/Met polymorphism has become the most widely studied polymorphism in psychiatry. In this review, we consider the evidence for and against the involvement of COMT in schizophrenia. The current data allow us to virtually exclude a simple relationship between schizophrenia and the Val/Met variant previously thought to dominate COMT function. However, recent data suggest a more complex pattern of genetic regulation of COMT function beyond that attributable to the Val/Met locus. Moreover, it is also clear that there is a complex nonlinear relationship between dopamine availability and brain function. These 2 factors, allied to phenotypic complexity within schizophrenia, make it difficult to draw strong conclusions regarding COMT in schizophrenia. Nevertheless, emerging research that takes greater account of all these levels of complexity is beginning to provide tantalizing, but far from definitive, support for the view that COMT influences susceptibility to at least some forms of psychosis.
Keywords: schizophrenia, COMT, prefrontal cortex, association, cognition, psychosis
Introduction
The traditional hypothesis, widely held for more than half a century, is that increased dopamine (DA) function is central to the pathophysiology of schizophrenia.1,2 More recently, the dopamine hypothesis has been revised, with excess mesolimbic dopamine function being proposed to be secondary to low dopamine function in the prefrontal cortex (PFC).3,4. In the context of either hypothesis, the COMT gene encoding catechol-O-methyl transferase (COMT) is a clear functional candidate gene for schizophrenia because COMT is involved in the catabolic clearance of dopamine.5 COMT maps to chromosome 22q11.2 and encodes 2 main well-characterized protein isoforms that display an altered affinity and capacity for their substrate. In most assayed tissues, a soluble cytoplasmic (S-COMT) isoform predominates,6 whereas in brain, a longer membrane-bound form (MB-COMT) is the major species.7 The 2 major protein isoforms differ by the inclusion of an extra 50 hydrophobic amino acids in MB-COMT. MB-COMT has approximately a 10-fold greater affinity for dopamine and noradrenaline than S-COMT, suggesting that MB-COMT is better suited to metabolizing catecholamines, including dopamine, at the physiological levels found in brain.8 Recently, a third isoform, which appears to be a larger variant of MB-COMT, has been reported,9,10 but as of yet, its functional properties are unknown.
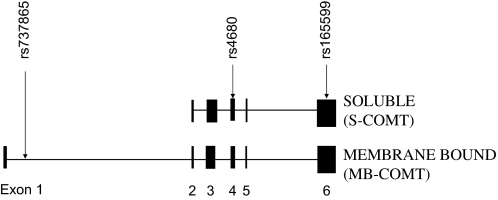
Within the coding sequence of COMT is a common G>A polymorphism (rs4680) (see figure 1) that produces a valine-to-methionine (Val/Met) substitution at codons 108 and 158 of S-COMT and MB-COMT, respectively.11 The polymorphism is usually referred to simply as the Val/Met locus but is also known by the reference sequence identification code rs4680 (previously also known as rs165688). The amino acid change results in altered activity of both S-COMT11,12 and MB-COMT13 and has generally been thought to be the main source of genetic variation in COMT enzyme activity. The Val (variant G) and Met (variant A) alleles, respectively, confer high and low activity. Based upon the traditional dopamine hypothesis, if COMT is an important regulator of dopamine function, it would be postulated that the low-activity Met variant would be associated with schizophrenia. It should be also noted that in Korean and Japanese populations, a second functional high-activity Alanine (low activity)-Serine (high activity) polymorphism exists (rs6267),14 a variant not found in Caucasian and African Americans who all (as far as is known) carry the Alanine allele.
Fig. 1.
