Abstract
Borrelia burgdorferi, the pathogen of Lyme disease, cycles in nature through Ixodes ticks and mammalian hosts. At least five Complement Regulator-Acquiring Surface Proteins (BbCRASPs) are produced by B. burgdorferi, which are thought to assist spirochetes in host immune evasion. Recent studies established that BbCRASP-2 is preferentially expressed in mammals, and elicits robust antibody response in infected hosts, including humans. We show that BbCRASP-2 is ubiquitously expressed in diverse murine tissues, but not in ticks, reinforcing a role of BbCRASP-2 in conferring B. burgdorferi defense against persistent host immune threats, such as complement. BbCRASP-2 immunization, however, fails to protect mice from B. burgdorferi infection and does not modify disease, as reflected by the development of arthritis. An infectious BbCRASP-2 mutant was generated, therefore, to examine the precise role of the gene product in spirochete infectivity. Similar to wild type B. burgdorferi, BbCRASP-2 mutants remain insensitive to complement-mediated killing in vitro, retain full murine infectivity and induce arthritis. Quantitative RT-PCR assessment indicates that survivability of BbCRASP-2-deficient B. burgdorferi is not due to altered expression of other BbCRASPs. Together, these results suggest that the function of a selectively expressed B. burgdorferi gene, BbCRASP-2, is not essential for complement resistance or infectivity in the murine host.
Introduction
Borrelia burgdorferi is the causative agent of Lyme disease, the most prevalent vector-borne disease in the United States and Europe [1], [2]. In nature, B. burgdorferi cycles between rodent reservoirs and Ixodes scapularis ticks. This complex enzootic life cycle requires successful colonization and coordinated transmission between strikingly different host and vector environments. It is thought that differential gene expression plays an important role in allowing the spirochete to navigate the transitions between hosts and in establishing persistent infection [3]–[6]. Due to the availability of excellent murine models of Lyme disease, B. burgdorferi gene expression through the tick-rodent transmission cycle can be examined in the laboratory [7]–[11]. These efforts may provide important clues for understanding functions of microbial gene products that support B. burgdorferi persistence in nature [3]–[6], [12], [13].
The genes encoding the Complement Regulator-Acquiring Surface Proteins (BbCRASPs) of B. burgdorferi are differentially expressed in the pathogen life cycle [14], [15]. As many as five BbCRASPs were identified that bind host proteins of the factor H (FH) family, and possibly contribute to the spirochete defense against host complement-mediating killing[16]–[18]. BbCRASP-1 (also known as cspA or bba68) and BbCRASP-2 (also called cspZ or bbh06), located on linear plasmids lp54 and lp28-3 respectively, share little sequence homology with other BbCRASP sequences. In contrast, BbCRASP-3, -4 and -5 are sequentially similar and belong to the erp paralog family and are known as erpP (bbn38), erpC and erpA (bbp38 and bbl39), respectively. Collectively these erp genes are also known as ospE, and are encoded on multiple cp32 plasmids [19]–[22]. The gene erpC (located on cp32-2) and one of the three erpA genes (located on cp32-5) currently lack TIGR database annotations, as the sequenced B31 M1 isolate lost these plasmids. Of all the BbCRASP genes, BbCRASP-2 is the only gene without paralogous family members, and is therefore unique in B. burgdorferi [17].
Evasion of host complement is especially important for B. burgdorferi, as it establishes an extracellular and disseminated infection in many tissue environments where the complement system is readily available through host vasculature or body fluids. The complement system includes soluble membrane binding proteins which, upon contact with foreign cells, become activated, and are then capable of direct chemical lysis via membrane disruption [23], [24]. Specific regulatory proteins, such as FH family proteins, protect the host from self-inflicted damage by preventing unwarranted complement activation. Pathogens such as Candida albicans, Neisseria meningiditis, and Streptococcus pneumonia have been shown to bind host FH, and that FH binding in N. gonorrhea and B. burgdorferi provides protection against complement killing in vitro [25]–[29]. BbCRASPs have been identified according to their ability to bind proteins of the FH family, although individual BbCRASPs vary in their affinities for particular FH family proteins. For example, only BbCRASP-1 and -2 preferentially bind factor H-like protein (FHL-1), while BbCRASP-3, -4 and -5 selectively bind factor H-related protein (FHR-1) [17], [30], [31]. BbCRASPs also vary in their interaction with uncharacterized serum proteins [14], [32]. Though the binding affinities and the expression profiles of the BbCRASPs have been studied, the independent role of each BbCRASP in B. burgdorferi infectivity is not clear. Recently studies using a non-infectious mutant demonstrated that the loss of BbCRASP-1 sensitized the B. burgdorferi to complement-mediated lysis in human serum, an effect that can be rescued with gene complementation [25]. While there is some disagreement as to the expression of BbCRASP-1 during mammalian infection, RT-PCR analysis indicate that it is only expressed transiently at the tick bite site and in ticks [14], but not expressed in mice [33]. BbCRASP-1 therefore, may not play an essential role in mammalian infection [33], but could be important in spirochete survival in feeding ticks. Although the above set of studies suggest an important role for BbCRASPs in spirochete immune evasion, the precise role of individual BbCRASPs, or their orchestrated role in the B. burgdorferi infection cycle is not clear, largely because infectious BbCRASP-deficient B. burgdorferi have not yet been successfully generated [14].
