Abstract
There is growing interest in the prodromal stage of psychotic disorders, with many services now providing care for these “ultra high risk” (UHR) individuals. However, a reduction in the rate of transition to psychosis has been suspected over the last few years. This has implications for the use of interventions in this population and for the validity of research findings. This study examined the transition rate in one UHR service, the Personal Assessment and Crisis Evaluation Clinic, over the years 1995–2000 and investigated possible causes for the transition rate reduction. There was evidence for a declining transition rate, with each successive year showing a rate 0.80 times that of the preceding year. Functioning and symptom level were not responsible for the change. The decreased transition rate was partly explained by a reduction in the duration of symptoms of patients prior to receiving help. That is, UHR individuals are being detected and provided with care earlier than in the past. Thus, the decline in transition rate may be due to treatment being more effective at this very early stage of illness or it may be due to finding more false positives who were never at risk of psychosis, ie, a “dilution” effect. Given that it is not possible to distinguish between these alternatives at least phenotypically at present, perhaps it is time to rethink the role and practice of UHR clinics. Patients presenting to them need help. It may be that we need to aim to prevent a range of target syndromes.
Keywords: schizophrenia, prodrome, prodromal, high risk, ultra high risk, early psychosis
Introduction
Many symptoms and a great deal of disability develop during the prodromal phase of schizophrenia and other psychotic disorders 1–3. It is also possible that neurobiological and neurocognitive changes occur during this period.4,5 This underlines the importance of recognizing emerging psychotic disorder. If the prodrome can be detected prospectively and treatment provided at this stage, then disability could be minimized, some recovery may occur before symptoms and poor functioning become entrenched, and the possibility of preventing, delaying, or ameliorating the onset of diagnosable psychotic disorder arises.
However, prodromal symptoms are nonspecific. Common prodromal symptoms include depression, anxiety, and social withdrawal.2 Even psychotic-like experiences (attenuated or subthreshold psychotic symptoms) have been found to occur commonly in the general population, especially among adolescents and young adults.6–10 Because of this lack of specificity, there are problems with using prodromal symptoms and signs alone to identify people thought to be at incipient risk of onset of psychotic disorder as this would result in a high false positive rate, ie, many people not actually at risk of psychosis would be falsely labeled as such. Thus, some added criteria are needed to identify those most likely to be at highest risk.
In order to address this issue, we used a “close-in strategy”11 which requires multiple risk factors to be combined to define a group at ultra high risk (UHR) of psychosis, that is putatively prodromal. Age in the period of highest risk of first onset of psychotic disorder is one intake criterion. In addition, 3 criteria are currently used to identify the UHR group.12,13 These are (1) Attenuated Psychotic Symptoms (APS) Group: have experienced subthreshold, attenuated positive psychotic symptoms during the past year; (2) Brief Limited Intermittent Psychotic Symptoms (BLIPS) Group: have experienced episodes of frank psychotic symptoms that have not lasted longer than a week and have been spontaneously abated; or (3) Trait plus State Risk Factor Group: have a first-degree relative with a psychotic disorder or the identified client has a schizotypal personality disorder and they have experienced a significant decrease in functioning during the previous year. Additionally, a perceived need for psychiatric help is also required as the person needs to have been referred to a specialized psychiatric service for management, the Personal Assessment and Crisis Evaluation (PACE) Clinic.14
Using these UHR criteria, we found a rate of transition to psychosis of over 34% within 6 months of referral to the PACE service and between 35% and 40% within 12 months.12,13 These rates are several thousand fold over the expected incidence rate for first-episode psychosis in the general population. This occurred despite the provision of supportive counseling, case management, and antidepressant medication if required.
The PACE UHR criteria have since been adopted and adapted by a number of other settings around the world.15,16 For example, the Prevention through Risk Identification, Management and Education Clinic at Yale University, USA, reported a 54% transition rate (7 of 13 subjects) within 12 months.17 The Psychological Assistance Service in Newcastle, Australia, described a 50% transition rate over 12 months,18 the TOPP Clinic in Norway reported a 12-month transition rate of 43%,19 the Early Identification and Intervention Evaluation Clinic in Manchester, UK, described a 22% transition rate,20 and the Cognitive Assessment and Risk Evaluation (CARE) Clinic in San Diego reported a 15% transition rate at 12 months.15 (For a more detailed review, see Haroun et al15, Olsen and Rosenbaum16, and McGorry et al.21)
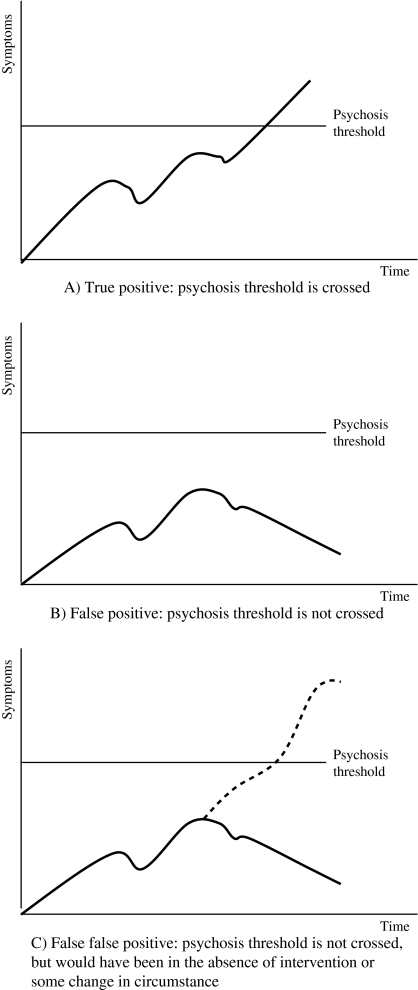