Schematic diagram to show the 2 major protein isforms of the COMT produced and the positions of the 3 polymorphisms used in the study of Shifman et al.49
COMT and Prefrontal Dopamine Function
Although expressed widely in mammalian brain, the importance of COMT in dopamine clearance and function varies by brain region. In some regions, for example striatum and nucleus accumbens, COMT appears to be a minor player in dopamine clearance compared with neuronal synaptic uptake by the dopamine transporter and subsequent monoamine oxidase metabolism.15 However, 1 region (and there may be others) where this does not apply is the PFC. These anatomical differences in the importance of COMT may reflect broader anatomical and functional variation between dopaminergic neurons depending on their nuclei of origin. Thus, in contrast to the nigrostriatal pathway, dopamine transporters are sparse at the dopaminergic synaptic termini of the mesocortical and mesothalamic pathways.16,17 As a result, diffusion, extrasynaptic function of DA, and COMT clearance may be more important in areas served by those pathways. The importance of COMT for dopaminergic clearance in the PFC has been confirmed in COMT knockout mice18 and by the effects on dopamine of pharmacological inhibition of COMT.19
Dopamine has been shown by pharmacological manipulation to play an important role in PFC-mediated cognition.20–22 Given this, if as suggested above, COMT is important in regulating PFC dopamine, a simple prediction would be that the Val/Met locus would show association with indices of PFC function. Such a relationship has repeatedly been demonstrated, first by Egan et al,23 using the Wisconsin Card Sorting Test and functional magnetic resonance imaging, and subsequently by others using a wide range of tasks. The detail of these studies is beyond the scope of this review, but 2 recent meta-analyses concluded broadly in favor of an association of very small effect.24,25 However, in neither were the findings significant after exclusion of the original study,23 suggesting that the relationship between variation in PFC functioning and COMT may not be quite as robust as is widely thought. More recently, a large prospective UK study has provided evidence that the Val/Met variant influences executive function and IQ in children, but, because the findings were restricted to males, it is not entirely clear how they tally with the earlier observations.25 Thus, while overall the data still support the functional relevance of COMT polymorphisms to individual differences in PFC function, some uncertainties remain in regard to the size of this effect and the question of sex differences. Interestingly, another study has recently revealed that haplotypes at COMT constructed of markers in addition to that of the Val/Met show a stronger relationship with prefrontal inefficiency of working memory than did the Val/Met locus alone.26 As will be discussed later, this most likely suggests that COMT function is influenced by polymorphisms other than the Val/Met as has been claimed by others.13,27,28
Genetic Evidence for COMT as a Schizophrenia Susceptibility Gene
The region of chromosome 22q11.2 containing COMT is also the location of a relatively common microdeletion syndrome which goes by several names, most commonly, in the psychiatric literature, velocardiofacial syndrome (VCFS) or 22q11Del syndrome. The microdeletions, typically about 3 Mb in size and encompassing 48 genes, result in a wide range of phenotypes including distinctive dysmorphology, congenital heart disease, and cognitive impairments that range in severity from minimal to severe.29 Interestingly, an increased prevalence in a broad spectrum of psychiatric disorders in children with VCFS has been reported, including anxiety, mood disorders, obsessive-compulsive disorder, and attention-deficit disorder.30–34 Of more relevance to the present discussion, adults with VCFS have high rates of psychosis,33,35–37 the majority of cases fulfilling the diagnostic criteria for schizophrenia with an estimated prevalence of 25%.38 Thus, in addition to the functional candidacy of COMT, the gene is also a positional candidate gene for schizophrenia. However, it should be noted that COMT is only one, albeit a priori the strongest, of several candidates within the deleted region.39 Weaker positional support for COMT also comes from linkage studies,40,41 although the implicated region of linkage is much broader than the 22q11Del region and therefore contains even more genes. Finally, the findings of association between COMT and PFC function may also be of relevance to schizophrenia because abnormal function of the PFC is a prominent feature in schizophrenia42,43 for which it has been proposed as a trait marker.23
In terms of position and function then, there are probably no genes with a better a priori case for involvement in schizophrenia than COMT. This, together with the existence of the functional polymorphism, has made the Val/Met polymorphism an almost irresistible target for genetic investigation, and combined worldwide, many thousands of cases and controls have now been studied. As would be expected for a putative risk allele of small effect, the numerous studies include positive and negative findings.44–49 This is potentially consistent with a true association where replication is hampered by lack of power. However, strongly arguing against this interpretation, 3 recent meta-analyses of the published case-control literature24,50,51 found no significant effect at the Val/Met locus. Moreover, large studies that have individually made a major contribution to the total number of samples studied worldwide but which were not included in the most recent meta-analysis24 also failed to find evidence for association in European52 and Asian51 populations. Based upon these data, the conclusion must be against a single locus effect of the Val/Met polymorphism on schizophrenia risk.
