Abstract
Recent studies have linked infectious agents to schizophrenia. The largest number of studies has involved the analysis of Toxoplasma gondii; these studies were subjected to a meta-analysis. Published articles and abstracts were identified by searches of MEDLINE, Ovid, and Google Scholar; by a search of Chinese publications; through letters to researchers; and by visiting China. Published and unpublished controlled studies that used serological methods for measuring T. gondii antibodies to assess inpatients and/or outpatients diagnosed with schizophrenia were selected for analysis, and source documents were translated as needed. Forty-two studies carried out in 17 countries over 5 decades were identified; 23 of these (6 unpublished) met selection criteria. The combined odds ratio (OR) was 2.73 (95% confidence interval, 2.10 to 3.60; chi-square with 1 df 263; P < .000001). Seven studies that included only patients with first-episode schizophrenia (OR 2.54) did not differ significantly from 16 studies that included patients in all clinical phases (OR 2.79). The results suggest that individuals with schizophrenia have an increased prevalence of antibodies to T. gondii. This association is consistent with other epidemiological studies as well as with animal studies. Although the OR of 2.73 is modest, it exceeds that for genetic or other environmental factors identified to date and suggests that Toxoplasma is in some way associated with a large number of cases of schizophrenia. If an etiological association can be proven, it would have implications for the design of measures for the prevention and treatment of this disease.
Keywords: apicomplexa, protozoa, infection
Introduction
In 1896, the Scientific American published an article, “Is Insanity Due to a Microbe?,” thereby initiating interest in a possible infectious etiology of schizophrenia. Interest in the hypothesis was widespread in the early years of the 20th century, then waned until the closing years of the century. Recent studies have linked schizophrenia with perinatal exposure to viruses such as influenza A virus,1 rubellavirus,2 herpes simplex virus type 2,3 and polioviruses4 and with postnatal exposure to viral and bacterial agents causing meningitis and encephalitis.5 The largest number of studies linking an infectious agent to schizophrenia, however, has involved Toxoplasma gondii.
Toxoplasma gondii, a coccidian protozoa of the apicomplexa family, was first described in 1908. In 1939, it was linked to a congenital syndrome that includes deafness, retinal damage, seizures, mental retardation, and intracranial calcifications.6 Postnatal transmission may produce lymphadenopathy and nonspecific symptoms of infection, but most cases are thought to be asymptomatic. The definitive hosts of this organism are cats and other felines. Transmission of T. gondii to humans may come about through ingestion or inhalation of oocysts shed by infected cats into litter boxes, gardens, sandboxes, or other children's play areas. The organism may also be transmitted through the ingestion of tissue cysts by the eating of undercooked meat containing tissue cysts from sheep, goats, or other animals that have been infected from cats.7 The availability of serological assays has allowed for the testing of exposure to T. gondii in large numbers of individuals. Studies using these assays have indicated that Toxoplasma infection is widespread and varies in geographic regions and among individuals with different demographic characteristics.
Given T. gondii's neurotropism and association with congenital brain dysfunction, there has been long-standing interest in investigating a possible association between exposure to this organism and the development of severe psychiatric disorders. The first study of T. gondii antibodies in psychiatric patients was published in 1953 by Kozar8 in Poland. Since that time, 41 additional published and unpublished studies have been identified by the authors and were subjected to a meta-analysis directed at defining the association between Toxoplasma exposure and the risk of schizophrenia.
METHOD
Data Sources
Through previous analysis of several Eastern European and Chinese publications directed at the association between Toxoplasma antibodies and psychiatric disorders,9 the authors were aware that many of the studies needed for a meta-analysis had been published in languages other than English. A keyword search of MEDLINE, Ovid, and Google Scholar found only 4 of the 34 published articles eventually identified. Most of the studies were identified through a survey of Chinese publications (Z.R. Lun, PhD, unpublished data, 2005), letters to Chinese and Eastern European researchers, a visit to China by two of us (EFT and RHY), and citations of earlier publications by those who published later. The earliest studies were published in Eastern Europe, and these studies were cited by the first researcher to carry out studies in China.10,11
Of the 42 studies ultimately identified, 35 were published and 7 were unpublished. Among those published, only 6 had been written in the English language. The studies were carried out between 1953 and 2005 in 17 countries: China (17); Germany (4); Australia, Bulgaria, Czechoslovakia, Italy, Mexico, and the United States (2 each); and Cuba, Egypt, Ireland, Korea, Peru, Poland, Russia, Spain, and Turkey (1 each).
The studies were translated as needed and then summarized regarding psychiatric diagnoses, control groups, and method of assessing exposure to T. gondii. It was decided to include in the meta-analysis only those studies that met the following criteria: (1) a clear diagnosis of schizophrenia, (2) inclusion of a defined control group, and (3) use of one of the following diagnostic assays: Sabin-Feldman dye test, complement fixation (CF), immune hemagglutination (IHA), immune fluorescence (IFA), or enzyme-linked immunosorbent assay (ELISA). The diagnostic criteria for schizophrenia used in the United States (Diagnostic and Statistical Manual of Mental Disorders), Europe (International Classification of Diseases), and China (Classification and Diagnostic Standards of Mental Disorders in China) are very similar.
In one study, antibodies were assessed serologically using both the dye test and CF.12 Because studies can be counted only once in a meta-analysis because analysis is based on the assumptions of independence, we utilized the results of the assay that showed the smaller difference between cases and controls.