However, a reduction in the rate of transition to psychosis has been suspected over the last few years. Recent data from the PACE Clinic found a 6-month transition rate of only 9.2%.22 Although a young person meeting UHR criteria still has significantly greater odds of becoming psychotic within a brief time period than someone not meeting the criteria,22 the apparently decreasing transition rate needs examination. It has implications for the use of interventions in this population. If a high proportion of those treated to prevent or delay psychosis are in fact “false positives” and were not actually at risk of developing psychotic disorder, at least in the short term, then intervention may not be justified. This would be particularly the case with biological treatments such as antipsychotics, which have potentially harmful side effects. Psychosocial treatment may be harmful too, if it involves educating individuals about psychosis and informing them that they are at risk, if they are not actually at risk. Treatment may needlessly alarm them, result in possible curtailment of activities, and cause stigma including self-stigmatization (eg, they may decide to cease attending university or work to avoid stress).23–25 The seeming reduction in transition rate is also relevant for research. Investigators have assumed that those meeting the UHR criteria are in the prodromal phase of either psychotic disorders in general and/or of schizophrenia in particular. Some even refer to “the prodrome” or “prodromals,” an attitude that reifies the syndrome.26 However, if the risk in this cohort has truly fallen to rates around 10%–15%, then the assumption that a high proportion of the UHR population are in the prodromal phase may no longer be valid. True positives (those making the transition) will be outnumbered by false positives. However, the possibility of “false false positives,” that is those who would have made the transition to psychotic disorder had it not been for some intervention or change in circumstances, needs to be remembered.12 At follow-up points, these false false positives are impossible to distinguish phenotypically from false positives: they have both failed to make the transition to full blown psychotic disorder (see figure 1). False false positives may differ from false positives genotypically or endophenotypically.
Fig. 1.
Theoretical Distinction Between False Positives and False False Positives. (A) True positive: psychosis threshold is crossed. (B) False positive: psychosis threshold is not crossed. (C) False false positive: psychosis threshold is not crossed but would have been in the absence of intervention or some change in circumstance.
Given the relatively low transition rate and our inability to distinguish between false positives and false false positives, the findings about a range of proposed endophenotypes in the UHR population, including neurocognitive, neurophysiological, and biological, cannot be assumed to be tenable for the “prodrome” or early stage of schizophrenia. Presumed endophenotypes for psychotic disorder in general, or schizophrenia in particular, may actually just be indicative of psychiatric distress, a feature of all young people seeking help at UHR or prodromal clinics.
Because of the importance of the issue, we set out to examine the transition rate over time in more detail. We aimed to explore possible clinical and demographic factors which may be contributing to a seeming reduction in the rate.
Aims
To determine if there is a change in transition rate over time among PACE subjects.
To examine factors which might account for any change over time
METHOD
Our sample was made up of UHR subjects recruited for 3 research studies during 3 separate periods (April 1995–October 1996, October 1996–January 1999, and February 1999–August 2000). Two of these studies were observational studies and one was a randomized control trial with a specific intervention. With the exception of those subjects who were randomized to the intervention group in the control trial, all other subjects in these 3 cohorts received the same treatment in PACE and underwent very similar research assessments. For the purpose of this article, we excluded those subjects who received the specific trial intervention and our sample consisted of all the other subjects.
Over the time period 1995–2000, referrals to PACE were taken from a range of sources including community mental health clinics, general practitioners (GPs) and other primary care services, school and University counseling services, drug and alcohol services, as well as from families/carers and young people themselves. Referrals were initially screened via telephone by a PACE clinician (either psychiatrist or clinical psychologist). If the referral seemed suitable, then a face-to-face assessment with the young person was arranged, again with either psychiatrist or clinical psychologist or both simultaneously. Intake criteria were applied using the Comprehensive Assessment of At Risk Mental States (CAARMS).27 From 1995 to 1996, the Brief Psychiatric Rating Scale (BPRS28) was also used to assess intake criteria in order to validate the CAARMS (see Yung et al27). The CAARMS has good to excellent interrater reliability and concurrent validity27.
Certain predictor variables were chosen for examination in this study because they had previously been shown to be significantly associated with transition to psychosis.12,13,27 These were duration of symptoms prior to seeking help, level of psychosocial functioning, positive and negative symptoms, level of depressive symptoms, clinically assessed attentional impairment (SANS Attention subscale), and meeting criteria for both the Trait plus State intake group and the APS intake group.
Measurement of Possible Predictor (Independent) Variables
Duration of symptoms was measured using the CAARMS,27 which has an initial overview section in which time of first onset of any psychiatric symptom is estimated from retrospective description. Both patient and family versions were used, and if accounts varied between these information sets, then the patient estimation was used, given that patients themselves can more accurately provide the date of first subjective change3,29 and that insight was not generally impaired in this cohort.