If it is correct that at a population level, the Val/Met locus is the major determinant of individual differences in COMT activity in brain, the data can be interpreted as providing no support for either the classic hypothesis that schizophrenia results from enhanced dopaminergic neurotransmission or for the modified dopamine hypothesis based upon low dopamine function in the PFC. However, there is now strong evidence for a more complex inverted “U”-shaped relationship between dopamine and at least some aspects of PFC function, with optimal functioning occurring within a narrow range of dopamine activity. Under this model, too little or too much dopamine has relatively deleterious effects.53,54 The model also implies that at the level of an individual, the effect at the Val/Met locus on PFC function depends upon the sum of the non-COMT-related influences on PFC dopamine levels. For individuals to the left of this curve, the low-activity allele may be beneficial (allowing increased dopamine activity), whereas for individuals to the right (too much dopamine), the high-activity Val allele should be beneficial. Nevertheless, we would still expect the direction of association to be dictated (within broadly similar populations) by the population mean position on the curve and therefore a consensus for a particular allele. The Val/Met data are then no more supportive of that model of schizophrenia than they are of the simpler dose-response relationships.
The conclusions reached above are, however, predicated on 2 main assumptions. First, within a given population, cases of schizophrenia can be ascertained that represent some baseline mean level of PFC dopamine activity that is typical of most cases. Second, that the Val/Met locus is the source of most of the genetic variance in COMT function. Regarding the first assumption, if somehow different strategies for ascertaining patients result in recruitment of samples that have different mean positions on this inverted U curve, then some samples may tend to show association to the Val allele and others to the Met. Such an ascertainment problem might conceivably occur if PFC dopamine influences symptom profile, probability of in-patient care, course, response to treatment, the degree to which subjects abuse drugs, and so on, and the net effect could fatally undermine meta-analyses based upon direction of effect. An effect might, however, be identified through alternative meta-analyses strategies based on combining P values for example. Although there is no convincing evidence at present that such an effect of phenotype is operating, it seems plausible given the repeated reports of association between COMT and almost every imaginable psychiatric phenotype. Importantly, these include reports of stronger association between COMT and unconventionally labeled psychosis-mood spectrum disorders55–57 and response to medication.58,59 Given the large numbers of studies of COMT undertaken, it is currently unclear whether these reports are substantially above chance levels, but nevertheless, this remains a potentially fruitful avenue for further exploration.
There is greater evidence suggesting that the second assumption is false, namely, that there are functional alleles in COMT beyond the Val/Met (and in some populations, the Ala/Ser). Several studies have now shown that variants that do not change the amino acid sequence of COMT can influence COMT mRNA expression,27 enzyme activity,13 and, very recently, the rate of COMT mRNA translation by altering mRNA structure.28 These findings in principle allow for highly complex and largely unpredictable patterns of association between disease status and the Val/Met locus in different populations as a result of population differences in allelic heterogeneity at the risk alleles, patterns of LD between the tested and true susceptibility alleles,60 allele frequencies at both sets of loci, phenotypic variation relevant to the associated alleles, or exposure to environmental variables with which the various risk alleles interact. The potential for this to influence the direction of reported associations in different populations has specifically been demonstrated for COMT.61 More complex still, it is now clear from exact mathematical analyses of the tripartite relationships between tag markers (markers that indirectly extract information from a true risk variant via linkage diequilibrium), true susceptibility variants, and affected status that, where the true effect size of a susceptibility allele is weak, opposite alleles may be associated with disease even in populations with similar LD measures, allele frequencies, and identical effect sizes at the functional loci.62
An even higher order of complexity is to be expected if risk alleles at the gene interact with other genes or with environmental factors. Several gene-gene interactions have been reported for COMT though they are not yet independently replicated.63 In mice, functional interaction has also been reported between COMT and PRODH, another putative susceptibility gene within the 22q11Del region.64 As yet, there is no genetic evidence that such an interaction increases risk of schizophrenia,65 though a recent study has reported that, in individuals with 22q11DS, risk of psychosis was increased in individuals who were hemizygous for the Met allele and who were hyperprolinaemic, though numbers were small and statistical significance was only achieved by the inclusion of 2 cases with autism in the “psychosis” group.66 Evidence for gene-environment interaction involving the COMT Val/Met polymorphism and schizophrenia has been obtained from a longitudinal study of the Dunedin birth cohort. Adult carriers of the COMT Val allele were most likely to exhibit psychotic symptoms and to develop schizophreniform disorder if they used cannabis, whereas cannabis use had no such adverse influence on individuals with 2 copies of the Met allele.67 The authors argue that their findings are unlikely to reflect reverse causation and this observation is consistent with COMT modulating the psychotogenic effects of cannabis exposure. Replication of this potentially important observation is eagerly awaited.