BbCRASP-2 is expressed by B. burgdorferi during murine infection [14], [34], and infected hosts, including human patients, readily generate BbCRASP-2-specific antibodies [17], [35]. This protein is conserved among B. burgdorferi isolates [22], reported to be localized on the spirochete surface [17] and has recently been suggested as a possible target for a second generation Lyme disease vaccine [17], [35]. The previous studies also suggest a possible functional role for BbCRASP-2 in immune evasion and pathogen survival [17], [22]. In order to test this hypothesis, we sought to determine whether BbCRASP-2 is consistently produced in diverse murine tissues throughout the infection, and whether BbCRASP-2 immunization could provide host immunity and influence disease outcome. To explore the precise role of BbCRASP-2 in B. burgdorferi infectivity of a mammalian host, we assessed how targeted deletion of BbCRASP-2 in an infectious isolate influences B. burgdorferi infection in the murine model of Lyme borreliosis. Functional characterization of microbial ligands that are differentially expressed in the complex enzootic cycle of B. burgdorferi is critical to understanding the adaptive strategies of a pathogen that has evolved to persist in diverse tissue environments resulting in multi-system disorders.
Materials and Methods
Bacteria, Mice and Ticks
Borrelia burgdorferi infectious isolate A3 [36], a clonal derivative of B31 M1, was used throughout the study. Female C3H/HeN mice between 4 and 6 weeks old purchased from the National Cancer Institute. Mice were inoculated with a single subcutaneous injection of 105 spirochetes per mouse. All animal procedures were in compliance with the guidelines set by the Institutional Animal Care and Use Committee. The ticks used in this study belong to a colony that is reared and maintained in the laboratory as described [37].
PCR
Mice were sacrificed following infection, and the heart, tibiotarsal joint, and skin samples were removed and frozen in liquid nitrogen. B. burgdorferi-infected ticks were isolated by allowing ticks to feed on an infected murine host as described [37]. RNA was extracted using TRIzol reagent (Invitrogen) and further treated with DNase I (Invitrogen), and finally purified using the RNeasy kit (Qiagen). RNA was used as a template for reverse-transcriptase polymerase chain reaction (RT-PCR) using the AffinityScript cDNA synthesis kit (Stratagene). The primers used for PCR reactions are indicated in Supplementary Table S1. Quantitative PCR analysis was performed using iQ SybrGreen Supermix (BioRad) as previously described [13]. For quantitative analysis of gene expression, the target transcripts were normalized to the number of flaB transcripts, whereas for quantitative measurement of B. burgdorferi burden in infected tissues, flaB transcripts were normalized to mouse or tick β-actin levels. All quantitative PCR results were checked for specificity by melting curve analysis.
Production of recombinant BbCRASP-2 protein and BbCRASP-2 antibodies
Recombinant BbCRASP-2 protein was produced in E. coli using the pET303/CT-His Champion vector (Invitrogen) using specific primers (Supplementary Table S1). Recombinant BbCRASP-2 was fused with a C-terminal 6-histidine tag for purification, and lacked the peptides encoding the lipidation signal. Polyclonal antibodies against recombinant BbCRASP-2 were generated in mice as described earlier [37], [38]. Briefly, recombinant BbCRASP-2 (10 μg/animal) was emulsified in complete Freund's adjuvant (Sigma) and injected into groups of 10 mice. The animals were boosted twice at 10 days intervals with the same dose of antigen in incomplete Freund's adjuvant (Sigma) and the sera were collected two weeks following the second boost. Titer and specificity of the serum was tested by ELISA and immunoblot blot as described previously [39].
Proteinase K accessibility assay
Proteinase K accessibility assays were performed as described [40]. Briefly, B. burgdorferi (2×108) were gently washed three times in 1 ml of PBS (pH 7.4) and collected by centrifugation at 4,000×g for 4 min. Washed spirochetes were then gently resuspended in 1 ml of PBS and split into two equal 500 μl volumes. One aliquot received 200 μg of proteinase K (PK) (Sigma) while the other aliquot received an equal volume of PBS without PK. Both aliquots were incubated for 1 h at room temperature before the addition of 10 μl of phenylmethylsulfonylfluoride (Sigma) to stop PK activity. Spirochete suspensions were subsequently pelleted by centrifugation at 10,000×g for 10 min and resuspended in PBS for immunoblot analysis using antibodies against BbCRASP-2, FlaB, or OspA.
Active immunization and infection studies
Groups of mice (6 animals/group) were immunized with adjuvant containing either recombinant BbCRASP-2, or adjuvant containing the same volume of phosphate buffered saline (PBS) in similar fashion as describe in above paragraph. Ten days after the final boost, mice were infected with a subcutaneous injection of B. burgdorferi (105 spirochetes/mouse). Mice were sacrificed after 7, 12, 15 and 30 days of infection. Heart, tibiotarsal joint, and skin samples were collected and frozen in liquid nitrogen. RNA was isolated from infected tissues and B. burgdorferi burden was measured using quantitative PCR. B. burgdorferi-infected mice were examined for swelling and histology of the tibiotarsal joints as detailed [13], [41], [42].
