Abstract
Kynurenic acid (KYNA) is a tryptophan metabolite that is synthesized and released by astrocytes and acts as a competitive antagonist of the glycine site of N-methyl-D-aspartate receptors at high concentrations and as a noncompetitive antagonist of the α7-nicotinic acetylcholine receptor at low concentrations. The discovery of increased cortical KYNA levels in schizophrenia prompted the hypothesis that elevated KYNA concentration may underlie the working memory dysfunction observed in this population that has been attributed to altered glutamatergic and/or cholinergic transmission. The present study investigated the effect of elevated endogenous KYNA on spatial working memory function in rats. Increased KYNA levels were achieved with intraperitoneal administration of kynurenine (100 mg/kg), the precursor of KYNA synthesis. Rats were treated with either kynurenine or a vehicle solution prior to testing in a radial arm maze task at various delays. Elevations of endogenous KYNA resulted in increased errors in the radial arm maze. In separate experiments, assessment of locomotor activity in an open field and latency to retrieve food reward from one of the maze arms ruled out the possibility that deficits in the maze were attributable to altered locomotor activity or motivation to consume food. These results provide evidence that increased KYNA levels produce spatial working memory deficits and are among the first to demonstrate the influence of glia-derived molecules on cognitive function. The implications for psychopathological conditions such as schizophrenia are discussed.
Keywords: schizophrenia, nicotinic receptor, acetylcholine, glia, maze, cholinergic
Introduction
Kynurenic acid (KYNA) is a tryptophan metabolite that is synthesized and released in the brain by astrocytes.1 Depending on concentration, KYNA can act as a competitive antagonist of the glycine site of N-methyl-D-aspartate receptors (NMDARs)2 or as a modulator of cholinergic function through noncompetitive blockade of α7-nicotinic acetylcholine receptors (α7nAChRs).3–5 Both NMDAR and nAChR-mediated neurotransmission are crucial for many types of cognitive functions,6–10 and both receptors have been implicated in the pathophysiology of schizophrenia.11–16
KYNA has recently been observed to be elevated in the brain tissue17 and cerebral spinal fluid18 of individuals with schizophrenia. This observation has prompted the hypothesis that increased KYNA levels in schizophrenia may contribute to the glutamatergic- and/or cholinergic-mediated cognitive impairments that characterize this population.3,19,20 Some of these cognitive impairments include deficits in verbal and spatial working memory,21–23 which reflect difficulty with the maintenance, updating, and manipulation of information in the face of competing distractors or as environmental contingencies change.24 Spatial working memory in particular has been linked to the integrity of glutamatergic and cholinergic function in rodent models.7,9 Thus, KYNA-induced blockade of NMDARs and/or α7nAChRs may contribute to impairment in spatial working memory function.
Several recent studies have examined the effects of increased KYNA concentration on behavior by administering kynurenine, the precursor of KYNA. Kynurenine readily crosses the blood-brain barrier where it is taken up by astrocytes and converted into KYNA,1 resulting in elevated KYNA concentration in brain regions where it is normally synthesized and released. Elevating the level of endogenous KYNA by this method has high face validity for modeling changes in KYNA concentration in schizophrenia in particular. For example, kynurenine is elevated in schizophrenia17 but not in other disorders such as Alzheimer disease, where increases in cerebral KYNA content may be a product of a compromised blood-brain barrier rather than alterations of kynurenine pathway degradation per se.25,26
The effects of kynurenine administration have been explored in studies of sensory gating, including prepulse inhibition and habituation of auditory evoked potentials. Prepulse inhibition is a reduction of the response to a startle-eliciting stimulus when it is preceded by a weaker prepulse stimulus. Prepulse inhibition is thought to reflect the ability to filter extraneous sensory information and is widely regarded as a model of schizophrenia due to frequent documentation of prepulse inhibition deficits in persons with schizophrenia.27 In rats, kynurenine administration disrupted prepulse inhibition of the acoustic startle response without affecting basal startle magnitude.20 A similar gating deficit was observed when 2 auditory stimuli were presented 500 ms apart; kynurenine disrupted the normal habituation of evoked potentials in hippocampal neurons following presentation of the second auditory stimulus, an effect that did not depend on blockade of the glycine site of NMDARs.19
In the current study, elevations of endogenous KYNA were achieved by intraperitoneal (IP) injection of kynurenine. The 100 mg/kg dose of kynurenine used in this study has been previously shown to result in a 37-fold increase of cerebral extracellular KYNA 2 hours postinjection28 and was the same dose that resulted in prepulse inhibition deficits.20 Kynurenine was administered 2 hours prior to testing in an 8-arm radial maze with varying delays between each arm entry. It was hypothesized that increased KYNA concentration would interfere with spatial working memory in the radial arm maze task, especially as the temporal delays between each arm entry were increased. At the conclusion of the working memory task, rats were tested in an open field to examine the possibility that KYNA-induced alterations in working memory could be explained by changes in locomotor activity. To address the possibility that elevated KYNA alters motivation to consume food reward, a separate group of rats was tested in the radial arm maze for latency to consume food reward.