The CAARMS was also used to assess threshold and subthreshold positive and negative symptoms and to determine which intake group (APS, BLIPS, and/or Trait plus State) the individual belonged to. The CAARMS was administered at baseline and at least every 6 months, depending on the study cohort (monthly for 12 months in the 1995–1996 and 1999–2000 cohorts, 6 monthly in the 1996–1999 cohort).
Psychopathology was further assessed using a range of instruments that were again administered at baseline and at least every 6 months. The BPRS28 was used to assess general psychopathology, and its psychotic subscales (unusual thought content, suspiciousness, hallucinations, and conceptual disorganization) were also analyzed separately to assess positive psychotic symptoms. Negative symptoms were assessed using the Schedule for Assessment of Negative Symptoms.30 Depression, anxiety, and manic symptoms were assessed using the Hamilton Rating Scale for Depression,31 Hamilton Rating Scale for Anxiety,32 and the Young Mania Rating Scale,33 respectively. Psychosocial functioning was measured using the Global Assessment of Functioning scale.34
Determination of Psychosis Status
Psychosis was a priori defined, as in previous articles, as the persistence of frank psychotic symptoms for over 1 week (see Yung et al12,13). The CAARMS27 was used to measure threshold and subthreshold forms of psychotic symptoms and hence to determine if a subject had developed a psychotic disorder. Subjects were followed up either monthly or at 6 and 12 months, and then at approximately 3 years follow-up time varied from 2.5 to 5.9 years with a mean of 3.8 years. If no CAARMS follow-up data were available, medical records were checked to ascertain if any psychiatric services within the state of Victoria were accessed by the individual, and if so, records were reviewed to determine psychosis status.
Data Analysis
In order to examine the possible change in transition rate over time, we initially divided the sample into yearly cohorts according to the year of entry. The number of subjects in each cohort were 22, 31, 32, 18, 22, and 17, respectively, for the years 1995–2000 (total 142). Because the number of subjects in each year was small, we decided to examine the effect of year in 3 ways. These were (1) treating year as a continuous variable, (2) categorizing year into 3 groups by combining 2 yearly cohorts, ie, 1995 and 1996, 1997 and 1998, and 1999 and 2000, and (3) categorizing year into 2 groups, 1995–1997 and 1998–2000. This was done to maximize our ability to impose some reasonable structure on year to enable the fitting of statistical models. Thus, any result which was consistent for all 3 ways was considered more robust and afforded more reliability than results which were not consistent across the different methods. Survival analysis and Cox regression was applied to analyze the association between risk of transition and year for each definition of year.
Results
Baseline Characteristics
A total of 142 UHR subjects were recruited into PACE research studies from 1995 to 2000. The mean age of the total sample was 19.3 years (standard deviation 3.4), and 50.7% of the sample was male.
Transition to Psychosis
Follow-up data regarding psychotic status were available on all 142 UHR subjects, 51 of whom are known to have become psychotic. In all but 3 cases, psychotic status was determined via interview. The Kaplan-Meier estimate of the 12-month transition rate is 0.31, 95% confidence interval (0.23, 0.39). The time from entry into PACE to development of psychotic disorder ranged from 4 days to 1218 days with a mean of 222.6 days. For each successive year, 1995 to 2000, the Kaplan-Meier estimates of the 12-month transition rate were, respectively, 0.50, 0.33, 0.32, 0.29, 0.21, and 0.12.
Effect of Year on Transition Rate
The association between risk of transition and year for each definition of year stated above was examined (see table 1). All 3 methods of assessing the effect of year were associated with estimated hazard ratios of less than 1, ie, risk of transition decreased as time passed. However, only the method of examining year as a continuous variable was significant at the 5% level. For the other methods, the P values were not significant at the 5% level but were still small (ranging from 0.064 to 0.21). The hazard ratio of 0.80 indicates that the estimated risk for each year is 0.80 times that of the previous year.
Table 1.
Hazard Ratios From Cox Regression With Year As the Only Predictor
| Estimated Hazard Ratio | 95% Confidence Interval | P Value | |
| yr1 | 0.80 | 0.66, 0.98 | .027 |
| yr2 | |||
| 97–98 vs 95–96 | 0.67 | 0.35, 1.26 | .21 |
| 99–00 vs 95–96 | 0.47 | 0.21, 1.04 | .064 |
| yr3 | |||
| 98–00 vs 95–97 | 0.59 | 0.32, 1.10 | .098 |
Note: yr1, year treated as a continuous variable; yr2, categorizing year into 3 groups 1995–1996, 1997–1998, and 1999–2000; yr3, categorizing year into 2 groups 1995–1997 and 1998–2000.
Factors Which May Account for the Reduction in Transition Rate Over Time
Next we investigated if the apparent drop in transition rate over time was due to subject characteristics being different in different years. Thus, the effect of year on transition rate was examined after adjusting for the effects of possible covariates, one at a time (see table 2).
Table 2.