Statistical Methods
The data summarized by meta-analysis in this report originate from a series of classic two-group, binary-event studies. For our study, we are looking at the exposure rate of positive T. gondii antibodies in individuals with a diagnosis of schizophrenia versus a group of controls without that diagnosis. The results of each study are reported in a classic two-by-two contingency table. The proportion of infected individuals in each group is denoted by pt and pc, respectively, for the exposed group (t), and the control group (c).
For two-by-two binary-event studies, the statistic summarized is the odds ratio (OR), defined as [pt/(1 - pt)]/[pc(1 - pc)]. An OR of unity implies no difference between the two groups. An OR of two, for example, implies that the numerator group is at a twice higher risk than the denominator group. The graphics in this report present the OR and the length of the confidence interval (CI) for each study as well as the combined results. The software program NCSS (NCSS Statistical System for Windows, Kaysville, UT: Number Cruncher Statistical Systems, 2004) was used to analyze the raw data for the meta-analysis. We used the random effects model, which incorporates a weighted method of analysis; this is not the inverse variance-weighted method that has known limitations. The random model is also more conservative than the fixed model with wider CIs, a decision supported by statistically significant chi-square heterogeneity tests. In addition, basic science supports this decision in that we expected the rate of positive test results to vary from site to site as it would on exposure, hence, the use of the random model. The Mantel-Haenszel method described in the NCSS software uses the stratified method and the raw counts for a combined OR estimate. We used the Woolf heterogeneity test for testing the general hypothesis that all ORs are equal but not necessarily equal to unity. Because opinions vary on the appropriate methods for performing a particular meta-analysis, we examined the robustness of the findings by using a sensitivity analysis. In addition, because statistically significant results are more likely to get published, this can distort the findings in a meta-analysis. Sensitivity was thus assessed by exploring the correlation association of the size of the OR and its CI versus the size of the study because smaller ORs can be statistically significant in larger studies.
Results
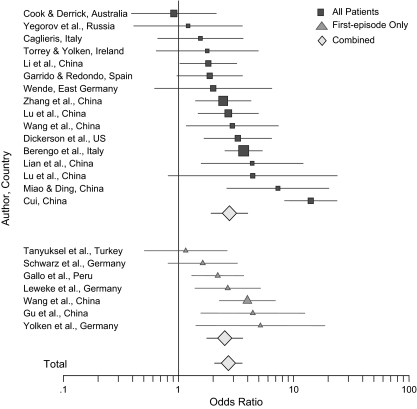
A total of 23 of the 42 identified studies met the criteria established for the meta-analysis (table 1).10,12–27 They included 3873 individuals with schizophrenia and 7046 controls. The reasons for excluding the other studies included one or more of the following: inclusion of patients with psychiatric diagnoses other than schizophrenia (eg, “psychiatric inpatients”) (8 studies28–35), use of skin testing and other nonserological measures of T. gondii antibodies (5 studies8,36–39), failure to include a control group (3 studies40–42), missing data (2 studies43,44), and selection of patients because of possible exposure to T. gondii (1 study45).
Table 1.
Serological Studies of Toxoplasma gondii Antibodies in Individuals With Schizophrenia and Controls
| Year | Authors and Country | Serological Test | Control Group | % Patients Antibody Positive | % Controls Antibody Positive | Odds Ratio |
| I. All schizophrenia patients | ||||||
| 1961 | Cook & Derrick,12 Australia | CF | “General population” | 6/53 (11%) | 99/760 (13%) | 0.88 |
| 1962 | Yegorov et al,13 Russia | CF | Healthy hospital employees | 12/37 (32%) | 7/25 (28%) | 1.23 |
| 1958 | Caglieris,14 Italy | dye test | Hospital employees, and blood donors | 13/61 (21%) | 12/81 (15%) | 1.55 |
| 2003 | Torrey & Yolken,a Ireland | ELISA | Hospital staff | 31/52 (60%) | 9/20 (45%) | 1.79 |
| 1999 | Li et al,15 China | ELISA | Normal persons, from same region | 22/152 (15%) | 34/396 (9%) | 1.81 |
| 1968 | Garrido & Redondo,16 Spain | CF | Medical clinic patients | 17/39 (44%) | 147/500 (29%) | 1.86 |
| 1956 | Wende,17 East Germany | dye test | Inpatients with neurological disorders | 3/38 (8%) | 24/520 (5%) | 1.88 |
| 1994 | Zhang et al,18 China | ELISA | Normal persons from same region | 17/92 (18%) | 118/1365 (9%) | 2.42 |
| 1990 | Lu et al,19 China | ELISA | Normal persons from same city | 35/418 (8%) | 16/512 (3%) | 2.81 |
| 1995 | Wang et al,20 China | ELISA | General population | 11/104 (11%) | 8/210 (4%) | 2.96 |
| 2005 | Dickerson et al,b United States | ELISA | Volunteers screened to rule out psychiatric disorders and matched to group | 71/405 (18%) | 10/170 (6%) | 3.33 |
| 1966 | Berengo et al,21 Italy | dye test | Blood donors | 76/560 (14%) | 49/1200 (4%) | 3.68 |
| 1996 | Lian et al,22 China | IHA | Hospital employees and their families | 20/67 (30%) | 5/60 (8%) | 4.51 |