MATERIALS AND METHODS
Subjects
Subjects included 52 male Long Evans rats (Harlan, Indianapolis, IN), approximately 3 months of age at the start of the experiment. Of these rats, 40 were used in the radial arm maze working memory experiment and the open field experiment, while 12 were used in the radial arm maze motivation experiment. Rats were individually housed, with free access to water. All rats were placed on a restricted feeding regimen to maintain them at 85% of their initial free-feeding weights. Some of the rats were previously used in a fear conditioning task, while other rats were used in an appetitive Pavlovian conditioning task prior to their inclusion in the current experiment. None of these rats received any prior pharmacological manipulation. Rats were cared for in accordance with all Institutional Care and Use Committee (IACUC) and Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines.
Radial Arm Maze Apparatus
Spatial working memory and motivation studies were conducted in a standard 8-arm radial arm maze (Med Associates, St Albans, VT). The maze consisted of an octagonal-shaped central platform area measuring 35 cm in diameter. All 8 arms radiated from the central platform and each arm measured 61 cm in length, 9 cm in width, and 16.8 cm in height and was placed at equal angles around the perimeter of the central platform. Food cups were located 2 cm from the end of each arm. In total, 2 sets of photobeams were located in each arm at a height of 2 cm. All components were constructed from clear polypropylene. The maze was located in a small, soundproof room with visually distinct cues mounted on the walls. Data from our laboratory indicate that rats use a hippocampal-dependent allothetic strategy in navigating this maze (A. C. Chess, unpublished observations, 2006).
Open Field Apparatus
Twenty-four-hours after completion of the maze task, locomotor activity in an open field was assessed. The open field chamber (43.2 × 43.2 cm) was composed of plexiglass walls and was connected to a personal computer running Open Field Activity Software (Med Associates, St Albans, VT). The chamber was equipped with 16 photobeams mounted on the sides at 2 different heights (3.5 and 7.5 cm from the floor) to monitor locomotor activity.
Drugs
L-kynurenine (L-KYN; Sigma, St Louis, MO) was prepared fresh daily. L-KYN was dissolved in 2N NaOH and brought to a final volume (30 mg/ml) with 0.1 M 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer. HCl (1N) was used to bring the pH to ∼8.4. Rats were given IP injections of 100 mg/kg 2 hours prior to testing. This dose produces a 37-fold increase in brain KYNA 2 hours postinjection,28 and because KYNA is the final metabolic product of this sidearm of the kynurenine pathway, the observed behavioral effects cannot be attributed to increases of downstream molecules. Further, L-KYN administration does not produce significant elevations of other molecules of kynurenine degradation (eg, quinolinic acid).19,29
Behavioral Procedure and Data Analysis
Radial Arm Maze: Working Memory
Once rats reached their target weights, they were habituated to the maze over 4 daily sessions. The first 2 days of habituation included 5 minutes of free exploration throughout the entire maze. Food pellets were scattered through the maze and in the food cups. The last 2 days of habituation included 5 minutes of free exploration with food pellets only present in the recessed food cups. Rats were monitored to ensure that they were eating food pellets out of all the food cups by the end of habituation training.
Following the 4 habituation sessions, all rats participated in 24 days of training. No drug was administered during this time. One trial was conducted each day, during which a rat was placed in the central hub of the maze and was always oriented in the same direction within the central hub. The doors for all arms were raised simultaneously. The rat could enter any of the 8 arms to obtain a food reward. Arm entry was defined by placement of all 4 paws inside the arm. Following an arm entry, the doors for all the arms were simultaneously closed, with the exception of the entered arm which remained open to the central hub. After consuming the food, the rat left the arm and returned to the central hub. The door to the arm that was just traversed was then closed. After a delay of 5 seconds, all doors opened again, and the rat was allowed to choose any of the 8 arms. This process was repeated until all arms were entered and all food was consumed or until 5 minutes elapsed. Rats were evaluated on 3 measures: errors of commission refer to reentering a previously entered arm on that trial, errors of omission refer to failure to enter an arm on a trial, and total errors refer to the sum of errors of commission and errors of omission.
Effects of cholinergic manipulations can be detected by sufficiently demanding task conditions30 (ie, a delay interposed between each arm entry instead of one delay after a series of arm entries). The radial arm maze procedure used in this study was employed because of its difficulty relative to other commonly used behavioral procedures.31,32 Rats were excluded from subsequent testing at the various delays if they exhibited more than one error on average during the last 3 days of training. This criterion was adopted to ensure that all rats participating in the test phase had acquired the task so that subsequent increases in the temporal delay between arm entries would yield measurable decrements in performance. To ensure that performance of L-KYN and Vehicle rats was equivalent prior to receiving drug, animals were assigned to drug conditions such that each groups' performance during the training segment of the experiment was equivalent.