Generation and phenotypic analysis of BbCRASP-2 mutant
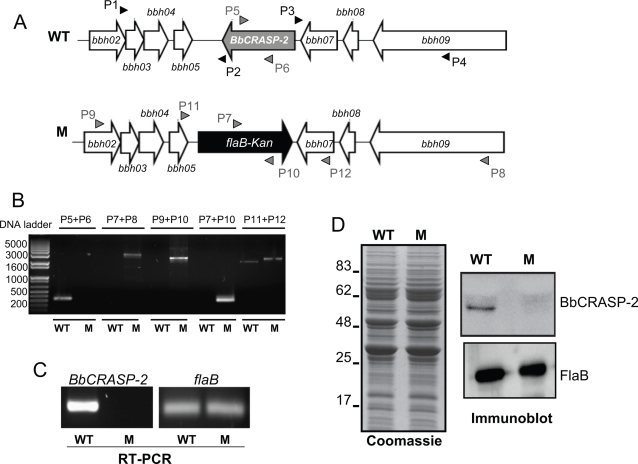
BbCRASP-2-deficient B. burgdorferi was generated by homologous recombination replacing the entire open reading frame of the BbCRASP-2 gene with a kanamycin resistance cassette [13], [38], [39], [43], [44] using primers as indicated in Supplementary Table S1. DNA fragments flanking the BbCRASP-2 open reading frame on the 5′ and 3′ sides were PCR-amplified and inserted into the plasmid pXLF10601. This plasmid was sequenced to confirm identity and electroporated into wild-type B. burgdorferi A3, and transformants were selected for growth in the presence of kanamycin. PCR analysis was used to confirm the intended recombination event using primers P5-P12. Immunoblotting analysis using BbCRASP-2 antibodies was performed to confirm the loss of BbCRASP-2. The plasmid profile of the mutant B. burgdorferi was assessed to confirm no loss of wild type plasmids [13], [38], [44].
The serum sensitivity of the BbCRASP-2 mutant was measured using a published procedure [16]. Briefly, triplicate samples of wild type B. burgdorferi or isogenic BbCRASP-2 and BbCRASP-1 mutant [25] were seeded into 1 ml tubes at a final concentration of 5×106 bacteria per ml. These aliquots were incubated in either 50% normal human serum or 50% heat-inactivated human serum. At 0, 1, 4, and 16 hours after the addition of serum, spirochete viability was examined using dark-field microscopy. Normal human serum (Sigma) collected from healthy, anonymous donors with no reactivity to B. burgdorferi after chemiluminescent immunoblot analyses were used in the assay.
To examine the phenotype of the BbCRASP-2 mutant in vivo, the mutant and wild type B. burgdorferi were separately inoculated into 2 groups of mice (6 animals/group, 105 spirochetes/mouse). A skin sample, the heart and the joints from infected mice were isolated at day 7, 12 and 18 after challenge, and B. burgdorferi burdens were measured by quantitative RT-PCR analysis. Before sacrifice, ear and spleen tissues from the mice were cultured in BSK medium for the presence of viable B. burgdorferi. Rear ankle joints of individual mice were measured before infection and at 7, 12 and 18 days after infection until sacrifice. Acquisition of wild type and mutant B. burgdorferi by nymphal I. scapularis ticks was performed as described earlier [37]. Briefly, groups of C3H mice (6 animals/group) were infected with wild type or BbCRASP-2 mutant B. burgdorferi (105 spirochetes/mouse). Following 12 days of infection, 25 I. scapularis nymphs were placed on each mouse. The ticks were forcibly detached from the mice following repletion and immediately stored in liquid nitrogen. B. burgdorferi burdens in each tick sample were measured as described earlier by quantitative RT-PCR analysis.
Statistical analysis
Results are expressed as the mean ± standard error of the mean (SEM). The significance of the difference between the mean values of the groups was evaluated by two-tailed Student t test.
Results
BbCRASP-2 is ubiquitously expressed during murine infection
To understand role of BbCRASP-2 in B. burgdorferi infectivity, we first assessed the transcript levels of BbCRASP-2 in multiple murine tissue locations where B. burgdorferi persists during infection, and in various stages of infected ticks. C3H mice were infected with B. burgdorferi, and heart, joints and skin samples were collected at days 5, 7, 12, 16, and 24 following infection. Larval and nymphal ticks were fed on parallel groups of 15 day-infected mice (25 ticks/mouse) and fully engorged ticks were isolated following 3 days of repletion. Batches of infected fed larvae were allowed to molt and then collected as unfed nymphs. Total RNA was prepared from murine and tick samples, and subjected to quantitative RT-PCR to measure the B. burgdorferi BbCRASP-2 transcript levels. BbCRASP-2 is ubiquitously and consistently expressed throughout infection (Fig. 1A), but was undetectable in larval or nymphal ticks. As reported earlier [17], the infected mice developed a specific antibody response against BbCRASP-2 (data not shown).
Figure 1. Ubiquitous expression of BbCRASP-2 in infected mice.
The relative expression levels of BbCRASP-2 in the murine host, and in representative life stages of ticks, are analyzed and presented as copies of BbCRASP-2 transcript per 1000 copies of flaB transcripts. Total RNA was isolated from multiple tissues of B. burgdorferi-infected mice (6 mice/group) at day 5, 7, 12, 16 and 24 following challenge, from infected fed larva (FL) after 3 days of feeding, unfed nymphs following larval molting (UN) and fed nymphs (FN) after 3 days of feeding. BbCRASP-2 transcripts were measured using quantitative RT-PCR. Error bars represent the mean ± SEM from four quantitative PCR analyses of two independent murine infection experiments. BbCRASP-2 transcripts were abundant in all murine tissues tested but were not detected in any stages of the ticks.