The premise that other functional alleles may be operating at COMT has led some investigators to expand their analysis to additional markers and haplotypes rather than the Val/Met locus alone. The first of these68 found no evidence for association, although haplotypes were not constructed. In contrast, Li et al69 reported stronger evidence for a multimarker haplotype than the Val/Met locus alone. The strongest evidence for COMT to date was reported in a large sample of Ashkenazi Jews49 in which modest evidence was found for association between schizophrenia and the Val allele but much stronger evidence was found with various combinations of haplotypes. This is compatible with the existence of alleles that modify the influence of the Val/Met locus, as is the observation that 1 haplotype carrying the Val allele was more common in cases, while 3 other haplotypes carrying the Val allele were underrepresented. Gender effects were also reported although these are difficult to interpret because much of the effect was driven by differences in the controls, not the cases. Since then, several studies have reported evidence for association at COMT that appears stronger with haplotypes than with the Val/Met locus alone.70–72 However, the largest study reported to date,52 comprising a total of around 1200 cases, did not find any evidence in favor of association. It should be noted that the latter study was designed to specifically test the associated haplotypes reported by Shifman et al49 that were also associated with altered gene expression27 rather than to comprehensively extract information from all variants in the gene.
The increased support for association from haplotype-based studies of schizophrenia provides some encouragement for the hypothesis that COMT is a susceptibility gene for schizophrenia, but again there are complexities. While for reasons indicated above it is reasonable to suspend the widely assumed requirement that replication studies must implicate the same alleles and haplotypes to provide support for association, this is no excuse for regarding any study with a nominally significant marker/haplotype as supportive. Instead, we must restrict claims of “significance” to those studies where any allele or haplotype survives appropriate correction for multiple testing (based upon the intended number of markers) and is based on a well-designed quality-controlled study. The latter point is particularly important in haplotype-based studies where trivial amounts of genotyping error can considerably inflate the false-positive rate.73 As only one of the positive studies, the smallest72 has formally addressed the multiple-testing issue, and only 1 each of the positive71 and negative52 studies has addressed the potential impact of genotyping error; the impact of multiple testing and genotyping error on the extant data is unknown and one can only gain an impression of significance. Our own judgment about the statistical evidence is that the findings are suggestive, but there are insufficient studies that would be clearly significant when corrected for multiple testing to draw stronger conclusions.
Conclusions
Because of its function, its position, and the apparent simplicity of the extent of the genetic variation influencing its function, COMT has become one of the most studied genes in psychiatric disorders. However, with respect to schizophrenia, it is still unclear whether the findings can all be written off to chance and technical issues in study design and implementation or whether they reflect the existence of a complex relationship between multiple functional variants of small effect size, the genetic markers used to tag them, and a heterogeneous phenotype, only some aspects of which are influenced by COMT. That no robust conclusions about the relationship between COMT and schizophrenia can be drawn after so many samples have been studied stresses the need for studies to adopt a more systematic approach to reporting data, with in particular more attention being paid to aspects of the phenotype, QC issues, and correction for multiple testing preferably using gene-wide tests of significance.74 That so much difficulty can be generated by a small gene with a clear and highly plausible functional relationship with schizophrenia also highlights the challenges that will face investigators tackling the newer generation of putative risk genes identified by positional cloning, not to mention the plethora of claimed associations that can be expected to emerge from whole-genome association studies. In most cases, researchers will have neither the luxury of known functional variation nor an obvious putative functional relationship with disease.
Acknowledgments
We are grateful to the Medical Research Council, which supports most of our research into schizophrenia via a program grant. We are also grateful to the Wellcome Trust and the National Institute of Mental health for their project grant support.