During the test phase, rats received injections of either L-KYN or Vehicle 2 hours prior to each trial. The test phase consisted of the introduction of 3 counterbalanced delays (5, 15, and 30 seconds) between each arm entry over 3 days of testing. Thus, rats in each group received a random sequence of delays during the test phase. Rats were allotted 300, 355, and 575 seconds to complete the 5-, 15-, and 30-second delay trials, respectively. Errors were recorded as described above. Typically, rats in this task vary the order in which arms are entered each day; thus, this training protocol engages working memory and not reference memory. Working memory requires maintenance and updating of information during a single trial while reference memory requires consolidation and retrieval of information across multiple trials.33
Analysis of Radial Arm Maze Working Memory Data
During the training portion of the experiment, a repeated-measures analysis of variance (ANOVA) was conducted on the total errors for each day of training, with Day as the repeated measure and Group as the between-subjects variable. During the testing portion of the experiment, a repeated-measures ANOVA was conducted on the number of errors of omission, errors of commission, and total errors (errors of omission + errors of commission), with Drug Treatment (L-KYN vs Vehicle) as a between-subjects variable and Delay (5, 15, or 30 seconds) as the repeated measure. A between-subjects analysis of errors at the 5-second delay was planned to address the possibility that addition of the drug after 24 days of training produced state-dependent effects.
It was expected that the majority of errors of commission would occur toward the end of a trial as the demands on working memory were progressively increased. To determine whether errors of commission were exhibited predominantly during the later portions of a given trial, the total arm entries were divided in half for each rat. Using a paired-samples t test, errors of commission exhibited during the first half of the arms entered were compared with the errors of commission exhibited during the second half of the arms that were entered.
Open Field
Twenty-four-hours after the last day of maze testing, rats received an injection of the same solution they received during the maze experiment. Two hours following injection, rats were placed individually in the open field chamber and were allowed to explore the chamber for 10 minutes, during which time the total distance traveled was monitored by a computer. The chamber was cleaned with a Quatricide solution between animals.
Analysis of Open Field Activity Data
Locomotor activity was analyzed using a repeated-measures ANOVA. The total distance traveled was averaged into five 2-minute blocks. Each block was treated as a repeated measure, while Group (L-KYN vs Vehicle) was the between-subjects factor. To further assess whether locomotor activity might explain the radial arm maze findings, separate Pearson correlation coefficients were calculated for the Vehicle and L-KYN groups to determine if activity levels in the open field predicted the total number of errors in the radial arm maze.
Radial Arm Maze: Motivation
A separate group of 12 rats were trained to eat from the recessed food cup at the end of one of the maze arms. On the first 3 days of training, rats were placed in the central platform of the maze. The door to one of the arms was raised, and rats were permitted to explore and eat food pellets scattered along the arm and in the food cup for 5 minutes. On 3 subsequent training days, pellets were available only in the food cup. Latency to eat food pellets from the start of the trial (opening of the door) was recorded. After 6 total days of training, rats were matched into equivalent groups based on training latency and were then given IP injections of either L-KYN or Vehicle 2 hours prior to each test day over 3 days. Test days were identical to the last 3 training days. Latency was recorded over these 3 test days.
Analysis of Radial Arm Maze Motivation Data
Latency to eat food pellets was analyzed over the 3 test days using a repeated-measures ANOVA with Day as the repeated measure and Group as the between-subjects variable. An alpha level of 0.05 was used for all analyses.
Results
Rats were excluded from testing at the various delays in the radial arm maze working memory study if they exhibited more than one error on average during the last 3 days of training. Thus, of the 40 rats trained on this task, 21 were excluded from the analyses because they failed to meet this strict criterion or because they received the wrong injection. In all, 19 rats remained in the study, resulting in the following final sample sizes: vehicle, N = 9; L-KYN, N = 10. In the motivation study, all 12 rats were retained for the analyses, resulting in the following final sample sizes: Vehicle, N = 6; L-KYN, N = 6.
Working Memory Training
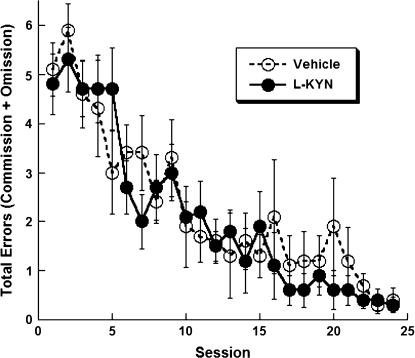
Rats gradually acquired the ability to obtain food rewards with very few errors at the standard 5-second delay (figure 1). Using total errors as the dependent variable, a repeated-measures ANOVA revealed a significant effect of Day, F23,391 = 18.2, P < 0.05, but no effect of Group or a Group × Day interaction, indicating that the rats' radial arm maze performance was roughly equivalent prior to receiving drug.
Fig. 1.
Total errors (errors of omission + commission) during the training portion of the radial arm maze task using a 5-second delay between each arm entry. Rats decreased total errors as training progressed over 24 daily sessions. No drug treatments were given during the training portion; the separate plots of Vehicle and L-KYN groups are intended to illustrate the equivalence of the groups' radial maze performance prior to receiving drug. All data are mean ± SEM.