P Values From Cox Regression Involving Year and 1 Covariate at a Time
| yr1 | yr2 | yr3 | ||||
| Covariate (x) | year* | year:x** | year* | year:x | year* | year:x |
| Age | .023 | .021 | .132 | .021 | .087 | .058 |
| Gender | .023 | .903 | .139 | .935 | .087 | .474 |
| Family history | .019 | .050 | .103 | .080 | .066 | .205 |
| Duration | .143 | .150 | .464 | .481 | .416 | .827 |
| log(Duration) | .066 | .249 | .286 | .562 | .237 | .839 |
| GAF | .019 | .137 | .102 | .382 | .074 | .500 |
| BPRST | .016 | .078 | .087 | .178 | .061 | .047 |
| BPRSP | .043 | .551 | .180 | .775 | .098 | .218 |
| Depression | .012 | .192 | .069 | .162 | .067 | .513 |
| Anxiety | .037 | .275 | .169 | .221 | .123 | .832 |
| Mania | .030 | .852 | .163 | .368 | .105 | .648 |
| SANST | .027 | .573 | .146 | .927 | .116 | .982 |
| SANS-Att | .003 | .098 | .018 | .198 | .020 | .098 |
| Intake gp (AT vs non-AT) | .007 | .019 | .048 | .010 | .023 | .001 |
| CAARMSM | .017 | .421 | .103 | .636 | .072 | .224 |
| CAARMSM1 | .015 | .682 | .073 | .545 | .061 | .910 |
| CAARMSM2 | .016 | .130 | .109 | .260 | .067 | .107 |
Note: yr1, year treated as a continuous variable; yr2, categorizing year into 3 groups 1995–1996, 1997–1998, and 1999–2000; yr3, categorizing year into 2 groups 1995–1997 and 1998–2000; year:x, interaction term between year and the covariate concerned;
values shown in the year column are P values for the effect of year on transition rate after adjusting for the covariate concerned;
values shown in the year:x column are P values for the interaction between year and the covariate concerned; family history, presence of family history of psychotic disorder in a first- or second-degree relative; duration, duration of psychiatric symptoms prior to seeking help; GAF, Global Assessment of Functioning; BPRST, total Brief Psychiatric Rating Scale score; BPRSP, Brief Psychiatric Rating Scale psychotic subscales score; depression, Hamilton Rating Scale for Depression total score; anxiety, Hamilton Rating Scale for Anxiety total score; mania, Young Mania Rating Scale total score; SANST, total Schedule for the Assessment of Negative Symptoms score; SANS-Att, Schedule for the Assessment of Negative Symptoms Attention subscale score; Intake gp (AT vs non-AT), membership of both Ultra High Risk Trait plus State and Attenuated Psychotic Symptoms intake groups compared with not being a member of both of these groups; CAARMSM, Comprehensive Assessment of At Risk Mental States total mean score; CAARMSM1, CAARMS mean score of positive symptom items; CAARMSM2, CAARMS mean score of negative symptom items.
The effect of year on transition rate remained significant, even after adjusting for a range of patient variables including age, gender, family history, baseline functioning, and level of psychopathology and psychiatric symptoms. However, year was no longer significantly associated with transition rate in any of the 3 methods used for assessing the effect of year once the duration of symptoms was adjusted for (see table 2).
The other notable finding from this analysis was that there was one interaction term, year by intake group, which was significant for all 3 methods of assessing year. This interaction term suggests that there might be a difference in transition rate over time but that difference may vary according to UHR intake group.
Duration of Symptoms and Transition Rate
Given that duration of symptoms appeared to have a nullifying effect on the association between year and transition, we then examined whether similar results would be obtained when duration of symptoms was used together with other covariates. After taking symptom duration into account, the effect of year appeared to have diminished substantially under each of the other covariates (see table 3). This provides further support to the notion that the drop in transition rate over time may at least be partly due to a change in duration of symptoms before reaching a psychiatric service.
Table 3.
P Values From Cox Regression Involving Year, Duration of Symptoms, and One Other Covariate
| Duration + yr1 | Duration + yr2 | Duration + yr3 | ||||
| year* | year:x | year* | year:x | year* | year:x | |
| Age | .150 | .022 | .496 | .040 | .429 | .080 |
| Gender | .141 | .880 | .478 | .928 | .407 | .473 |
| Family history | .124 | .037 | .383 | .055 | .353 | .188 |
| GAF | .097 | .153 | .371 | .513 | .282 | .463 |
| BPRST | .116 | .078 | .376 | .220 | .324 | .036 |
| BPRSP | .201 | .496 | .542 | .835 | .392 | .158 |
| Depression | .106 | .314 | .357 | .283 | .380 | .658 |
| Anxiety | .238 | .408 | .602 | .347 | .544 | .940 |
| Mania | .182 | .963 | .527 | .764 | .481 | .824 |
| SANST | .134 | .891 | .472 | .864 | .425 | .735 |
| SANS-Att | .031 | .071 | .124 | .158 | .145 | .080 |
| Intake gp (AT vs non-AT) | .061 | .021 | .254 | .008 | .164 | .001 |
| CAARMSM | .105 | .473 | .408 | .712 | .341 | .313 |
| CAARMSM1 | .087 | .617 | .330 | .659 | .300 | .864 |
| CAARMSM2 | .105 | .170 | .432 | .406 | .338 | .150 |
Note: Abbreviations are explained in the footnote to table 2.