References
- 1.Van Rossum JM. The significance of dopamine-receptor blockade for the mechanism of action of neuroleptic drugs. Arch Int Pharmacodyn Ther. 1966;160:492–49. [PubMed] [Google Scholar]
- 2.Carlsson A. Mechanism of Action of Neuroleptic Drugs. New York, NY: Raven Press; 1978. [Google Scholar]
- 3.Daniel DG, Berman KF, Weinberger DR. The effect of apomorphine on regional cerebral blood flow in schizophrenia. J Neuropsychiatry Clin Neurosci. 1989;1:377–384. doi: 10.1176/jnp.1.4.377. [DOI] [PubMed] [Google Scholar]
- 4.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 5.Axelrod J, Tomchick R. Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem. 1958;233:702–705. [PubMed] [Google Scholar]
- 6.Jeffery DR, Roth JA. Characterization of membrane-bound and soluble catechol-O-methyltransferase from human frontal cortex. J Neurochem. 1984;42:826–832. doi: 10.1111/j.1471-4159.1984.tb02755.x. [DOI] [PubMed] [Google Scholar]
- 7.Tenhunen J, Salminen M, Lundstrom K, et al. Genomic Organization of the human catechol O-methyltransferase gene and its expression from 2 distinct promoters. Eur J Biochem. 1994;223:1049–1059. doi: 10.1111/j.1432-1033.1994.tb19083.x. [DOI] [PubMed] [Google Scholar]
- 8.Roth JA. Membrane-bound catechol-O-methyltransferase: a reevaluation of its role in the O-methylation of the catecholamine neurotransmitters. Rev Physiol Biochem Pharmacol. 1992;120:1–29. doi: 10.1007/BFb0036121. [DOI] [PubMed] [Google Scholar]
- 9.Tunbridge EM, Weinberger DR, Harrison PJ. A novel protein isoform of catechol O-methyltransferase (COMT): brain expression analysis in schizophrenia and bipolar disorder and effect of Val158Met genotype. Mol Psychiatry. 2006;11:116–117. doi: 10.1038/sj.mp.4001767. [DOI] [PubMed] [Google Scholar]
- 10.Tunbridge EM, Weickert CS, Kleinman JE, et al. Catechol-o-methyltransferase enzyme activity and protein expression in human prefrontal cortex across the postnatal lifespan. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl032. doi:10.1093/cercor/bhl032. [DOI] [PubMed] [Google Scholar]
- 11.Lachman HM, Papolos DF, Saito T, et al. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Lotta T, Vidgren J, Tilgmann C, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Lipska BK, Halim N, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SG, Joo Y, Kim B, et al. Association of Ala72Ser polymorphism with COMT enzyme activity and the risk of schizophrenia in Koreans. Hum Genet. 2005;116:319–328. doi: 10.1007/s00439-004-1239-y. [DOI] [PubMed] [Google Scholar]
- 15.Huotari M, Gogos JA, Karayiorgou M, et al. Brain catecholamine metabolism in catechol-O-methyltransferase (COMT)-deficient mice. Eur J Neurosci. 2002;15:246–256. doi: 10.1046/j.0953-816x.2001.01856.x. [DOI] [PubMed] [Google Scholar]
- 16.Sesack SR, Hawrylak VA, Matus C, et al. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melchitzky DS, Erickson SL, Lewis DA. Dopamine innervation of the monkey mediodorsal thalamus: location of projection neurons and ultrastructural characteristics of axon terminals. Neuroscience. 2006;143:1021–1030. doi: 10.1016/j.neuroscience.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 18.Gogos JA, Morgan M, Luine V, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- 21.Jentsch JD, Andrusiak E, Tran A, Bowers MB, Jr, Roth RH. Delta 9-tetrahydrocannabinol increases prefrontal cortical catecholaminergic utilization and impairs spatial working memory in the rat: blockade of dopaminergic effects with HA966. Neuropsychopharmacology. 1997;16:426–432. doi: 10.1016/S0893-133X(97)00018-3. [DOI] [PubMed] [Google Scholar]
- 22.Gasparini M, Fabrizio E, Bonifati V, Meco G. Cognitive improvement during Tolcapone treatment in Parkinson's disease. J Neural Transm. 1997;104:887–894. doi: 10.1007/BF01285556. [DOI] [PubMed] [Google Scholar]