Working Memory Testing
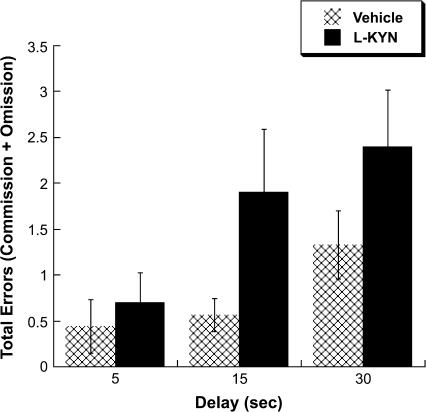
Figure 2 illustrates radial arm maze performance during the test phase. Examination of total errors revealed a main effect of Delay, F2,34 = 4.183, P < 0.05, and a main effect of Group, F1,17 = 4.618, P < 0.05, but no Delay × Group interaction. Heterogeneity of variance was detected using Levene's test for equality of variance. Thus, an independent-samples t test was calculated assuming unequal variance. This test revealed a significant difference between Vehicle- and L-KYN–treated rats, t(43) = −2.3, P < 0.05, with Vehicle-treated rats exhibiting fewer total errors (M = 0.78, SD = 0.93) than L-KYN–treated rats (M = 1.67, SD = 1.88). Comparison of the 3 delay conditions revealed a significant difference between the 5- and 30-second delays, t(18) = −3.19, P < 0.05, with fewer errors exhibited during the 5-second delay (M = 0.58, SD = 0.96) compared with the 30-second delay (M = 1.89, SD = 1.66). Comparisons of the 5- and 15-second delays and the 15- and 30-second delays failed to reach significance. To address the possibility of state dependence in the second phase of the task, an independent-samples t test was conducted at the 5-second delay. There was no significant difference between Vehicle- and L-KYN–treated rats at the 5-second delay, suggesting that impaired performance at the longer delays in the L-KYN–treated group was not simply a product of state-dependent effects.
Fig. 2.
Total errors (errors of omission + commission) during the testing portion of the radial arm maze task at 3 counterbalanced delays (5, 15, and 30-seconds). L-KYN rats exhibited significantly more total errors than rats in the Vehicle condition. All data are mean ± SEM.
The main effect of Group described above does not appear to be driven by errors of commission (see table 1 for separate listing of errors of commission and omission). For errors of commission, there was a significant main effect of Delay, F2,34 = 4.02, P < 0.05, but no significant effect of Group or a Group × Delay interaction. In contrast, a significant main effect of Group was observed for errors of omission, F1,17 = 5.36, P < 0.05. Nevertheless, there was no significant effect of Delay nor was there a significant interaction for errors of omission. Levene's test revealed heterogeneity of variance, and thus, a t test was conducted assuming unequal variances. This comparison revealed significantly more errors of omission in the L-KYN group (M = 0.97, SD = 1.43) compared with the Vehicle group (M = 0.22, SD = 0.51), t(37) = −2.678, P < 0.05. To test whether the elevation of errors of omission in the L-KYN group was due to differences in the total number of arms entered, t tests were calculated to compare the groups on the total number of arm entries at each delay. There were no significant differences between the total number of arm entries made in the Vehicle or L-KYN groups at any of the delays. To address whether the increase in errors of omission in the L-KYN group was due to differences in latency to complete the task, independent-samples t tests were conducted on latency at each of the 3 delays. There were no differences in latency between the groups at any of the tested delays.
Table 1.
Average Errors of Commission and Omission in Vehicle and L-KYN Groups at Each of 3 Temporal Delays.
| Commission |
Omission |
|||||
| 5 - second | 15 - second | 30 - second | 5 - second | 15 - second | 30 - second | |
| Vehicle | 0.33 ± 0.24 | 0.44 ± 0.18 | 0.89 ± 0.2 | 0.11 ± 0.11 | 0.11 ± 0.11 | 0.44 ± 0.24 |
| L-KYN | 0.4 ± 0.22 | 0.5 ± 0.22 | 1.2 ± 0.44 | 0.3 ± 0.21 | 1.4 ± 0.65 | 1.2 ± 0.33 |
Note: Data are mean number of errors ± SEM.
Errors of commission during the first and second halves of each trial were compared. Collapsed across delays, both Vehicle, t(26) = −3.328, P < 0.05, and L-KYN–treated rats, t(29) = −3.2, P < 0.05, exhibited significantly more errors during the second half of the arm entries on a given trial (Vehicle: M = 0.59, SD = 0.75; L-KYN: M = 0.82, SD = 1.34) compared with the first half (Vehicle: M = 0.19, SD = 0.48; L-KYN: M = 0.15, SD = 0.40). This suggests that both groups had difficulty maintaining spatial information as the trial progressed. There were no between-groups differences in errors of commission during either the first or second portion of the trial at any of the delays.