BbCRASP-2 immunization failed to evoke protective immunity in mice
Because BbCRASP-2 is surface-exposed [17], immunogenic, and expressed throughout the murine infection, we next assessed if immunization of mice using recombinant BbCRASP-2 could elicit protective immunity and influence the outcome of Lyme disease. To accomplish this, we produced recombinant BbCRASP-2 in E. coli and immunized the murine host with purified BbCRASP-2. Separate groups of C3H mice (6 animals/group) were immunized with either BbCRASP-2 or PBS (control) mixed with similar volume of adjuvant. ELISA (data not shown) and immunoblotting performed after final boosting indicated that immunized mice had developed strong antibody titer that specifically recognized recombinant and native BbCRASP-2 (Fig. 2A). Although previous immunofluorescence studies indicated that BbCRASP-2 antibodies recognized native antigen on the surface of the intact spirochetes [17], our proteinase K accessibility assay indicated that BbCRASP-2 is not significantly exposed on the spirochete surface (Fig. 2B). Ten days after the final immunization mice were needle-inoculated with B. burgdorferi (105 spirochetes/mouse). B. burgdorferi levels were measured by quantitative PCR from skin, heart and joint samples collected at 7, 12, 15 and 30 days after infection. Results indicated no significant difference in spirochete burden between mice immunized with BbCRASP-2 or the control at any time points (Fig. 2C). Quantitative RT-PCR analysis further showed no difference between the transcript levels of BbCRASP-2 in immunized and control groups (data not shown). These results indicated that BbCRASP-2 immunization did not influence the ability of B. burgdorferi to establish infection. Development of ankle swelling (Fig. 2D) or histopathological changes in the joint tissue (data not shown) in B. burgdorferi-infected mice immunized with BbCRASP-2 did not differ from the control, suggesting that host BbCRASP-2 antibodies fail to influence the ability of B. burgdorferi to induce arthritis in the murine host.
Figure 2. BbCRASP-2 immunization does not interfere with B. burgdorferi infectivity.
A, Recognition of recombinant and native BbCRASP-2 proteins by immunized murine serum, as assessed by immunoblotting. Recombinant BbCRASP-2 protein (50 ng) or B. burgdorferi lysates (200 ng) were probed with BbCRASP-2 antiserum or normal mouse serum (NMS). The arrow indicates the position of BbCRASP-2. Migration of protein standards is shown to the left. B, BbCRASP-2 is not sensitive to proteinase K-mediated degradation of B. burgdorferi surface proteins. Viable spirochetes (2×108 cells) were incubated with (+) or without (−) proteinase K for removal of protease sensitive surface proteins and processed for immunoblot analysis using BbCRASP-2 antibodies. B. burgdorferi OspA and FlaB antibodies were utilized as controls for surface-exposed and sub-surface proteins, respectively. C, Comparable levels of B. burgdorferi in rBbCRASP-2-immunized or control mice. Groups of mice (6 animals/group) were immunized with either rBbCRASP-2 or PBS (control) mixed with adjuvant, and 10 days after final immunization mice were infected with B. burgdorferi (105 spirochetes/mouse). The spirochete burdens in both groups of mice were assessed by measuring copies of the B. burgdorferi flaB gene at 7, 12 and 30 days following infection. Amounts of murine ß-actin were determined in each sample and used to normalize the quantities of spirochete RNA. Bars represent the mean measurements ± SEM from four quantitative PCR analyses from two independent infection experiments. Levels of B. burgdorferi were similar in BbCRASP-2-immunized (black bars) and control mice (white bars). The burdens found in BbCRASP-2-immunized mice were not statistically significant from the control burdens in any tissue or time point (P>0.05, n = 4). D, Severity of joint swelling in BbCRASP-2-immunized and infected mice. Groups of mice (6 animals/group) were immunized with BbCRASP-2 (black bars) or PBS (control, white bars) and infected with B. burgdorferi. Development of joint swelling was assessed after 12 and 30 days of spirochete challenge by measuring the largest diameter of the rear tibiotarsal joints using a digital caliper.
Generation of an infectious isolate of BbCRASP-2-deficient B. burgdorferi
Since BbCRASP-2 immunization did not influence spirochete infection, we created a BbCRASP-2-deficient B. burgdorferi to more directly assess the role of the gene product in B. burgdorferi survival and infectivity. An infectious isogenic mutant was created by replacing the BbCRASP-2 open reading frame with a kanamycin resistance cassette via homologous recombination (Fig. 3A). PCR analysis was performed to ensure that the antibiotic cassette was appropriately inserted into the intended chromosomal locus (Fig. 3B), and that the plasmid profile of the mutant was unchanged. Out of 4 transformed clones that grew in antibiotic-containing media, 2 clones contained the desired integration of the antibiotic cassette and retained the same set of plasmid as in the parental isolate. One of the mutant clones was chosen for further study. RT-PCR analysis showed that BbCRASP-2 mRNA was absent in the mutant (Fig. 3C), and that BbCRASP-2 mutagenesis did not impose polar effects on the transcription of surrounding genes, bbh05 and bbh07 (data not shown). The BbCRASP-2 mutant spirochetes displayed a similar protein profile to that of the wild type (Fig. 3D, left panel), except that the BbCRASP-2 mutant failed to produce BbCRASP-2 protein (Fig. 3D, upper right panel).
Figure 3. Construction and analysis of the BbCRASP-2 mutant B. burgdorferi.