- 23.Egan MF, Goldberg TE, Kolachana BS, et al. Effect of Comt Val(108/158) Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munafo MR, Bowes L, Clark TG, Flint J. Lack of association of the COMT (Val158/108 Met) gene and schizophrenia: a meta-analysis of case-control studies. Mol Psychiatry. 2005;10:765–770. doi: 10.1038/sj.mp.4001664. [DOI] [PubMed] [Google Scholar]
- 25.Barnett JH, Heron J, Ring SM, et al. Gender-specific effects of the catechol-O-methyltransferase Val108/158Met polymorphism on cognitive function in children. Am J Psychiatry. 2007;164:142–149. doi: 10.1176/ajp.2007.164.1.142. [DOI] [PubMed] [Google Scholar]
- 26.Meyer-Lindenberg A, Nichols T, Callicott JH, et al. Impact of complex genetic variation in COMT on human brain function. Mol Psychiatry. 2006;11:867–877. doi: 10.1038/sj.mp.4001860. [DOI] [PubMed] [Google Scholar]
- 27.Bray NJ, Buckland PR, Williams NM, et al. A haplotype implicated in schizophrenia susceptibility is associated with reduced COMT expression in human brain. Am J Hum Genet. 2003;73:152–161. doi: 10.1086/376578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nackley AG, Shabalina SA, Tchivileva IE, et al. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314:1930–1933. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- 29.Scambler PJ. The 22q11 deletion syndromes. Hum Mol Genet. 2000;9:2421–2426. doi: 10.1093/hmg/9.16.2421. [DOI] [PubMed] [Google Scholar]
- 30.Swillen A, Devriendt K, Legius E, et al. Intelligence and psychosocial adjustment in velocardiofacial syndrome: a study of 37 children and adolescents with VCFS. J Med Genet. 1997;34:453–458. doi: 10.1136/jmg.34.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerdes M, Solot C, Wang PP, et al. Cognitive and behavior profile of preschool children with chromosome 22q11.2 deletion. Am J Med Genet. 1999;85:127–133. [PubMed] [Google Scholar]
- 32.Golding-Kushner KJ, Weller G, Shprintzen RJ. Velo-cardio-facial syndrome: language and psychological profiles. J Craniofac Genet Dev Biol. 1985;5:259–266. [PubMed] [Google Scholar]
- 33.Papolos DF, Faedda GL, Veit S, et al. Bipolar spectrum disorders in patients diagnosed with velo-cardio-facial syndrome: does a hemizygous deletion of chromosome 22q11 result in bipolar affective disorder? Am J Psychiatry. 1996;153:1541–1547. doi: 10.1176/ajp.153.12.1541. [DOI] [PubMed] [Google Scholar]
- 34.Feinstein C, Eliez S, Blasey C, Reiss AL. Psychiatric disorders and behavioral problems in children with velocardiofacial syndrome: usefulness as phenotypic indicators of schizophrenia risk. Biol Psychiatry. 2002;51:312–318. doi: 10.1016/s0006-3223(01)01231-8. [DOI] [PubMed] [Google Scholar]
- 35.Shprintzen RJ, Goldberg R, Golding-Kushner KJ, Marion RW. Late-onset psychosis in the velo-cardio-facial syndrome. Mol Psychiatry. 2005;10:765–770. doi: 10.1002/ajmg.1320420131. [DOI] [PubMed] [Google Scholar]
- 36.Pulver AE, Nestadt G, Goldberg R, et al. Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. J Nerv Ment Dis. 1994;182:476–478. doi: 10.1097/00005053-199408000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Bassett AS, Hodgkinson K, Chow EWC, et al. 22q11 deletion syndrome in adults with schizophrenia. Am J Med Genet. 1998;81:328–337. [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- 39.Owen MJ, Craddock N, O'Donovan MC. Schizophrenia: genes at last? Trends Genet. 2005;21:518–525. doi: 10.1016/j.tig.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Badner JA, Gershon ES. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry. 2002;7:405–411. doi: 10.1038/sj.mp.4001012. [DOI] [PubMed] [Google Scholar]