Open Field
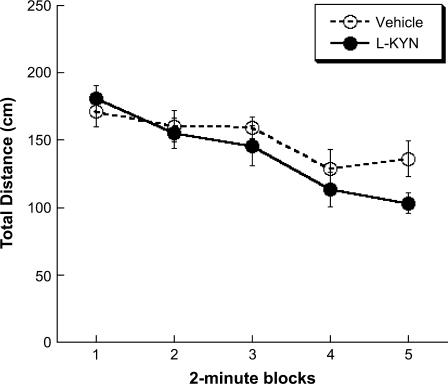
In all, 2 rats were excluded from these analyses due to technical difficulties with the data collection leaving sample sizes of 7 and 10 for the Vehicle and L-KYN groups, respectively. A repeated-measures ANOVA was conducted using the Greenhouse-Geisser correction for violation of sphericity. There was a significant main effect of Block, F3, 41 = 13.504, P < 0.05, but no main effect of Group or a Group × Block interaction, indicating that both groups habituated locomotor activity in the open field at the same rate (figure 3). Although it was not statistically significant, there appeared to be greater activity in the Vehicle group compared with the L-KYN group during the fifth block. Nevertheless, the fifth block of locomotor activity was not significantly correlated with total errors in the radial arm maze task in either the Vehicle or L-KYN groups, suggesting that any slight variations in locomotor activity were unlikely to account for radial arm maze performance.
Fig. 3.
Locomotor activity in an open field. L-KYN and Vehicle rats did not differ in the total distance traveled during a 10-minute free exploration test. All data are mean ± SEM.
Motivation Task
A repeated-measures ANOVA revealed no significant main effects of Day or Group; thus, the latency to consume food reward did not differ between Vehicle (M = 6.56, SD = 3.36) and L-KYN–treated rats (M = 8.78, SD = 7.02). In addition, there was no Day × Group interaction, suggesting that elevated KYNA does not affect motivation to eat from the recessed food cup in the maze and that this lack of effect is stable across 3 days of drug administration.
Discussion
The present results indicate that increases in endogenous KYNA concentration disrupt spatial working memory and that this disruption is not attributable to general alterations in locomotor activity or motivation to consume food reward. These findings are consistent with both the basic science literature which has documented disruption of spatial working memory following either NMDA or nicotinic receptor blockade, and the clinical literature, which describes similar deficits in individuals with schizophrenia, a population exhibiting increases in KYNA concentration. Furthermore, the results support the hypothesis that increased concentration of KYNA may contribute to the observed deficits in working memory in persons with schizophrenia.
The observation that L-KYN and Vehicle rats did not differ on errors of commission in the present study suggests that the impaired performance of L-KYN rats was not driven by perseverative responding on any particular arm or subset of arms. Instead, L-KYN rats exhibited a significant increase in errors of omission by failing to enter arms they had not yet entered on that particular trial. This effect did not depend on locomotor activity levels, total number of arm entries required to complete the trial, latency to complete the trial, or motivation to eat from the recessed food cup. It is unclear whether this increase in errors of omission in the L-KYN group reflects an inability to update spatial information as the trial progresses or an inability to maintain information about spatial locations visited earlier in the trial. In addition, the increased number of errors in L-KYN–treated rats does not appear to be simply the result of state-dependent effects because Vehicle- and L-KYN–treated rats exhibited comparable performance at the 5-second delay.
Increased errors of omission in L-KYN–treated rats may be understood in the context of experiments involving other NMDA and nicotinic receptor compounds. For instance, the nicotinic receptor antagonist mecamylamine increased errors of omission in a water maze discrimination task34 and in a sustained attention task.35 Similar increases of omission errors were observed in a sustained attention task following nicotine withdrawal or dopamine receptor blockade in rats.36 The latter effect suggests the possibility that administration of KYNA in the current study may have increased omission errors through blockade of presynaptic α7nAChRs on dopaminergic neurons. Intrahippocampal infusions of the specific NMDA glycine site antagonist 7-chlorokynurenic acid similarly increased errors of omission in a water maze discrimination task.37 Thus, it is unclear whether the increased errors of omission in the current study were a consequence of NMDA or nicotinic receptor mechanisms, although the lack of an effect at the 5-second delay provides indirect evidence against KYNA-induced NMDAR antagonism.
Several previous studies examined the behavioral effects of increased KYNA concentration by administering exogenous KYNA. Peripheral KYNA administration did not disrupt the ability to use olfactory cues to differentiate between novel and familiar conspecifics.38 A similar study reported enhancements in social and object recognition following peripheral KYNA administration.39 These results are difficult to interpret, however, because peripherally administered KYNA is essentially unable to cross the blood-brain barrier.2
Another study found that intracerebroventricular infusions of exogenous KYNA did not affect the formation of simple associations between a visual cue and food, although KYNA administration for 2 days prior to conditioning altered subsequent conditioned orienting behavior compared with rats not receiving drug preexposure.40 Posttraining infusions of KYNA into the shell of the nucleus accumbens increased working memory and reference memory errors in a 4-arm baited 8-arm radial maze task in one study41 but not in another similar study.42 Although administration of KYNA directly into the brain circumvents the problems associated with peripheral injection, the application of exogenous KYNA results in a ubiquitous elevation of KYNA that is distributed homogeneously throughout the tissue, which can also confound interpretation. In contrast, administration of the precursor kynurenine as in the current study and other recent reports19,20 results in elevations of endogenous KYNA concentration in regions where it is normally synthesized and released, more accurately modeling clinical conditions and likely producing more valid results.