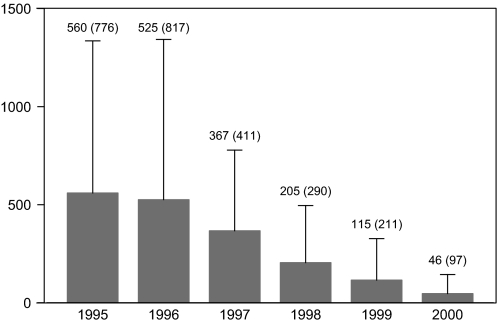
Given that long duration of symptoms was associated with transition to psychosis in previous cohorts, we suspected that duration of symptoms may be decreasing over time. This was borne out by examination of the duration of symptoms over the years. From 1995 to 2000, duration of symptoms reduced significantly, with successive reduction over each year (P value < 0.001, log-transformed values in analysis of variance). In the 1995 cohort, the mean number of days that a patient experienced psychiatric symptoms before receiving help was 559.6 (over 18 months). This decreased to just 46.5 days (less than 2 months) in 2000 (see figure 2).
Fig. 2.
Mean Duration of Symptoms [days (standard deviation)] Prior to Receiving Psychiatric Help in Successive Personal Assessment and Crisis Evaluation ultra high risk Cohorts 1995–2000.
UHR Intake Group and Transition Rate
As noted above, the year by intake group interaction was significant for all 3 methods of assessing year (see table 2). This interaction term remained significant after adjusting for duration of symptoms for all 3 methods of assessing year (table 3). This indicates that the difference in transition rate between the years depends on which intake group (being a member of both Trait plus State and APS intake groups or not being a member of both of these groups) is under consideration. That is, the difference in transition rate over time varied according to intake group.
Next we estimated the hazard ratios for transition to psychosis from the Cox regression model involving duration of symptoms, intake group, year, and the interaction between intake group and year (see table 4).
Table 4.
Hazard Ratios for Year From Cox Regression Involving Duration of Symptoms, Intake Group and Year, and the Interaction Between Intake Group and Year
| Estimated Hazard Ratio | 95% Confidence Interval | P Value | |||
| yr1 | AT | 0.63 | 0.45, 0.88 | .006 | |
| Non-AT | 1.00 | 0.78, 1.28 | .966 | ||
| yr2 | AT | 97–98 vs 95–96 | 0.58 | 0.15, 2.26 | .433 |
| 99–00 vs 95–96 | 0.07 | 0.01, 0.53 | .011 | ||
| Non-AT | 97–98 vs 95–96 | 0.94 | 0.42, 1.85 | .742 | |
| 99–00 vs 95–96 | 1.29 | 0.50, 3.33 | .598 | ||
| yr3 | AT | 98–00 vs 95–97 | 0.10 | 0.02, 0.46 | .003 |
| Non-AT | 98–00 vs 95–97 | 1.23 | 0.60, 2.54 | .570 |
As shown in table 4, there was a significant drop in transition rate over time in the AT group, but this was not as marked in the non-AT group.
We also examined whether mean age decreased in successive yearly cohorts, as would be expected if young people were being referred earlier (ie, with a shorter duration of symptoms). There was a slight but nonsignificant decrease (P = .56) in mean age over the years, especially between 1997 and 2000. The mean ages for the different yearly cohorts were (standard deviation in parentheses) 1995, 20.3 (3.5); 1996, 18.8 (3.9); 1997, 20.2 (3.0);1998, 19.9 (3.1); 1999, 19.6 (3.0); 2000, 19.2 (3.0).
Discussion
It seems that there has been a drop in the rate of transition to psychotic disorder in the PACE Clinic over time, from 1995 to 2000. This was not due to patients more recently attending PACE having higher levels of functioning, lower levels of subthreshold psychotic symptoms, less depression, or fewer negative symptoms.
The decreased transition rate seems at least partly due to a reduction in the duration of symptoms of patients prior to receiving help. That is, we are detecting UHR individuals and providing them with care much earlier than in the past. Thus, the ultimate level of transition may not be so low if we follow these patients up for longer. Alternatively, it may be that detection this early enables intervention to be more effective and perhaps psychotic disorder is more readily prevented. This is consistent with a staging model in psychiatry, which proposes that the earlier disorder is identified, the more benign the treatment and the better the outcome.35 Thus, very early detection of UHR status may enable avoidance of progression to psychosis even without antipsychotic medication.20
Another relevant factor is the provision of treatment to the UHR group, which may reduce transition rate. Routine treatment of UHR individuals involves supportive therapy and antidepressants and anxiolytics if indicated. These could reduce distress and decrease likelihood of transition. Antidepressants may have a neuroprotective effect.36 Although antipsychotics are not prescribed outside clinical trials, they are occasionally used if a patient has marked suicidality or aggression related to subthreshold psychotic symptoms.23,37 In line with a staging model,35 these treatments may be more effective in the early stages of illness. Additionally, clinicians may be becoming more confident and adept at managing UHR patients, resulting in a decline in transition rate. Thus, we may be seeing an increase in the number of false false positives in the cohorts because transition to psychotic disorder is prevented in larger numbers of individuals thought to be at risk due to earlier and more effective treatment.