- 41.Lewis CM, Levinson DF, Wise LH, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: schizophrenia. Am J Hum Genet. 2003;73:34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cannon TD, Huttunen MO, Lonnqvist J, et al. The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. Am J Hum Genet. 2000;67:369–382. doi: 10.1086/303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldberg TE, Ragland JD, Torrey EF, et al. Neuropsychological assessment of monozygotic twins discordant for schizophrenia. Arch Gen Psychiatry. 1990;47:1066–1072. doi: 10.1001/archpsyc.1990.01810230082013. [DOI] [PubMed] [Google Scholar]
- 44.Liou YJ, Tsai SJ, Hong CJ, Wang YC, Lai IC. Association analysis of a functional catechol-o-methyltransferase gene polymorphism in schizophrenic patients in Taiwan. Neuropsychobiology. 2001;43:11–14. doi: 10.1159/000054858. [DOI] [PubMed] [Google Scholar]
- 45.Daniels JK, Williams NM, Williams J, et al. No evidence for allelic association between schizophrenia and a polymorphism determining high or low catechol O-methyltransferase activity. Am J Psychiatry. 1996;153:268–270. doi: 10.1176/ajp.153.2.268. [DOI] [PubMed] [Google Scholar]
- 46.Strous RD, Bark N, Woerner M, Lachman HM. Lack of association of a functional catechol-O-methyltransferase gene polymorphism in schizophrenia. Biol Psychiatry. 1997;41:493–495. doi: 10.1016/s0006-3223(96)00474-x. [DOI] [PubMed] [Google Scholar]
- 47.Norton N, Kirov G, Zammit S, et al. Schizophrenia and functional polymorphisms in the MAOA and COMT genes: no evidence for association or epistasis. Am J Med Genet. 2002;114:491–496. doi: 10.1002/ajmg.10517. [DOI] [PubMed] [Google Scholar]
- 48.Wonodi I, Stine OC, Mitchell BD, Buchanan RW, Thaker GK. Association between Val108/158 Met polymorphism of the COMT gene and schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2003;120:47–50. doi: 10.1002/ajmg.b.20037. [DOI] [PubMed] [Google Scholar]
- 49.Shifman S, Bronstein M, Sternfeld M, et al. A highly significant association between a Comt haplotype and schizophrenia. Am J Hum Genet. 2002;71:1296–1302. doi: 10.1086/344514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glatt SJ, Faraone SV, Tsuang MT. Association between a functional catechol O-methyltransferase gene polymorphism and schizophrenia: meta-analysis of case-control and family-based studies. Am J Psychiatry. 2003;160:469–476. doi: 10.1176/appi.ajp.160.3.469. [DOI] [PubMed] [Google Scholar]
- 51.Fan JB, Zhang CS, Gu NF, et al. Catechol-O-methyltransferase gene Val/Met functional polymorphism and risk of schizophrenia: a large-scale association study plus meta-analysis. Biol Psychiatry. 2005;57:139–144. doi: 10.1016/j.biopsych.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 52.Williams HJ, Glaser B, Williams NM, et al. No association between schizophrenia and polymorphisms in COMT in two large samples. Am J Psychiatry. 2005;162:1736–1738. doi: 10.1176/appi.ajp.162.9.1736. [DOI] [PubMed] [Google Scholar]
- 53.Goldman-Rakic PS, Muly EC, III, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 54.Mattay VS, Goldberg TE, Fera F, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Craddock N, Owen MJ, O'Donovan MC. The catechol-O-methyl transferase (COMT) gene as a candidate for psychiatric phenotypes: evidence and lessons. Mol Psychiatry. 2006;11:446–458. doi: 10.1038/sj.mp.4001808. [DOI] [PubMed] [Google Scholar]
- 56.DeRosse P, Funke B, Burdick KE, et al. COMT genotype and manic symptoms in schizophrenia. Schizophr Res. 2006;87:28–31. doi: 10.1016/j.schres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 57.McClay JL, Fanous A, van den Oord EJ, et al. Catechol-O-methyltransferase and the clinical features of psychosis. Am J Med Genet B Neuropsychiatr Genet. 2006;141:935–938. doi: 10.1002/ajmg.b.30401. [DOI] [PubMed] [Google Scholar]
- 58.Bertolino A, Caforio G, Blasi G, et al. Interaction of COMT (Val(108/158)Met) genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am J Psychiatry. 2004;161:1798–1805. doi: 10.1176/ajp.161.10.1798. [DOI] [PubMed] [Google Scholar]