Spatial working memory deficits are one manifestation of the core neuropsychological working memory deficits characterizing schizophrenia.21 For example, visuospatial working memory deficits are observed in this population using a task previously shown to recruit Walker area 46 in nonhuman primates,23 an area that is homologous to dorsolateral prefrontal cortex (DLPFC) in humans. Recent human imaging studies confirm involvement of DLPFC in both the maintenance and manipulation of spatial information over time and demonstrate less recruitment of this region in schizophrenic subjects compared with controls.43 Elevated KYNA concentration has been observed in the DLPFC of schizophrenic brains,17 suggesting the possibility that compromised spatial working memory ability in schizophrenia may occur through blockade of NMDARs and/or α7nAChRs in prefrontal cortex.
Alternatively, KYNA-induced spatial working memory impairments could be mediated by the hippocampus, a structure that has been extensively implicated in the ability to maintain spatial locations in working memory.44 The current study required that the rats use a win-shift strategy that is known to depend on the integrity of the hippocampus. Both NMDARs and α7nAChRs are highly concentrated in the hippocampus,7,45 and thus, KYNA-induced blockade of these hippocampal receptors could disrupt spatial working memory function. Consistent with the present findings, intrahippocampal infusion of the nonselective nicotinic receptor antagonist mecamylamine46 or the specific NMDA glycine site antagonist 7-chlorokynurenic acid6 increased incorrect panel choices in a 3-panel runway task. Similarly, infusion of either α7nAChR or α4β2nAChR antagonists into the ventral hippocampus decreased the number of arm entries before reentry of a previously entered arm.10
Although our experimental design did not allow us to directly determine whether the results were produced by the action of KYNA at NMDARs or α7nAChRs, several factors suggest that the observed deficits in working memory were due to blockade of α7nAChRs. First, a slightly higher dose of kynurenine (150 mg/kg) than the one used in the current study (100 mg/kg) was found to impair sensory gating, an effect that was not attributable to blockade of the glycine site of the NMDAR.19 Secondly, administration of 100 mg/kg kynurenine in rats has been previously shown to produce up to 37-fold increases in extracellular KYNA concentration from basal levels of 17.1 ± 1.1 nM.28 Thus, a 100 mg/kg dose of kynurenine may maximally increase KYNA concentration to ∼0.5 μM, which is in the range of endogenous KYNA concentration,1 and has been shown to preferentially affect α7nAChR rather than NMDAR function in vitro.3 For example, 0.1 and 1.0 μM KYNA-reduced α7nAChR activity by 20% and 40%, respectively, while these same concentrations had no effect on NMDARs in the presence of added glycine. In the absence of glycine, 0.1 and 1.0 μM KYNA-reduced NMDAR activity by 10% and 20%, respectively.3 Furthermore, KYNA primarily targets α7nAChRs at physiologically relevant concentrations in knockout mice lacking the biosynthetic enzyme for KYNA.4 Nevertheless, caution should be exercised in using results from in vitro studies to make inferences regarding the receptor mechanisms involved in the current study.
The current results provide evidence that KYNA, a glia-derived molecule, is capable of influencing cognitive functions that depend on intact NMDA or nicotinic receptor transmission. These findings suggest that increased KYNA concentration observed in schizophrenia may contribute to cognitive impairment. This line of reasoning would also suggest that cognitive symptoms reminiscent of schizophrenia would be expected in diseases affecting immune function and consequent KYNA concentration. Interestingly, individuals infected with HIV-1 can develop HIV-associated dementia, in addition to exhibiting reactive astrocytes47 and increases in kynurenine, kynurenic acid, and its biosynthetic enzyme kynurenine aminotransferase.26 These findings lend support to the notion that upregulation of kynurenine and KYNA may contribute to symptoms that are similar to those observed in schizophrenia and extend the implications of our data to cases in which the source of this upregulation is immune activated. Further research is required to determine the neural regions mediating the behavioral effects of KYNA. Of additional importance is the development of therapeutic tools capable of targeting the kynurenine pathway, with the goal of attenuating cognitive impairments induced by increased KYNA concentration.
Acknowledgments
This research was supported by National Institute of Mental Health (NIMH) grants R03MH066941 and R21MH069670 to David J. Bucci and NIMH grant F31MH 73304 to Amy C. Chess.