| 2002 | Lu et al,23 China | IHA | Healthy persons for routine physicals | 17/222 (7%) | 1/78 (1%) | 5.19 |
| 1999 | Miao & Ding,24 China | ELISA | Clinic patients for routine physicals | 56/146 (38%) | 4/56 (7%) | 7.66 |
| 1984 | Cui,10 China | IHA | Local population | 133/257 (52%) | 19/274 (7%) | 14.22 |
| Pooled estimate for all schizophrenia patients 2.79 | ||||||
| II. First-episode schizophrenia patients only | ||||||
| 2005 | Tanyuksel et al,c Turkey | ELISA | Healthy individuals with no physical or psychiatric disease | 23/64 (36%) | 13/40 (33%) | 1.16 |
| 2005 | Schwarz et al,d Germany | ELISA | Volunteers screened to rule out psychiatric disorders | 27/100 (27%) | 16/87 (18%) | 1.63 |
| 2005 | Gallo et al,e Peru | ELISA | Individually matched controls, no psychiatric or neurological illness | 64/120 (53%) | 41/120 (34%) | 2.19 |
| 2004 | Leweke et al,25 Germany | ELISA | Healthy volunteers from same area | 38/113 (34%) | 16/102 (16%) | 2.70 |
| 2006 | Wang et al,26 China | ELISA | 200 for routine physical exams and 200 hospitalized for nonpsychiatric illnesses | 82/600 (14%) | 15/400 (4%) | 4.01 |
| 2001 | Gu et al,f China | ELISA | Normal individuals matched to group | 45/135 (33%) | 4/43 (9%) | 4.63 |
| 2001 | Yolken et al,27 Germany | ELISA | Healthy individuals matched by block design | 16/38 (42%) | 3/27 (11%) | 5.45 |
| Pooled estimate for first-episode schizophrenia patients only 2.54 | ||||||
| Pooled estimate for all 2.73 | ||||||
Note:CF, complement fixation; ELISA, enzyme-linked immunosorbent assay; IHA, immune hemagglutination.
E. F. Torrey, MD, and R. H. Yolken, MD, unpublished data, 2003.
F. Dickerson, PhD, et al, unpublished data, 2005.
M. Tanyuksel, MD, et al, unpublished data, 2005.
M. J. Schwarz, MD, and N. Mueller, MD, PhD, unpublished data, 2005.
C. Gallo, MSc, et al, unpublished data, 2005.
H. Gu, PhD, et al, unpublished data, 2001.
Seven of the studies involved the analysis of outpatients; the remainder were directed largely at hospitalized populations. Eight studies included individuals who were undergoing their first episode of schizophrenia, with 7 of these meeting the criteria for inclusion in the meta-analysis; the remainder of the studies included patients with varying lengths of illness duration.
Table 1 lists the 23 studies, divided by first episode, and other studies, with ORs. The chi-square test for heterogeneity across the 23 studies is 25.3, 3 df and P < .000001, strongly supporting the use of the random model, which thus was used throughout the statistical analyses. Figure 1 is a forest plot of these studies. The overall combined OR is 2.73 (95% CI, 2.10 to 3.60; chi-square with 1 df 263; P < .000001). The OR for the combined first-episode studies is 2.54 and for the other combined studies 2.79 (Mentel-Haenszel chi-square with 1 df 0.81; P < .36); thus, there is no significant difference between the two ORs 2.54 and 2.79 and consequently the two studies. Dividing the studies by the serological test yields ORs of 1.38 for CF (3 studies), 2.61 for ELISA (14 studies), 2.54 for the dye test (3 studies), and 8.27 for IHA (3 studies) (Mantel-Haenszel chi-square with 3 df 25.3, P < .0001).
Figure 1.
Forest Plot of Odds Ratio for the 23 Studies in Table 1.
Among the 23 studies used in the meta-analysis, 17 have been published and 6 are unpublished. The OR for published studies is 2.97 and for unpublished studies 2.16. The chi-square of equality with 1 df is 4.8, P < .03. Thus, there appears to be some evidence that studies with a higher OR are more likely to have been published. In addition, further sensitivity analysis for the 23 studies was explored using the Pearson product moment correlation between the size of the study and the size of the OR. For all 23 studies, it was -0.01, P < .96; for the 17 published studies, it was -0.06, P < .81; and for the 6 unpublished studies, it was 0.17, P < .71, all statistically nonsignificant, indicating no association between the size of the study and the size of the OR in any of the comparisons.
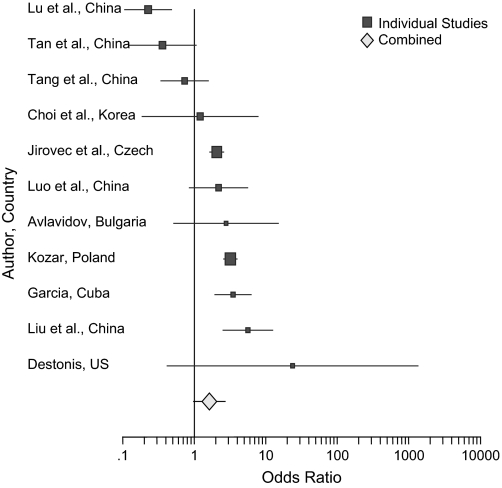
Among the 19 studies not meeting the criteria used for this meta-analysis, 11 had analyzable data for cases and controls.8,28–34,36,37,39 Because they included some psychiatric patients with diagnoses other than schizophrenia in their sample and/or used nonserological measures of T. gondii antibodies, one would predict greater variability in the results. These studies are listed in table 2 with ORs; figure 2 is a forest plot of these studies. The combined OR is 1.60 (95% CI, 0.98 to 2.69; chi-square with 1 df 158, P < .000001). A chi-square test with 10 df for variability among the 11 studies is 73.06, P < .000001. Thus, in these 11 studies not used in the meta-analysis, the individuals with mixed psychiatric diagnoses had significantly more antibodies to T. gondii than the controls although, as expected, there was much greater variability among the studies.