- 59.Weickert TW, Goldberg TE, Mishara A, et al. Catechol-O-methyltransferase val108/158met genotype predicts working memory response to antipsychotic medications. Biol Psychiatry. 2004;56:677–682. doi: 10.1016/j.biopsych.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 60.Palmatier MA, Pakstis AJ, Speed W, et al. COMT haplotypes suggest P2 promoter region relevance for schizophrenia. Mol Psychiatry. 2004;9:859–870. doi: 10.1038/sj.mp.4001496. [DOI] [PubMed] [Google Scholar]
- 61.Lin PI, Vance JM, Pericak-Vance MA, Martin ER. No gene is an island: the flip-flop phenomenon. Am J Hum Genet. 2007;80:531–538. doi: 10.1086/512133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moskvina V, O'Donovan MC. Detailed analysis of the relative power of direct and indirect association studies and the implications for their interpretation. Hum Hered. 2007 doi: 10.1159/000101424. In press. [DOI] [PubMed] [Google Scholar]
- 63.Nicodemus KK, Kolachana BS, Vakkalanka R, et al. Evidence for statistical epistasis between catechol-O-methyltransferase (COMT) and polymorphisms in RGS4, G72 (DAOA), GRM3, and DISC1: influence on risk of schizophrenia. Hum Genet. 2007;120:889–906. doi: 10.1007/s00439-006-0257-3. [DOI] [PubMed] [Google Scholar]
- 64.Paterlini M, Zakharenko SS, Lai WS, et al. Transcriptional and behavioral interaction between 22q11.2 orthologs modulates schizophrenia-related phenotypes in mice. Nat Neurosci. 2005;8:1586–1594. doi: 10.1038/nn1562. [DOI] [PubMed] [Google Scholar]
- 65.Glaser B, Moskvina V, Kirov G, et al Analysis of ProDH, COMT and ZDHHC8 risk variants does not support individual or interactive effects on schizophrenia susceptibility. Schizophr Res. 2006;87:21–27. doi: 10.1016/j.schres.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 66.Raux G, Bumsel E, Hecketsweiler B, et al. Involvement of hyperprolinemia in cognitive and psychiatric features of the 22q11 deletion syndrome. Hum Mol Genet. 2007;16:83–91. doi: 10.1093/hmg/ddl443. [DOI] [PubMed] [Google Scholar]
- 67.Caspi A, Moffitt TE, Cannon M, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 68.Karayiorgou M, Gogos JA, Galke BL, et al. Identification of sequence variants and analysis of the role of the catechol-O-methyl-transferase gene in schizophrenia susceptibility. Biol Psychiatry. 1998;43:425–431. doi: 10.1016/s0006-3223(97)00202-3. [DOI] [PubMed] [Google Scholar]
- 69.Li T, Ball D, Zhao J, et al. Family-based linkage disequilibrium mapping using SNP marker haplotypes: application to a potential locus for schizophrenia at chromosome 22q11. Mol Psychiatry. 2000;5:77–84. doi: 10.1038/sj.mp.4000638. [DOI] [PubMed] [Google Scholar]
- 70.Chen X, Wang X, AF O'neill, et al. Variants in the catechol-O-methyltransferase (Comt) gene are associated with schizophrenia in Irish high-density families. Mol Psychiatry. 2004;9:1359–4184. doi: 10.1038/sj.mp.4001519. [DOI] [PubMed] [Google Scholar]
- 71.Sanders AR, Rusu I, Duan J, et al. Haplotypic association spanning the 22q11.21 genes COMT and ARVCF with schizophrenia. Mol Psychiatry. 2005;10:353–365. doi: 10.1038/sj.mp.4001586. [DOI] [PubMed] [Google Scholar]
- 72.Handoko HY, Nyholt DR, Hayward NK, et al. Separate and interacting effects within the catechol-O-methyltransferase (COMT) are associated with schizophrenia. Mol Psychiatry. 2005;10:589–597. doi: 10.1038/sj.mp.4001606. [DOI] [PubMed] [Google Scholar]
- 73.Moskvina V, Craddock N, Holmans P, Owen MJ, O'Donovan MC. Effects of differential genotyping error rate on the type I error probability of case-control studies. Hum Hered. 2006;61:55–64. doi: 10.1159/000092553. [DOI] [PubMed] [Google Scholar]
- 74.Neale BM, Sham PC. The future of association studies: gene-based analysis and replication. Am J Hum Genet. 2004;75:353–362. doi: 10.1086/423901. [DOI] [PMC free article] [PubMed] [Google Scholar]