References
- 1.Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- 2.Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309–379. [PubMed] [Google Scholar]
- 3.Hilmas C, Pereira EFR, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alkondon M, Pereira EFR, Yu P, et al. Targeted deletion of the kynurenine aminotransferase II gene reveals a critical role of endogenous kynurenic acid in the regulation of synaptic transmission via alpha7 nicotinic receptors in the hippocampus. J Neurosci. 2004;24:4635–4648. doi: 10.1523/JNEUROSCI.5631-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopes C, Pereira EFR, Wu H, Rassoulpour A, Schwarcz R, Albuquerque EX. In Vitro and In Vivo Interactions Between Kynurenic Acid and The Nicotinic Allosteric Potentiating Ligand Galantamine: Clinical Relevance. Program No. 953.12 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2005. [Google Scholar]
- 6.Ohno M, Yamamoto T, Watanabe S. Intrahippocampal administration of a glycine site antagonist impairs working memory performance of rats. Eur J Pharmacol. 1994;253:183–187. doi: 10.1016/0014-2999(94)90776-5. [DOI] [PubMed] [Google Scholar]
- 7.Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004;5:361–372. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- 8.Lee I, Kesner RP. Differential contribution of NMDA receptors in hippocampal subregions to spatial working memory. Nat Neurosci. 2002;5:162–168. doi: 10.1038/nn790. [DOI] [PubMed] [Google Scholar]
- 9.Levin E, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology. 1998;138:217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- 10.Felix R, Levin ED. Nicotinic antagonist administration into the ventral hippocampus and spatial working memory in rats. Neuroscience. 1997;81:1009–1017. doi: 10.1016/s0306-4522(97)00224-8. [DOI] [PubMed] [Google Scholar]
- 11.Coyle JT, Tsai G, Goff D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Ann N Y Acad Sci. 2003;1003:318–327. doi: 10.1196/annals.1300.020. [DOI] [PubMed] [Google Scholar]
- 12.Millan MJ. N-methyl-D-aspartate receptors as a target for improved antipsychotic agents: novel insights and clinical perspectives. Psychopharmacology (Berl) 2005;179:30–53. doi: 10.1007/s00213-005-2199-1. [DOI] [PubMed] [Google Scholar]
- 13.Woodruff-Pak DS, Gould TJ. Neuronal nicotinic acetylcholine receptors: involvement in Alzheimer's disease and schizophrenia. Behav Cogn Neurosci Rev. 2002;1:5–20. doi: 10.1177/1534582302001001002. [DOI] [PubMed] [Google Scholar]
- 14.Sacco KA, Bannon KL, George TP. Nicotinic receptor mechanisms and cognition in normal states and neuropsychiatric disorders. J Psychopharmacol. 2004;18:457–474. doi: 10.1177/0269881104047273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacco KA, Termine A, Seyal A, et al. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry. 2005;62:649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- 16.Sawa A, Snyder SH. Schizophrenia: neural mechanisms for novel therapies. Mol Med. 2003;9:3–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Schwarcz R, Rassoulpour A, Wu H-Q, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521–530. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- 18.Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313:96–98. doi: 10.1016/s0304-3940(01)02242-x. [DOI] [PubMed] [Google Scholar]
- 19.Shepard PD, Joy B, Clerkin L, Schwarcz R. Micromolar brain levels of kynurenic acid are associated with a disruption of auditory sensory gating in the rat. Neuropsychopharmacology. 2003;28:1454–1462. doi: 10.1038/sj.npp.1300188. [DOI] [PubMed] [Google Scholar]
- 20.Erhardt S, Schwieler L, Emanuelsson C, Geyer M. Endogenous kynurenic acid disrupts prepulse inhibition. Biol Psychiatry. 2004;56:255–260. doi: 10.1016/j.biopsych.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. Am J Psychiatry. 2003;160:1809–1816. doi: 10.1176/appi.ajp.160.10.1809. [DOI] [PubMed] [Google Scholar]
- 22.McGurk SR, Green MF, Wirshing WC, et al. Antipsychotic and anticholinergic effects on two types of spatial memory in schizophrenia. Schizophr Res. 2004;68:225–233. doi: 10.1016/S0920-9964(03)00123-3. [DOI] [PubMed] [Google Scholar]
- 23.Keefe RS, Roitman SE, Harvey PD, et al. A pen-and-paper human analogue of a monkey prefrontal cortex activation task: spatial working memory in patients with schizophrenia. Schizophr Res. 1995;17:25–33. doi: 10.1016/0920-9964(95)00027-j. [DOI] [PubMed] [Google Scholar]
- 24.Smith EE, Jonides J. Executive control and thought. In: Squire LR, Bloom FE, McConnell SK, Roberts JL, Spitzer NC, Zigmond MJ, editors. Fundamental Neuroscience. San Diego, Calif: Academic Press; 2003. pp. 1377–1394. [Google Scholar]
- 25.Baran H, Jellinger K, Deecke L. Kynurenine metabolism in Alzheimer's disease. J Neural Transm. 1999;106:165–181. doi: 10.1007/s007020050149. [DOI] [PubMed] [Google Scholar]