Table 2.
Studies Not Used
| Year | Author and Country | Reason for Nonuse | Cases | Controls | Odds Ratio |
| 1995 | Lu et al,28 China | Inpatients with mental disease | 182/754 (24%) | 17/29 (59%) | 0.23 |
| 1995 | Tan et al,29 China | Inpatients with nervous system disease | 43/674 (6%) | 4/26 (15%) | 0.36 |
| 2003 | Tang et al,30 China | Inpatients with psychosis | 64/455 (14%) | 9/50 (18%) | 0.73 |
| 1983 | Choi et al,31 Korea | Inpatients | 11/573 (1.9%) | 1/76 (1.3%) | 1.20 |
| 1957 | Jirovec et al,36 Czech | Skin test | 238/501 (48%) | 296/970 (29%) | 2.06 |
| 2005 | Luo et al,32 China | Inpatients | 38/413 (9%) | 5/117 (4%) | 2.18 |
| 1962 | Avlavidov,33 Bulgaria | Inpatients | 3/14 (21%) | 3/34 (9%) | 2.78 |
| 1953 | Kozar,8 Poland | Skin test, inpatients | 495/961 (52%) | 170/681 (25%) | 3.19 |
| 1979 | Garcia,37 Cuba | Skin test, psychiatric patients | 60/100 (60%) | 30/100 (30%) | 3.48 |
| 2004 | Liu et al,34 China | Inpatients with nervous system disease | 37/93 (40%) | 9/87 (10%) | 5.60 |
| 1966 | Destounis,39 United States | Skin test | 5/45 (12%) | 0/45 (0%) | 23.61 |
| Pooled estimate for 11 studies 2.29 | |||||
Figure 2.
Forest Plot of Odds Ratio for the 11 Studies in Table 2.
Discussion
We found that the prevalence of antibodies to T. gondii in individuals with schizophrenia is significantly higher than the prevalence of antibodies in control populations, with an OR of 2.73. This difference was found in studies carried out over 5 decades in 17 countries employing several different methods of antibody measurement.
The findings in the present study are consistent with other studies linking T. gondii to schizophrenia. A study of maternal sera of women who gave birth to offspring who later developed schizophrenia spectrum disorders46 and a study of newborn sera of individuals who later developed schizophrenia47 both reported increased antibodies to T. gondii in cases versus controls. Two other studies reported that children who were later diagnosed with schizophrenia or other psychoses had had more childhood exposure to cats, but not dogs.48,49 It has also been observed that some individuals who develop adult-onset toxoplasmosis exhibit delusions and hallucinations.50
It is noteworthy that the largest number of studies have been done in China, where, until recently, the keeping of cats as pets has been uncommon and where the prevalence of T. gondii antibodies in the general population has been low.7 In such instances, the higher prevalence of antibodies in patients with schizophrenia may be more apparent. By contrast, in a country like Ireland, where cats are ubiquitous and where the prevalence of T. gondii antibodies in the general population is known to be high,51 a modest increase in patients with schizophrenia may be less apparent.
Given what is known about the causes of schizophrenia, is it plausible that T. gondii could play a role in its etiology? There are four major considerations. First, schizophrenia is known to have a genetic component, with concordance among monozygotic twins of 35%–50%. Genes are also known to influence the susceptibility of animals to T. gondii,52,53 and in mice, T. gondii has also been shown to be transplacentally transmitted for as many as 5 successive generations in a pseudogenetic pattern.54 Second, schizophrenia is known to include abnormalities of neurotransmitters; animal studies have demonstrated an effect of T. gondii on dopamine and serotonin.55 Third, schizophrenia is widely believed to be a disease of neurodevelopment; this is consistent with T. gondii's known ability to cause prenatal infections and then remain latent for many years before becoming reactivated. Fourth, an association between Toxoplasma infections and schizophrenia is consistent with animal models indicating persistent behavioral changes in Toxoplasma-infected animals.56
In other aspects of plausibility, T. gondii is neurotrophic, with a special affinity for glia,57,58 now thought to be centrally involved in the schizophrenia disease process. It is also of interest that some antipsychotic drugs used to treat schizophrenia have been shown to inhibit the growth of T. gondii in cell culture.59 Finally, individuals who develop schizophrenia are known to have had an excess number of winter and spring births60; toxoplasmosis, like many infectious diseases, also occurs more commonly in the winter and spring months.61–63
Despite many attractive aspects, there are three major problems with the plausibility of T. gondii being etiologically linked to schizophrenia. One is the fact that these studies are serological in nature and are not based on the direct detection of Toxoplasma organisms or DNA in infected body fluids. This is an inherent limitation of studies in Toxoplasma biology because the organism is difficult to detect in non–immune-compromised individuals. However, numerous studies have indicated that properly performed serological assays are accurate indicators of prior Toxoplasma infection.64,65 The second is epidemiological; the seropositivity rate of T. gondii is very high in countries such as France and Ethiopia, where undercooked or raw meat is regularly consumed, yet schizophrenia has not been found to be unusually prevalent in these countries. Possible explanations include the fact that transmission by eating tissue cysts in undercooked meat is a more benign mode of infection, and there is some evidence to support this66; thus, it may pose less of a risk for the development of schizophrenia than the consumption of oocysts shed by cat feces. There may also be differences in terms of the neuropathogenicity of strains of Toxoplasma prevalent in different areas of the world67 as well as differences in the genetic susceptibility of different human populations.