Another possibility is that by early “diagnosis” of the at-risk mental state, we are including more false positives. That is, the apparent UHR phenotype may have a number of different outcome trajectories, and that early detection increases the probability that we will be identifying those never destined to develop a psychotic disorder. In other words, the number truly at risk is being “diluted” with false positives: those not at risk of psychotic disorder (they may be at risk of other outcomes, however). Many individuals who at one stage meet UHR criteria diverge from the path leading to psychosis—some may have resolution of symptoms and difficulties, others may develop nonpsychotic disorders. Of course, these outcomes are commonly seen in all UHR cohorts, not just those detected especially early, but it may be that these alternative pathways are more common earlier on.
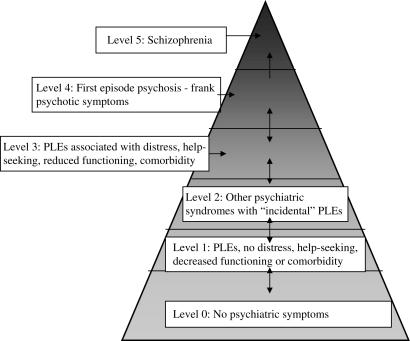
Another finding was that subjects who met criteria both for APS and State plus Trait (“AT” subjects) were affected differently over time compared with those not meeting both these criteria simultaneously (“non-AT” subjects). The transition rate in non-AT subjects did not reduce as much as that in AT subjects. This may be because, in the light of the early finding of the very high transition rate, staff at PACE became more attuned to picking up people who had both attenuated symptoms and a family history of psychotic disorder. Through the PACE community development and education forums with other agencies and clinical services, we also highlighted the apparent high risk of young people with a family history of psychotic disorder and subthreshold psychotic symptoms. Thus, other potential referrers may also have been more focused on this population. This could have led to these people being referred earlier and also some of these young people being referred to the PACE Clinic who were not distressed by their psychotic-like experiences or who were perhaps not actually seeking help for psychiatric problems. For example, a young person with a family history of psychosis may seek help from a GP due to depressive symptoms around a relationship break up. He or she may then be questioned about psychotic-like experiences because the well-meaning doctor is keen to detect any hint of risk for psychosis. Such questioning may not have occurred in the past before attention was drawn to the “AT” group. Similarly, a teenager with a family history of schizophrenia may seek help from a school counselor about school pressures or difficulties dealing with a mentally ill relative. If aware of the data about risk of psychosis in “AT” individuals, the school counselor may enquire about psychotic-like experiences. These young people are different from those who present to mental heath services with distressing psychotic-like experiences and who are seeking help because of these. They may be more likely to be false positives. We have previously discussed the importance of these factors in predicting psychosis.22 That is, the population from which subjects are drawn is important when considering their risk of transition to psychosis because the expected base rate of psychosis in each population will be different. Figure 3 illustrates this point. The first individual described who presented to a GP is conceptualized as coming from level 2. The second young person who presented to a school counselor comes from level 1.
Fig. 3.
Theoretical Model of Different Samples. PLEs = psychotic-like experiences. Level 0 individuals are most common, level 5 least common. Subjects in early Personal Assessment and Crisis Evaluation cohorts were recruited mainly from level 3. Later cohorts probably included individuals from both level 3 and level 2. It is possible that some young people from level 1 were also included (reproduced with permission of Schizophrenia Research22).
Another possibility is that AT subjects may have responded to interventions. It is our experience at the PACE Clinic that many young people experience relief, reduction in stress levels, and decrease in symptoms early in their management. Some report feeling better after an initial session at the service because they feel that they have made a decision to get help, someone has listened to their problems, and they have been assessed as being not currently psychotic. Young people with a parent with schizophrenia often find that reassurance that developing the same illness is not inevitable is beneficial. They are also told that even if a psychotic disorder does become manifest, then early intervention can improve outcome. This provides relief to many PACE patients. Focus on their current difficulties and attention to improving functioning can also be of great help to this group. Thus, there may be many false false positives, especially in the AT group, who would have otherwise progressed to psychotic disorder, had it not been for their attendance at PACE.
The limitations of this study need to be acknowledged. As noted in the Introduction, we suspected a declining transition rate in the UHR group and used existing data to investigate this “hunch.” Thus, we did not set out in 1995 to examine this issue prospectively, and data collected for other research studies within PACE were used for this study. The issue of possible reduction in transition rate needs further examination, both within PACE and within other “prodromal” services around the world. It must also be acknowledged that our thoughts about the causes of the decline in transition rate are speculative and are included in this article to promote discussion in the research and clinical community involved with these services.