A, Schematic drawings of the wild type isolate (WT) and the BbCRASP-2 mutant (M) at the BbCRASP-2 (bbh06) locus. Genes bbh02, bbh03, bbh04, bbh05, bbh07, bbh08 and bbh09 (white box arrows) and the kanamycin-resistance cassette driven by the B. burgdorferi flaB promoter (flaB-Kan, black box arrow) are indicated. Primers P1-P4 (black arrow-heads) amplified 5′ and 3′ arms for homologous recombination, regions flanking up- and down-stream of the BbCRASP-2 locus and ligated on either side of the flaB-Kan cassette as detailed in the text. B, Integration of the mutagenic construct, flab-Kan, in the intended genomic locus. Primers 5–12 (gray arrow-heads, positions indicated in Fig. 3A) were used for PCR analysis using isolated DNA from wild type (WT) or BbCRASP-2 mutant B. burgdorferi (M) and subjected to gel electrophoresis. The combination of primers used for PCR is indicated at the top. Migration of DNA ladder is shown on the left. C, RT-PCR analysis of BbCRASP-2 transcripts. Total RNA was isolated from either the wild type (WT) or BbCRASP-2 mutant (M) B. burgdorferi, converted to cDNA and used to PCR-amplify regions within BbCRASP-2 and flaB, and these amplicons visualized on a gel. D, Protein analysis of wild type (WT) or BbCRASP-2 mutant (M) B. burgdorferi. Equal amounts of protein from wild type or BbCRASP-2 mutant spirochetes were separated on an SDS-PAGE gel, and either stained with coomassie blue (left panel) or transferred onto a nitrocellulose membrane and probed with BbCRASP-2 or FlaB antibodies. Migration of protein standards is shown to the left in kDa.
BbCRASP-2-deficiency did not affected serum resistance of B. burgdorferi in vitro
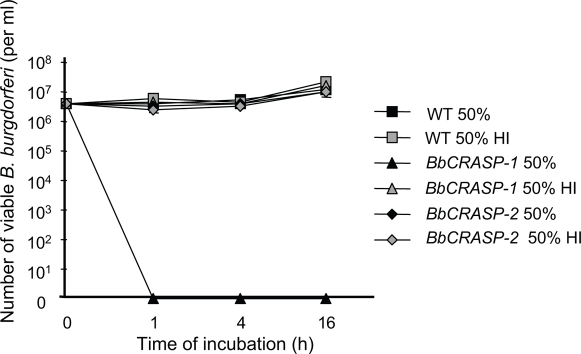
B. burgdorferi is known to be resistant to complement-mediated lysis in serum, and deficiency of BbCRASP-1 has been shown to render B. burgdorferi highly susceptible to serum-mediated killing in vitro [25]. Because BbCRASP-2 is expressed by wild type spirochetes grown in culture, we assessed whether BbCRASP-2 deficiency affects the serum resistance of the spirochetes. Equal concentrations of wild type and BbCRASP-2 mutant B. burgdorferi were separately incubated with human serum containing active complement and the serum sensitivity of each isolate was assessed. While the isogenic BbCRASP-1 mutant were readily killed within 1 hour of serum exposure, the viability of the BbCRASP-2 mutant did not differ significantly from that of the wild type (Fig. 4) indicating that BbCRASP-2 is not essential for B. burgdorferi resistance to complement-mediated lysis in serum.
Figure 4. BbCRASP-2 mutant B. burgdorferi is resistant to complement-mediated killing in vitro.
Triplicate wells of spirochetes (5×106 cells/ml) were exposed to 50% normal human serum or 50% heat-inactivated human serum (HI) and the viability of B. burgdorferi was assessed using dark-field microscopy. An isogenic B. burgdorferi BbCRASP-1 mutant served as a serum-sensitive positive control, which were killed within 1 hour of incubation with complement active serum as expected (P<0.05, compared to corresponding HI control), while both wild type and BbCRASP-2 remained fully resistant to serum (P>0.05).
BbCRASP-2-deficient B. burgdorferi retain full murine infectivity
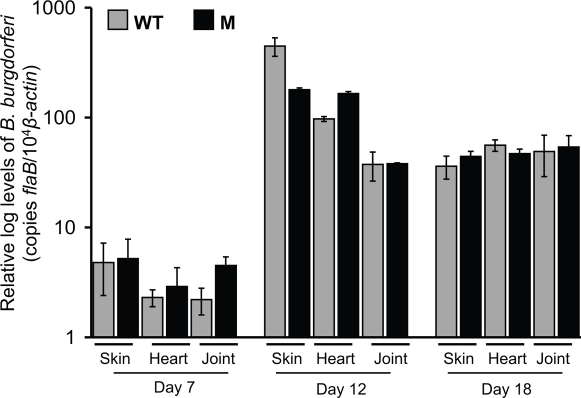
To examine whether the lack of BbCRASP-2 influences B. burgdorferi infectivity in a mammalian host, C3H mice were infected with wild type or BbCRASP-2 mutant B. burgdorferi. Both the mutant and wild type spirochetes were readily cultured from ear and spleen tissues taken from mice 12 days after the inoculum (data not shown). When nymphal ticks were allowed to feed on infected mice, BbCRASP-2 mutant B. burgdorferi were able to migrate into fed ticks at a similar level to the wild type spirochetes (data not shown). Quantitative RT-PCR further showed that the BbCRASP-2 mutant established infection in mice in comparable levels to the parental isolate. No significant differences in the burdens of BbCRASP-2 mutant and wild type isolates were detected in murine skin, heart and joint samples isolated after 7, 12 and 18 days of infection (Fig. 5). Development of swelling in the murine joints infected with either the BbCRASP-2 mutant or the wild type B. burgdorferi was also similar (data not shown). Overall, these results suggest that BbCRASP-2 is not essential for establishment of B. burgdorferi infection in the mouse model of Lyme disease.
Figure 5. BbCRASP-2 mutant B. burgdorferi retain full pathogenicity in the murine host.
The B. burgdorferi burdens in multiple tissues of the infected mice are shown. Mice (6 animals/group) were infected with either the wild type or the BbCRASP-2 mutant B. burgdorferi and spirochete burdens were analyzed as before by measuring copies of B. burgdorferi flaB RNA at day 7, 12 and 18 following infection. Amounts of murine ß-actin were determined in each sample and used to normalize the quantities of spirochete RNA. Bars represent the mean measurements ± SEM from four quantitative PCR measurements from two independent infection experiments. No difference between wild type and BbCRASP-2 mutant levels was statistically significant in any tissue or time point measured (P>0.05, n = 4).