The third problem with plausibility is that the majority of individuals with schizophrenia do not have measurable antibodies to T. gondii. This fact may be related to the relative insensitivity of available serological assays or to the heterogeneity of disease pathogenesis. It is also possible that other environmental factors may initiate neuropathogenic pathways similar to those employed by T. gondii, such as the ones involving the activation of interferon gamma.68 The identification of pathways initiated by T. gondii within the central nervous system might thus lead to an increased understanding of schizophrenia pathogenesis in individuals without specific evidence of Toxoplasma infection.
A question for future research is the specificity of the finding. To date, only one study of T. gondii and schizophrenia has also included a large number of individuals with another psychiatric diagnosis; Wang et al26 in China reported that 40/600 (7%) of patients with first-episode affective disorder had antibodies to T. gondii compared with 82/600 (14%) of those with first-episode schizophrenia and 15/400 (4%) of controls.
Future research should also focus on the timing of the infection. Individuals with schizophrenia may behave in ways that increase the likelihood of becoming infected with T. gondii either prior to or after the onset of the disease. An example of the latter was speculation by the authors of a Spanish study that the patients with schizophrenia had a high rate of seropositivity to T. gondii because they regularly worked in the hospital gardens that had been fecally contaminated by the hospital's cats.16 Alternatively, institutionalized psychiatric patients may be fed undercooked meat, thereby increasing their exposure to T. gondii. Such post-onset explanations seem unlikely in view of the similar findings in first-episode schizophrenia patients who had not had a previous hospitalization and were unlikely to have undergone unusual environmental exposures after illness onset. The likelihood of infection occurring as an artifact of behavioral effects of the disease or its treatment is also not consistent with studies indicating an increased risk of schizophrenia in the offspring following maternal infection.46,47
Another alternate explanation of the association is that the increased T. gondii antibodies in individuals with schizophrenia are secondary to immune system abnormalities, such as are seen in individuals infected with HIV. In such a scenario, another infectious agent could be a primary cause of schizophrenia, with the reactivation of T. gondii tissue cysts and generation of antibody being merely a secondary manifestation. Although immune, and specifically lymphocyte, abnormalities have been described in schizophrenia, their magnitude is not impressive and seems unlikely to account for the increase in T. gondii antibodies. In addition, increases in antibodies to other infections associated with immunodeficiency, such as Epstein-Barr virus, have generally not been found in individuals with schizophrenia.25 Finally, the possible effects of antipsychotic medication must be considered; however, antipsychotics have been shown to inhibit T. gondiiin vitro,59 and the one study of never-treated individuals with schizophrenia had an OR of 2.70.25
The schizophrenia-associated OR of Toxoplasma found in this meta-analysis, 2.73, while modest in extent, is greater than the ORs associated with individual genes (1.6–1.8)69 and with other environmental factors such as obstetrical complications (∼2).70 Given the high prevalence of Toxoplasma infection in healthy individuals, Toxoplasma seropositivity has a relatively low predictive value for the development of schizophrenia, and most individuals who are Toxoplasma seropositive do not have manifestations of this disorder.
If the association between T. gondii infection and schizophrenia is, in fact, etiological, why should only some individuals who are exposed to the infectious agent develop schizophrenia? Possible reasons include differences in genetic susceptibility, organism strain differences, route of infection (eg, ingestion of oocysts from infected cats versus tissue cysts from meat), and timing of the infection (eg, in utero, early postnatal, childhood, adulthood). Each of these factors is known to lead to different disease outcomes for other infectious agents. The study of these factors will be important in further defining the relationship between T. gondii and schizophrenia.
Acknowledgments
We gratefully acknowledge the following lead authors for permission to use their unpublished studies: Faith Dickerson, Carla Gallo, Hongbin Gu, Norbert Mueller, Markus Schwarz, and Mehmet Tanyuksel. We are also indebted to the following persons for helping to identify or translate the studies: Jaroslav Flegr, Ida Llenos, Ana Ramos, Inna Ruslanova, Serge Weis, and Shen Zhong.