Conclusion
A factor contributing to the decline in transition rate in the PACE Clinic seems to be the earlier detection of UHR individuals. Depending on whether the decline is due to more effective treatment (greater numbers of false false positives in whom psychotic disorder has been prevented or at least delayed) or dilution (greater numbers of false positives who are never really at risk of psychosis in the first place), one could either argue for or against early intervention. Given that it is not possible to distinguish between these groups at least phenotypically at present, perhaps it is time to rethink the role and practice of UHR or prodromal clinics. Young people presenting to PACE by and large still had psychiatric symptoms and difficulties with psychosocial functioning. Many of them were experiencing nonpsychotic disorders such as major depression or had emerging nonpsychotic disorders. They still need some form of help. A new model could involve a period free of specific antipsychotic treatment and instead a period of observation, monitoring, and treatment of psychiatric disorders, such as depression, anxiety disorders, and substance use problems. Evidence of deterioration and worsening of subthreshold psychotic symptoms would lead to more specific treatment, involving perhaps cognitive therapy in the first instance, or antipsychotics if rapid worsening occurred. Failure to respond to nonspecific treatment, prolonged subthreshold psychotic symptoms, and suicidality and dangerousness coupled with subthreshold psychosis could be other reasons to institute low-dose atypical anitpsychotics. The onset of nonpsychotic disorders would lead to treatment of these illnesses. Thus, our ability to detect young people with mental health problems earlier could be of considerable benefit in detecting a range of disorders close to their onset and could reduce the burden of these other illnesses. Even subthreshold nonpsychotic disorders could be targeted for specific treatment if deterioration is occurring, especially since subthreshold nonpsychotic disorders are known to cause a great deal of disability.38,39 The primary prevention of secondary disorders, eg, treatment of depression to prevent a substance use problem from developing, is another preventive strategy possible through this expanded focus.40 This type of service is consistent with the clinical staging model in psychiatry35 which emphasizes that less differentiated early phases of mental illnesses may benefit from broad spectrum and simpler treatments. As clearer target syndromes such as schizophreniform disorder and mania emerge, they can attract more specific interventions. This new service model could enable young people to receive the help they need in a timely manner, with the potential for improved outcomes across several fronts.
References
- 1.Hafner H, Loffler W, Maurer K, Hambrecht M, an der Heiden WA. Depression, negative symptoms, social stagnation and social decline in the early course of schizophrenia. Acta Psychiatr Scand. 1999;100:105–118. doi: 10.1111/j.1600-0447.1999.tb10831.x. [DOI] [PubMed] [Google Scholar]
- 2.Yung AR, McGorry PD. The prodromal phase of first episode psychosis: past and current conceptualisations. Schizophr Bull. 1996;22:353–370. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]
- 3.Yung AR, McGorry PD. The initial prodrome in psychosis: descriptive and qualitative aspects. Aust N Z J Psychiatry. 1996;30:587–599. doi: 10.3109/00048679609062654. [DOI] [PubMed] [Google Scholar]
- 4.Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI study. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 5.Brewer WJ, Wood SJ, Phillips LJ, et al. Generalised and specific cognitive performance in clinical high-risk cohorts: a review highlighting potential vulnerability markers for psychosis. Schizophr Bull. 2006;32:538–555. doi: 10.1093/schbul/sbj077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yung AR, Buckby JA, Cotton SM, et al. Psychotic-like experiences in non-psychotic help-seekers: associations with distress, depression and disability. Schizophr Bull. 2006;32:352–359. doi: 10.1093/schbul/sbj018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Os J, Hanssen M, Bijl RV, Vollebergh W. Prevalence of psychotic disorder and community level of psychotic symptoms: an urban-rural comparison. Arch Gen Psychiatry. 2001;58:663–668. doi: 10.1001/archpsyc.58.7.663. [DOI] [PubMed] [Google Scholar]
- 8.Tien AY. Distributions of hallucinations in the population. Soc Psychiatry Psychiatr Epidemiol. 1991;26:287–292. doi: 10.1007/BF00789221. [DOI] [PubMed] [Google Scholar]
- 9.Eaton WW, Romanoski A, Anthony JC, Nestadt G. Screening for psychosis in the general population with a self-report interview. J Nerv Ment Dis. 1991;179:689–693. doi: 10.1097/00005053-199111000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Verdoux H, van Os J. Psychotic symptoms in non-clinical populations and the continuum of psychosis. Schizophr Res. 2002;54:59–65. doi: 10.1016/s0920-9964(01)00352-8. [DOI] [PubMed] [Google Scholar]
- 11.Bell RQ. Multiple-risk cohorts and segmenting risk as solutions to the problem of false positives in risk for the major psychoses. Psychiatry. 1992;55:370–381. doi: 10.1080/00332747.1992.11024610. [DOI] [PubMed] [Google Scholar]
- 12.Yung AR, Phillips LJ, Yuen HP, et al. Psychosis prediction: 12-month follow up of a high-risk (“prodromal”) group. Schizophr Res. 2003;60:21–32. doi: 10.1016/s0920-9964(02)00167-6. [DOI] [PubMed] [Google Scholar]