Loss of BbCRASP-2 function is not compensated by augmented or new expression of other BbCRASPs
BbCRASP-2 deficiency did not affect the ability of the BbCRASP-2 mutant to survive complement-mediated lysis in vitro or establish infection in a mammalian host in vivo. Since B. burgdorferi encodes multiple BbCRASPs that are capable of binding host complement regulators, we explored the possibility that the loss of BbCRASP-2 could be compensated by altered expression of other potential BbCRASP genes, such as BbCRASP-1, -3 and -5. We did not examine the expression of BbCRASP-4, as the parental B. burgdorferi isolate A3[36] lacks the non-essential cp32-2 plasmid housing the gene. To examine BbCRASP expression, groups of 6 C3H mice were needle-inoculated with wild type or BbCRASP-2-deficient B. burgdorferi (105 spirochetes/mouse). Infected murine skin and heart samples were isolated 7, 12 and 18 days after infection, and expression of each BbCRASP was measured by quantitative RT-PCR. In vitro expression of BbCRASP genes was also assessed by growing wild type and mutant B. burgdorferi in BSK medium to various cell densities (106–108 spirochetes/ml) and analyzed by quantitative RT-PCR. The expression profiles of BbCRASP-1, -3, and -5 remained unaltered in the BbCRASP-2 mutant when compared to the wild type spirochetes, both in vitro and in vivo, such as in the murine skin and heart tissues at all time points. BbCRASP expression in cultured spirochetes grown in vitro to a density of 107/ml and in infected murine skin and heart tissue samples at 12 days of infection is presented (Fig. 6). These results suggest that the loss of BbCRASP-2 function is not compensated by alteration or new expression of other BbCRASP genes (Fig. 6).
Figure 6. BbCRASP-2 mutant B. burgdorferi express other BbCRASP genes at similar levels to the wild type spirochetes.
The relative expression levels of BbCRASP-1, -2, -3 and -5 were examined in the BbCRASP-2 mutant and wild type spirochetes in vitro and in vivo by quantitative RT-PCR, and are represented as copies of gene per 1000 copies of flaB transcripts. Total RNA was isolated from B. burgdorferi isolates grown in culture (107/ml), as well as multiple tissues of B. burgdorferi-infected mice following 12 days of infection. The experiments were replicated thrice, and bars represent the mean measurements ± SEM from four representative quantitative PCR measurements. Differences between BbCRASP transcripts in the wild type and mutant were not statistically significant (P>0.05, n = 4).
Discussion
B. burgdorferi express up to five BbCRASPs that are either structurally unique, such as BbCRASP-1 and -2, or closely related, BbCRASP-3, -4 and -5 [17], [18], [21], [45]. These BbCRASPs are differentially expressed and are postulated to confer defense against host-derived complements via specific interaction with FH family proteins [16], [17], [31], [32]. The precise role of individual BbCRASPs in the B. burgdorferi infection cycle, however, is currently unclear. BbCRASP-2 is specifically produced in the mammalian host including humans, and is immunogenic, and thus, is thought to be important in spirochete pathogenesis and may be useful in a future Lyme disease vaccine [17], [35]. Here, we show that BbCRASP-2 is ubiquitously expressed throughout murine infection, evoking a detectable antibody response. However, BbCRASP-2 immunization fails to protect the host against B. burgdorferi infection or influence the genesis of disease. Targeted deletion of BbCRASP-2 did not impair the ability of the mutants to resist serum-mediated killing in vitro, establish infectivity in vivo, or the severity of disease. Deficiency of BbCRASP-2 expression in mutants is not functionally compensated by the enhanced expression of other BbCRASP genes. BbCRASP-2, therefore, is not essential for B. burgdorferi survivability in vitro, and based on the time periods covered in the present study, we conclude that BbCRASP-2 function is dispensable for B. burgdorferi infectivity mice and in feeding ticks.
Immunization of murine hosts against specific B. burgdorferi antigens, such as DbpA [46], [47], OspC [48] and OspA [49] can elicit production of borreliacidal antibodies, and thus confer protective host immunity possibly by killing spirochetes in vivo when administered prior to spirochete infection. In contrast, other B. burgdorferi antigens are also described, such as BmpA/B [13] and Arp [50], [51], that fail to protect host against B. burgdorferi infection, but influence pathogenesis either by reduction of the B. burgdorferi burden in selected tissues [13] or by modifying the disease without affecting spirochete load [50], [51]. The failure of BbCRASP-2-specific host immunity to influence B. burgdorferi pathogenesis indicates that BbCRASP-2 antibodies lack significant neutralizing effects on spirochetes, possibly due to the lack of significant surface-exposure of the antigen. This is further confirmed by the observation that the BbCRASP-2-deficient B. burgdorferi displayed no phenotypic defects in their ability to infect the murine host or induce disease. Nevertheless, as BbCRASP-2 is abundantly produced by B. burgdorferi throughout infection and evokes development of specific antibody, our data reinforces an earlier contention that BbCRASP-2 could be a reliable marker for the serodiagnosis of Lyme disease [17], [35].