- 26.Baran H, Hainfellner JA, Kepplinger B, Mazal PR, Schmid H, Budka H. Kynurenic acid metabolism in the brain of HIV-1 infected patients. J Neural Transm. 2000;107:1127–1138. doi: 10.1007/s007020070026. [DOI] [PubMed] [Google Scholar]
- 27.Geyer MA, Krebs-Thompson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- 28.Swartz KJ, During MJ, Freese A, Beal MF. Cerebral synthesis and release of kynurenic acid: an endogenous antagonist of excitatory amino acid receptors. J Neurosci. 1990;10:2965–2973. doi: 10.1523/JNEUROSCI.10-09-02965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu H-Q, Guidetti P, Goodman JH, Varasi M, Ceresoli-Borroni G, Speciale C, Scharfman HE, Schwarcz R. Kynurenergic manipulations influence excitatory synaptic function and excitotoxic vulnerability in the rat hippocampus in vivo. Neuroscience. 2000;97:243–251. doi: 10.1016/s0306-4522(00)00030-0. [DOI] [PubMed] [Google Scholar]
- 30.Sarter M, Bruno JP, Givens B. Attentional functions of cortical cholinergic inputs: what does it mean for learning and memory? Neurobiol Learn Mem. 2003;80:246–256. doi: 10.1016/s1074-7427(03)00070-4. [DOI] [PubMed] [Google Scholar]
- 31.Wrenn CC, Lappi DA, Wiley RG. Threshold relationship between lesion extent of the cholinergic basal forebrain in the rat and working memory impairment in the radial maze. Brain Res. 1999;847:284–298. doi: 10.1016/s0006-8993(99)02099-5. [DOI] [PubMed] [Google Scholar]
- 32.Vuckovich JA, Semel ME, Baxter MG. Extensive lesions of cholinergic basal forebrain neurons do no impair spatial working memory. Learn Mem. 2004;11:87–94. doi: 10.1101/lm.63504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galani R, Obis S, Coutureau E, Jarrard L, Cassel J-C. A comparison of the effects of fimbria-fornix, hippocampal, or entorhinal cortex lesions on spatial reference and working memory in rats: short versus long postsurgical recovery period. Neurobiol Learn Mem. 2002;77:1–16. doi: 10.1006/nlme.2000.3998. [DOI] [PubMed] [Google Scholar]
- 34.Steckler T, Holsboer F. Interaction between the cholinergic system and CRH in the modulation of spatial discrimination learning in mice. Brain Res. 2001;906:46–59. doi: 10.1016/s0006-8993(01)02555-0. [DOI] [PubMed] [Google Scholar]
- 35.Turski J, Holley LA, Sarter M. Effects of nicotinic acetylcholine receptor ligands on behavioral vigilance in rats. Psychopharmacology. 1995;118:195–205. doi: 10.1007/BF02245840. [DOI] [PubMed] [Google Scholar]
- 36.Shoaib M, Bizarro L. Deficits in a sustained attention task following nicotine withdrawal in rats. Psychopharmacology. 2005;178:211–222. doi: 10.1007/s00213-004-2004-6. [DOI] [PubMed] [Google Scholar]
- 37.Carli M, Balducci C, Samanin R. Stimulation of 5-HT1A receptors in the dorsal raphe ameliorates the impairment of spatial learning caused by intrahippocampal 7-chloro-kynurenic acid in naïve and pretrained rats. Psychopharmacology. 2001;158:39–47. doi: 10.1007/s002130100837. [DOI] [PubMed] [Google Scholar]
- 38.Hliňák Z, Krejčí I. Kynurenic acid prevented social recognition deficits induced by MK-801 in rats. Physiol Res. 2003;52:805–808. [PubMed] [Google Scholar]
- 39.Hliňák Z, Krejčí I. Kynurenic acid and 5,7-dichlorokynurenic acids improve social and object recognition in male rats. Psychopharmacology. 1995;120:463–469. doi: 10.1007/BF02245819. [DOI] [PubMed] [Google Scholar]
- 40.Chess AC, Bucci DJ. Increased concentration of cerebral kynurenic acid alters stimulus processing and conditioned responding. Behav Brain Res. 2006;170:326–332. doi: 10.1016/j.bbr.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Klein S, Hadamitzky M, Koch M, Schwabe K. Role of glutamate receptors in nucleus accumbens core and shell in spatial behaviour of rats. Neuroscience. 2004;128:229–238. doi: 10.1016/j.neuroscience.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 42.Schacter GB, Yang CR, Innis NK, Mogenson GJ. The role of the hippocampal-nucleus accumbens pathway in radial-arm maze performance. Brain Res. 1989;494:339–349. doi: 10.1016/0006-8993(89)90602-1. [DOI] [PubMed] [Google Scholar]
- 43.Cannon TD, Glahn DC, Kim J, et al. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Arch Gen Psychiatry. 2005;62:1071–1080. doi: 10.1001/archpsyc.62.10.1071. [DOI] [PubMed] [Google Scholar]
- 44.Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 45.Tribollet E, Bertrand D, Marguerat A, Raggenbass M. Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: an autoradiographic study in the rat brain. Neuroscience. 2004;124:405–420. doi: 10.1016/j.neuroscience.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 46.Ohno M, Yamamoto T, Watanabe S. Blockade of hippocampal nicotinic receptors impairs working memory but not reference memory in rats. Pharmacol Biochem Behav. 1993;45:89–93. doi: 10.1016/0091-3057(93)90091-7. [DOI] [PubMed] [Google Scholar]
- 47.Wang T, Rumbaugh JA, Nath A. Viruses and the brain: from inflammation to dementia. Clin Sci. 2006;1:393–407. doi: 10.1042/CS20050278. [DOI] [PubMed] [Google Scholar]