- 13.Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67:131–142. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 14.Yung AR, McGorry PD, McFarlane CA, Jackson HJ, Patton GC, Rakkar A. Monitoring and care of young people at incipient risk of psychosis. Schizophr Bull. 1996;22(2):283–303. doi: 10.1093/schbul/22.2.283. [DOI] [PubMed] [Google Scholar]
- 15.Haroun N, Dunn L, Haroun A, Cadenhead K. Risk and protection in prodromal schizophrenia: ethical implications for clinical practice and future research. Schizophr Bull. 2006;32:166–178. doi: 10.1093/schbul/sbj007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsen KA, Rosenbaum B. Prospective investigations of the prodromal state of schizophrenia: review of studies. Acta Psychiatr Scand. 2006;113:247–272. doi: 10.1111/j.1600-0447.2005.00697.x. [DOI] [PubMed] [Google Scholar]
- 17.Miller TJ, McGlashan TH, Rosen JL, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- 18.Mason O, Startup M, Halpin S, Schall U, Conrad A, Carr V. State and trait predictors of transition to first episode psychosis among individuals with at risk mental states. Schizophr Res. 2004;71:227–237. doi: 10.1016/j.schres.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Larsen TK. The transition from premorbid period to psychosis: how can it be described? Acta Psychiatr Scand. 2002;106(suppl):10–11. [Google Scholar]
- 20.Morrison AP, Bentall RP, French P, et al. Randomised controlled trial of early detection and cognitive therapy for preventing transition to psychosis in high-risk individuals: study design and interim analysis of transition rate and psychological risk factors. Br J Psychiatry. 2002;181(suppl 43):s78–s84. doi: 10.1192/bjp.181.43.s78. [DOI] [PubMed] [Google Scholar]
- 21.McGorry PD, Yung AR, Phillips LJ. The ‘close-in’ or ultra high risk model: a safe and effective strategy for research and clinical intervention in prepsychotic mental disorder. Schizophr Bull. 2003;29:771–790. doi: 10.1093/oxfordjournals.schbul.a007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yung AR, Stanford C, Cosgrave E, et al. Testing the ultra high risk (prodromal) criteria for the prediction of psychosis in a clinical sample of young people. Schizophr Res. 2006;84:57–66. doi: 10.1016/j.schres.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Yung AR, Phillips LJ, McGorry PD. Treating Schizophrenia in the Prodromal Phase. London: Taylor and Francis; 2004. [Google Scholar]
- 24.Corcoran C, Malaspina D, Hercher L. Prodromal interventions for schizophrenia vulnerability: the risks of being “at risk”. Schizophr Res. 2005;73:173–184. doi: 10.1016/j.schres.2004.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinssen RK, Perkins DO, Appelbaum PS, Fenton WS. Informed consent in early psychosis research: National Institute of Mental Health Workshop, November 15, 2000. Schizophr Bull. 2001;27:571–584. doi: 10.1093/oxfordjournals.schbul.a006897. [DOI] [PubMed] [Google Scholar]
- 26.Yung AR. Commentary: the schizophrenia prodrome: a high-risk concept. Schizophr Bull. 2003;29:859–865. doi: 10.1093/oxfordjournals.schbul.a007052. [DOI] [PubMed] [Google Scholar]
- 27.Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis—the Comprehensive Assessment of At Risk Mental States (CAARMS) Aust N Z J Psychiatry. 2005;39:964–971. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 28.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 29.Moller P, Husby R. The initial prodrome in schizophrenia: searching for naturalistic core dimensions of experience and behavior. Schizophr Bull. 2000;26:217–232. doi: 10.1093/oxfordjournals.schbul.a033442. [DOI] [PubMed] [Google Scholar]
- 30.Andreasen NC. The scale for the assessment of negative symptoms (SANS) Iowa City: The University of Iowa; 1983. [Google Scholar]
- 31.Hamilton M. A rating scale for depression. J Neurol, Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamilton M. The assessment of anxiety states by rating. Br J Psychiatry. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 33.Young RC, Biggs JT, Ziegler VE, Myer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 34.Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. American Psychiatric Association. [Google Scholar]
- 35.McGorry PD, Hickie IB, Yung AR, Pantelis C, Jackson HJ. Clinical staging of psychiatric disorders: a heuristic framework for choosing earlier, safer and more effective interventions. Aust N Z J Psychiatry. 2006;40:616–622. doi: 10.1080/j.1440-1614.2006.01860.x. [DOI] [PubMed] [Google Scholar]
- 36.Berger G, Wood SJ, McGorry PD. Incipient neurovulnerability and neuroprotection in early psychosis. Psychopharmacol Bulletin. 2003;37:79–101. [PubMed] [Google Scholar]
- 37.RANZCP Clinical Practice Guidelines Team. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of schizophrenia and related disorders. Aust N Z J Psychiatry. 2005;39:1–30. doi: 10.1080/j.1440-1614.2005.01516.x. [DOI] [PubMed] [Google Scholar]
- 38.Kessler RC, Merikangas KR, Berglund P, Eaton WW, Koretz DS, Walters EE. Mild disorders should not be eliminated from the DSM-V. Arch Gen Psychiatry. 2003;60:1117–1122. doi: 10.1001/archpsyc.60.11.1117. [DOI] [PubMed] [Google Scholar]
- 39.Angold A, Costello EJ, Farmer E, Burns BJ, Erkanli A. Impaired but undiagnosed. J Am Acad Child Adolesc Psychiatry. 1999;38:129–137. doi: 10.1097/00004583-199902000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Merikangas KR, Avenevoli S. Implications of genetic epidemiology for the prevention of substance use disorders. Addict Behav. 2000;25:807–820. doi: 10.1016/s0306-4603(00)00129-5. [DOI] [PubMed] [Google Scholar]