References
- 1.Limosin F, Rouillon F, Payan C, Cohen J-M, Strub N. Prenatal exposure to influenza as a risk factor for adult schizophrenia. Acta Psychiatr Scand. 2003;107:331–335. doi: 10.1034/j.1600-0447.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- 2.Brown AS, Cohen P, Greenwald S, Susser E. Nonaffective psychosis after prenatal exposure to rubella. Am J Psychiatry. 2000;157:438–443. doi: 10.1176/appi.ajp.157.3.438. [DOI] [PubMed] [Google Scholar]
- 3.Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry. 2001;58:1032–1037. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- 4.Suvisaari J, Haukka J, Tanskanen A, Hovi T, Lonnqvist J. Association between prenatal exposure to poliovirus infection and adult schizophrenia. Am J Psychiatry. 1999;156:1100–1102. doi: 10.1176/ajp.156.7.1100. [DOI] [PubMed] [Google Scholar]
- 5.Koponen H, Rantakallio P, Veijola J, Jones P, Jokelainen J, Isohanni M. Childhood central nervous system infections and risk for schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2004;254:9–13. doi: 10.1007/s00406-004-0485-2. [DOI] [PubMed] [Google Scholar]
- 6.Wolf A, Cowen D, Paige BH. Human toxoplasmosis. Science. 1939;89:226–227. doi: 10.1126/science.89.2306.226. [DOI] [PubMed] [Google Scholar]
- 7.Remington JS, McLeod R, Thulliez P, Desmonts G. Toxoplasmosis. In: Remington RS, Klein JO, editors. Infectious Diseases in the Fetus and Newborn Infant. Philadelphia, PA: W.B. Saunders; 2001. pp. 224–227. [Google Scholar]
- 8.Kozar Z. Badania nad toksoplazmoza wsrod umyslowo chorych. Bull Inst Mar Trop Med Gdansk. 1953;5:146–173. Summary in English: Studies of toxoplasmosis among the mentally sick. Bull Inst Marit Trop Med Gdansk 1953;5:142–145. [PubMed] [Google Scholar]
- 9.Torrey EF, Yolken RH. Toxoplasma gondii and schizophrenia. Emerg Infect Dis. 2003;9:1375–1380. doi: 10.3201/eid0911.030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui JZ. Epidemiological investigation and etiological discussion of Toxoplasma gondii infection in psychiatric patients. Chin J Neurol. 1984;17:224. [in Chinese] [Google Scholar]
- 11.Cui JZ. Ninety year study of Toxoplasma gondii. J Pract Parasit Dis. 2000;8:75–78. [in Chinese] [Google Scholar]
- 12.Cook I, Derrick EH. The incidence of Toxoplasma antibodies in mental hospital patients. Australas Ann Med. 1961;10:137–141. doi: 10.1111/imj.1961.10.2.137. [DOI] [PubMed] [Google Scholar]
- 13.Yegorov IF, Kovalyukh AI, Smagah MF, Pavlova EE. Comparative results of the complement binding reaction and the intradermal test in toxoplasmosis diagnostics. Microbiol Epidemiol Immunol. 1962;10:51–54. [in Russian] [Google Scholar]
- 14.Caglieris AN. Risultati del test tintoriale in individui sani ed ammalati. Boll Soc Med Chir Pavia. 1958;72:647–661. [Google Scholar]
- 15.Li QY, Luo XN, Li L, Tong F. The control study of schizophrenia and affective disorders and Toxoplasma infections. Acta Acad Med Hubei. 1999;20:222–223. [in Chinese] [Google Scholar]
- 16.Garrido JA, Redondo VP. Toxoplasmosis y enfermedades mentales. Arch Neurobiol. 1968;31:161–172. [PubMed] [Google Scholar]
- 17.Wende S. Die Bedeutung der Toxoplasmose fuer die Neurologie und Psychiatrie. Arch Psychiatr Z Neurol. 1956;194:179–199. doi: 10.1007/BF00342840. [DOI] [PubMed] [Google Scholar]
- 18.Zhang A, Xiong TY, Ding JY. Serological detection of Toxoplasma gondii infection from 108 cases of schizophrenia. Chin J Neurol Ment Dis. 1994;20:52. [in Chinese] [Google Scholar]
- 19.Lu C, Zhou M, Ma F, et al. Toxoplasma antibody detection and screening in 418 cases of schizophrenia. J Tianjin Med. 1990;12:720–723. [in Chinese] [Google Scholar]
- 20.Wang SJ, Xu SY, Luo M, Chen JQ. Detection of antibody against Toxoplasma gondii in schizophrenia patients by methods ELISA and IHA. Chin J Parasit Dis Control. 1995;8:240. [in Chinese] [Google Scholar]
- 21.Berengo A, Bechelli G, de Lalla F, et al. Richerche sierologiche sulla diffusione della toxoplasmosi. Studio su 1720 ricoverati in un Ospedale psichiatrico. Minn Med. 1966;57:2292–2305. [PubMed] [Google Scholar]
- 22.Lian D, Duan RZ, Xu LR, Qiu HJ. An investigation on antibody of Toxoplasma gondii among patients with mental diseases. Chin J Parasit Dis Control. 1996;9:299–300. [in Chinese] [Google Scholar]
- 23.Lu ZM, Zhang HB, Zhang JS, Ma WY. Serological investigation of Toxoplasma gondii infection in schizophrenia patients. Chin J Parasit Dis Control. 2002;15:299–300. [in Chinese] [Google Scholar]
- 24.Miao JS, Ding WL. Study of the correlation of Toxoplasma gondii infection and schizophrenia. Chin J Parasit Dis Control. 1999;12:69–70. [in Chinese] [Google Scholar]