B. burgdorferi expresses select lipoproteins [19] in the mammalian host [52], including BbCRASP-2. Owing to its ubiquitous expression in the host and known affinity for FH family proteins [17], BbCRASP-2 appears to be important for B. burgdorferi protection against persistent host immune threats, such as complement system. BbCRASP-2 is conserved among infectious B. burgdorferi isolates, which also suggests an important role for this protein in the infectivity of B. burgdorferi [22]. The plasmid lp28-3 that houses BbCRASP-2 is retained in most of the B. burgdorferi clones isolated from experimentally infected hosts [53]–[56]. Previous studies attempting to identify specific plasmids required for B. burgdorferi infectivity indicate that lp28-3 plasmid may not be strictly necessary for spirochete infectivity [55] while other studies suggest that several plasmids, including lp28-3, in the correct combinations, may be required to mediate B. burgdorferi infection of the mammals [54]. Nevertheless, our data conclusively show that the function of BbCRASP-2 is not essential for B. burgdorferi survival against serum-mediated killing in vitro or host infectivity. The BbCRASP-2 mutant fails to produce both BbCRASP-1 and BbCRASP-2 in the murine host, yet still retains full infectivity, which suggests that complement evasion in the host, if relevant, could be mediated by BbCRASP-3 and -5, which remain fully expressed by the mutant. Alternatively, binding to certain FH family proteins may not be essential to the spirochetes, as previous studies indicate that B. burgdorferi are able to infect and cause disease in FH-deficient mice [57].
In summary, we present direct evidence that B. burgdorferi adapts for the loss of a differentially expressed and abundant lipoprotein during mammalian infection. Past studies also identified additional B. burgdorferi genes encoding potential lipoproteins, such as BBA64 [58], [59] or OspD [60], [61] that display highly regulated expression in vivo but lack an essential role in B. burgdorferi persistence in tick-mouse infection cycle. Here we show that BbCRASP-2 function is also dispensable for infectivity of the murine hosts or in feeding ticks. Together, these results highlight B. burgdorferi as a unique pathogen that has evolved versatile adaptive strategies to survive and establish infection in a diverse array of host species, including humans.
Supporting Information
(0.05 MB DOC)
Acknowledgments
We sincerely thank Aravinda de Silva and Katie Tyson for the advice and critical comments. We are grateful to Patricia Rosa for providing us the B. burgdorferi B31-A3 clone. We sincerely thank Youn K. Lee for excellent technical help.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported in part by grants from Arthritis Foundation, Maryland Chapter (U.P), National Institutes of Health AR055323 (U.P), AI059373 (D.R.A) and AI064629 (D.R.A).
References
- 1.Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J Clin Invest. 2004;113:1093–1101. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orloski KA, Hayes EB, Campbell GL, Dennis DT. Surveillance for Lyme disease–United States, 1992–1998. MMWR CDC Surveill Summ. 2000;49:1–11. [PubMed] [Google Scholar]
- 3.Rosa P. Lyme disease agent borrows a practical coat. Nat Med. 2005;11:831–832. doi: 10.1038/nm0805-831. [DOI] [PubMed] [Google Scholar]
- 4.de Silva AM, Fikrig E. Arthropod- and host-specific gene expression by Borrelia burgdorferi. J Clin Invest. 1997;99:377–379. doi: 10.1172/JCI119169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwan TG, Piesman J. Vector interactions and molecular adaptations of Lyme disease and relapsing fever spirochetes associated with transmission by ticks. Emerg Infect Dis. 2002;8:115–121. doi: 10.3201/eid0802.010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neelakanta G, Li X, Pal U, Liu X, Beck DS, et al. Outer Surface Protein B Is Critical for Borrelia burgdorferi Adherence and Survival within Ixodes Ticks. PLoS Pathog. 2007;3:e33. doi: 10.1371/journal.ppat.0030033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barthold SW, DeSouza M, Fikrig E, Persing DH. Lyme borreliosis in the laboratory mouse. In: Schuster SE, editor. Lyme disease. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1992. pp. 223–242. [Google Scholar]
- 8.Barthold SW, Beck DS, Hansen GM, Terwilliger GA, Moody KD. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 9.Barthold SW, deSouza MS, Janotka JL, Smith AL, Persing DH. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:959–971. [PMC free article] [PubMed] [Google Scholar]
- 10.Schaible UE, Kramer MD, Wallich R, Tran T, Simon MM. Experimental Borrelia burgdorferi infection in inbred mouse strains: antibody response and association of H-2 genes with the resistance and susceptibility to development of arthritis. Eur J Immunol. 1991;21:2397–2405. doi: 10.1002/eji.1830211016. [DOI] [PubMed] [Google Scholar]
- 11.Simon MM, Schaible UE, Wallich R, Kramer MD. A mouse model for Borrelia burgdorferi infection: approach to a vaccine against Lyme disease. Immunol Today. 1991;12:11–16. doi: 10.1016/0167-5699(91)90106-4. [DOI] [PubMed] [Google Scholar]