- 25.Leweke FM, Gerth CW, Koethe D, et al. Antibodies to infectious agents in individuals with recent onset schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2004;254:4–8. doi: 10.1007/s00406-004-0481-6. [DOI] [PubMed] [Google Scholar]
- 26.Wang H-L, Wang G-H, Li Q-Y, Shu C, Jiang M-S, Guo Y. Prevalence of Toxoplasma infection in first-episode schizophrenia and comparison between Toxoplasma-seropositive and Toxoplasma-seronegative schizophrenia. Acta Psychiatr Scand. 2006;114:40–48. doi: 10.1111/j.1600-0447.2006.00780.x. [DOI] [PubMed] [Google Scholar]
- 27.Yolken RH, Bachmann S, Rouslanova I, et al. Antibodies to Toxoplasma gondii in individuals with first-episode schizophrenia. Clin Infect Dis. 2001;32:842–844. doi: 10.1086/319221. [DOI] [PubMed] [Google Scholar]
- 28.Lu YC, Cui JZ, Zheng T, Xie LQ. Investigation of Toxoplasma infection in patients with mental disorders. J Clin Psychol Med. 1995;5:86–87. [in Chinese] [Google Scholar]
- 29.Tan YH, Du WH, Zhang B. An investigation on Toxoplasma infection to nervous system. Endemic Dis Bull. 1995;3:86–88. [in Chinese] [Google Scholar]
- 30.Tang W, Tu XD, Zhang SN. Investigation of Toxoplasma gondii on the patients with psychosis. J Clin Psychol Med. 2003;13:262. [in Chinese] [Google Scholar]
- 31.Choi WY, Yoo JE, Chung CS, Paik KK, Cho SN. Toxoplasma antibodies by indirect latex agglutination tests in National Seoul Mental Hospital patients. Kisaengchunghak Chapchi. 1983;21:281–285. doi: 10.3347/kjp.1983.21.2.281. [in Korean] [DOI] [PubMed] [Google Scholar]
- 32.Luo SG, Shu CH, Ma ZG, Luo JF, Huang RM, Lin WX. Investigation of Toxoplasma gondii infection in patients with psychosis. J Guangxi Med Univ. 2005;22:141–142. [in Chinese] [Google Scholar]
- 33.Avlavidov TP. Toxoplasmosis among certain groups of the population of the Varna region. Suvr med. 1962;13:18–23. [in Bulgarian] [Google Scholar]
- 34.Liu L, Li YJ, Jing LX, Li P, Jiang ZH, Li YL. A survey of Toxoplasma gondii infection in 93 cases of nerve system diseases in Dalian region. Pract Prev Med. 2004;11:757–758. [in Chinese] [Google Scholar]
- 35.Amminger GP, McGorry PD, Wade D. Antibodies to infectious agents in individuals at ultra-high risk for psychosis. Biol Psychiatry. doi: 10.1016/j.biopsych.2006.09.034. In press. [DOI] [PubMed] [Google Scholar]
- 36.Jirovec O, Jindrich J, Fuchs V, Peter R. Studien mit dem Toxoplasmintest. Z Bakteriol. 1957;169:129–159. [PubMed] [Google Scholar]
- 37.Garcia GD. Toxoplasmosis y enfermedades mentales. Rev Cuba Med Trop. 1979;31:127–131. [PubMed] [Google Scholar]
- 38.Buentello E. Comunicacion preliminary sobre las relaciones entre toxoplasmosis, acido lisergico y esquizofrenia. Gac Med Mex. 1958;88:693–708. [PubMed] [Google Scholar]
- 39.Destounis N. The relationship between schizophrenia and toxoplasmosis: a critical study. Del Med J. 1966;38:349–354. [PubMed] [Google Scholar]
- 40.Rifaat MA, Nagaty HF. Toxoplasmosis in Egypt: a toxoplasmin-skin-testing survey among a group of Cairo population. J Egypt Public Health Assoc. 1959;34:121–135. [Google Scholar]
- 41.Hui HP, Liang LM, Chen Y. The situation of Toxoplasma gondii infection in schizophrenia patients. J First Mil Med Univ. 1996;16:53–55. [in Chinese] [Google Scholar]
- 42.Chen ZX, Luo MQ, Xiao P, Gao ZS, Li ZR. The relationship between Toxoplasma infection and schizophrenia in 136 cases. Neo Med. 1999;4:179–180. [in Chinese] [Google Scholar]
- 43.Guigof A. Étude sur la toxoplasmose en Bulgarie. Bull Soc Pathol Exot. 1964;57:205–208. [PubMed] [Google Scholar]
- 44.Vojtechovska M, Vojtechovsky M, Petru M. Some problems of parasitology in mental patients. Cas Lek Cesk. 1956;95:559–566. [in Czechoslovakian] [PubMed] [Google Scholar]
- 45.Roch E, Varela G. Diversos aspectos de la investigacion sobre toxoplasmosis en Mexico: resultados obtenidos en 29 883 reacciones de Sabin y Feldman efectuadas de 1953 a 1965. Rev Invest Salud Publica (Mex) 1966;26:31–49. [PubMed] [Google Scholar]
- 46.Brown AS, Schaefer CA, Quesenberry CP, Jr, Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162:767–773. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- 47.Mortensen PB, Nørgaard-Pedersen B, Waltoft BL, et al. Toxoplasma gondii as a risk factor for schizophrenia: analysis of filter paper blood samples obtained at birth. Biol Psychiatry. doi: 10.1016/j.biopsych.2006.05.024. In press. [DOI] [PubMed] [Google Scholar]
- 48.Torrey EF, Yolken RH. Could schizophrenia be a viral zoonosis transmitted from house cats? Schizophr Bull. 1995;21:167–171. doi: 10.1093/schbul/21.2.167. [DOI] [PubMed] [Google Scholar]
- 49.Torrey EF, Rawlings R, Yolken RH. The antecedents of psychoses: a case-control study of selected risk factors. Schizophr Res. 2000;46:17–23. doi: 10.1016/s0920-9964(99)00237-6. [DOI] [PubMed] [Google Scholar]
- 50.Kramer W. Frontiers of neurological diagnosis in acquired toxoplasmosis. Psychiatr Neurol Neurochir. 1966;69:43–64. [PubMed] [Google Scholar]
- 51.Stanford CF, Connolly JH, Ellis WA, et al. Zoonotic infections in Northern Ireland farmers. Epidemiol Infect. 1990;105:565–570. doi: 10.1017/s0950268800048196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson J, Suzuki Y, Mack D, et al. Genetic analysis of influences on survival following Toxoplasma gondii infection. Int J Parasitol. 2002;32:179–185. doi: 10.1016/s0020-7519(01)00321-6. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki Y. Host resistance in the brain against Toxoplasma gondii. J Infect Dis. 2002;185(suppl):S58–S65. doi: 10.1086/337999. [DOI] [PubMed] [Google Scholar]
- 54.Beverley JKA. Congenital transmission of toxoplasmosis through successive generations of mice. Nature. 1959;183:1348–1349. doi: 10.1038/1831348a0. [letter] [DOI] [PubMed] [Google Scholar]
- 55.Stibbs HH. Changes in brain concentrations of catecholamines and indoleamines in Toxoplasma gondii infected mice. Ann Trop Med Parasitol. 1985;79:153–157. doi: 10.1080/00034983.1985.11811902. [DOI] [PubMed] [Google Scholar]
- 56.Webster JP. Rats, cats, people and parasites: the impact of latent toxoplasmosis on behaviour. Microbes Infect. 2001;3:1037–1045. doi: 10.1016/s1286-4579(01)01459-9. [DOI] [PubMed] [Google Scholar]
- 57.Creuzet C, Robert F, Roisin MP, et al. Neurons in primary cultures are less efficiently infected by Toxoplasma gondii than glial cells. Parasitol Res. 1998;84:25–30. doi: 10.1007/s004360050351. [DOI] [PubMed] [Google Scholar]
- 58.Halonen SK, Lyman WD, Chiu FC. Growth and development of Toxoplasma gondii in human neurons and astrocytes. J Neuropathol Exp Neurol. 1996;55:1150–1156. doi: 10.1097/00005072-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Jones-Brando L, Torrey EF, Yolken R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res. 2003;62:237–244. doi: 10.1016/s0920-9964(02)00357-2. [DOI] [PubMed] [Google Scholar]
- 60.Torrey EF, Miller J, Rawlings R, Yolken RH. Seasonality of births in schizophrenia and bipolar disorder: a review of the literature. Schizophr Res. 1997;28:1–38. doi: 10.1016/s0920-9964(97)00092-3. [DOI] [PubMed] [Google Scholar]
- 61.Bannister B. Toxoplasmosis 1976–80: review of laboratory reports to the Communicable Disease Surveillance Centre. J Infect. 1982;5:301–306. [Google Scholar]
- 62.Chatterton JMW, Skinner LJ, Moir IL, Joss AWL, Ho-Yen DO. Toxoplasmosis 1983–1987: season, sex, and behaviour. Commun Dis Scotland. 1988;12:5–7. [Google Scholar]
- 63.Tizard IR, Rish NA, Quinn JP. Some observations on the epidemiology of toxoplasmosis in Canada. J Hyg (Camb) 1976;77:11–21. doi: 10.1017/s0022172400055467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Innes EA. Related toxoplasmosis: comparative species susceptibility and host immune response. Comp Immunol Microbiol Infect Dis. 1997;20:131–138. doi: 10.1016/s0147-9571(96)00038-0. [DOI] [PubMed] [Google Scholar]
- 65.Cubitt WD, Ades AE, Peckham CS. Evaluation of five commercial assays for screening antenatal sera for antibodies to Toxoplasma gondii. J Clin Pathol. 1992;45:435–438. doi: 10.1136/jcp.45.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ledgerwood LG, Ewald PW, Cochran GM. Genes, germs, and schizophrenia: an evolutionary perspective. Perspect Biol Med. 2003;46:317–348. doi: 10.1353/pbm.2003.0038. [DOI] [PubMed] [Google Scholar]
- 67.Grigg ME, Bonnefoy S, Hehl AB, Suzuki Y, Boothroyd JC. Success and virulence in Toxoplasma as the result of sexual recombination between two distinct ancestries. Science. 2001;294:161–165. doi: 10.1126/science.1061888. [DOI] [PubMed] [Google Scholar]
- 68.Suzuki Y, Claflin J, Wang X, Lengi A, Kikuchi T. Related microglia and macrophages as innate producers of interferon-gamma in the brain following infection with Toxoplasma gondii. Int J Parasitol. 2005;35:83–90. doi: 10.1016/j.ijpara.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 69.Fanous AH, Kendler KS. Genetic heterogeneity, modifier genes, and quantitative phenotypes in psychiatric illness: searching for a framework. Mol Psychiatry. 2005;10:6–13. doi: 10.1038/sj.mp.4001571. [DOI] [PubMed] [Google Scholar]
- 70.Kendler KS. “A gene for…”: the nature of gene action in psychiatric disorders. Am J Psychiatry. 2005;162:1243–1252. doi: 10.1176/appi.ajp.162.7.1243. [DOI] [PubMed] [Google Scholar]