- 12.Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, et al. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc Natl Acad Sci U S A. 2004;101:3142–3147. doi: 10.1073/pnas.0306845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pal U, Wang P, Bao F, Yang X, Samanta S, et al. Borrelia burgdorferi basic membrane proteins A and B participate in the genesis of Lyme arthritis. J Exp Med. 2008;205:133–141. doi: 10.1084/jem.20070962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bykowski T, Woodman ME, Cooley AE, Brissette CA, Brade V, et al. Coordinated expression of Borrelia burgdorferi complement regulator-acquiring surface proteins during the Lyme disease spirochete's mammal-tick infection cycle. Infect Immun. 2007;75:4227–4236. doi: 10.1128/IAI.00604-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hefty PS, Jolliff SE, Caimano MJ, Wikel SK, Radolf JD, et al. Regulation of OspE-related, OspF-related, and Elp lipoproteins of Borrelia burgdorferi strain 297 by mammalian host-specific signals. Infect Immun. 2001;69:3618–3627. doi: 10.1128/IAI.69.6.3618-3627.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alitalo A, Meri T, Ramo L, Jokiranta TS, Heikkila T, et al. Complement evasion by Borrelia burgdorferi: serum-resistant strains promote C3b inactivation. Infect Immun. 2001;69:3685–3691. doi: 10.1128/IAI.69.6.3685-3691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartmann K, Corvey C, Skerka C, Kirschfink M, Karas M, et al. Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol Microbiol. 2006;61:1220–1236. doi: 10.1111/j.1365-2958.2006.05318.x. [DOI] [PubMed] [Google Scholar]
- 18.McDowell JV, Wolfgang J, Tran E, Metts MS, Hamilton D, et al. Comprehensive analysis of the factor H binding capabilities of Borrelia species associated with Lyme disease: delineation of two distinct classes of factor h binding proteins. Infect Immun. 2003;71:3597–3602. doi: 10.1128/IAI.71.6.3597-3602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casjens S, Palmer N, van Vugt R, Huang WM, Stevenson B, et al. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:490–516. doi: 10.1046/j.1365-2958.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- 20.Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 21.Kraiczy P, Hartmann K, Hellwage J, Skerka C, Kirschfink M, et al. Immunological characterization of the complement regulator factor H-binding CRASP and Erp proteins of Borrelia burgdorferi. Int J Med Microbiol 293 Suppl. 2004;37:152–157. doi: 10.1016/s1433-1128(04)80029-9. [DOI] [PubMed] [Google Scholar]
- 22.Rogers EA, Marconi RT. Delineation of species-specific binding properties of the CspZ protein (BBH06) of Lyme disease spirochetes: evidence for new contributions to the pathogenesis of Borrelia spp. Infect Immun. 2007;75:5272–5281. doi: 10.1128/IAI.00850-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 24.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 25.Brooks CS, Vuppala SR, Jett AM, Alitalo A, Meri S, et al. Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J Immunol. 2005;175:3299–3308. doi: 10.4049/jimmunol.175.5.3299. [DOI] [PubMed] [Google Scholar]
- 26.Jarva H, Janulczyk R, Hellwage J, Zipfel PF, Bjorck L, et al. Streptococcus pneumoniae evades complement attack and opsonophagocytosis by expressing the pspC locus-encoded Hic protein that binds to short consensus repeats 8–11 of factor H. J Immunol. 2002;168:1886–1894. doi: 10.4049/jimmunol.168.4.1886. [DOI] [PubMed] [Google Scholar]
- 27.Meri T, Hartmann A, Lenk D, Eck R, Wurzner R, et al. The yeast Candida albicans binds complement regulators factor H and FHL-1. Infect Immun. 2002;70:5185–5192. doi: 10.1128/IAI.70.9.5185-5192.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ram S, Mackinnon FG, Gulati S, McQuillen DP, Vogel U, et al. The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B Neisseria meningitidis. Mol Immunol. 1999;36:915–928. doi: 10.1016/s0161-5890(99)00114-5. [DOI] [PubMed] [Google Scholar]
- 29.Ram S, McQuillen DP, Gulati S, Elkins C, Pangburn MK, et al. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J Exp Med. 1998;188:671–680. doi: 10.1084/jem.188.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haupt K, Kraiczy P, Wallich R, Brade V, Skerka C, et al. Binding of human factor H-related protein 1 to serum-resistant Borrelia burgdorferi is mediated by borrelial complement regulator-acquiring surface proteins. J Infect Dis. 2007;196:124–133. doi: 10.1086/518509. [DOI] [PubMed] [Google Scholar]
- 31.Haupt K, Kraiczy P, Wallich R, Brade V, Skerka C, et al. FHR-1, an additional human plasma protein, binds to complement regulator-acquiring surface proteins of Borrelia burgdorferi. Int J Med Microbiol 2008 [Google Scholar]
- 32.Hovis KM, Tran E, Sundy CM, Buckles E, McDowell JV, et al. Selective binding of Borrelia burgdorferi OspE paralogs to factor H and serum proteins from diverse animals: possible expansion of the role of OspE in Lyme disease pathogenesis. Infect Immun. 2006;74:1967–1972. doi: 10.1128/IAI.74.3.1967-1972.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDowell JV, Hovis KM, Zhang H, Tran E, Lankford J, et al. Evidence that the BBA68 protein (BbCRASP-1) of the Lyme disease spirochetes does not contribute to factor H-mediated immune evasion in humans and other animals. Infect Immun. 2006;74:3030–3034. doi: 10.1128/IAI.74.5.3030-3034.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bykowski T, Woodman ME, Cooley AE, Brissette CA, Wallich R, et al. Borrelia burgdorferi complement regulator-acquiring surface proteins (BbCRASPs): Expression patterns during the mammal-tick infection cycle. Int J Med Microbiol. 2007 doi: 10.1016/j.ijmm.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kraiczy P, Seling A, Brissette CA, Rossmann E, Hunfeld KP, et al. Borrelia burgdorferi complement regulator-acquiring surface protein 2 (CspZ) as a serological marker of human Lyme disease. Clin Vaccine Immunol. 2008;15:484–491. doi: 10.1128/CVI.00415-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elias AF, Stewart PE, Grimm D, Caimano MJ, Eggers CH, et al. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect Immun. 2002;70:2139–2150. doi: 10.1128/IAI.70.4.2139-2150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pal U, Li X, Wang T, Montgomery RR, Ramamoorthi N, et al. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell. 2004;119:457–468. doi: 10.1016/j.cell.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 38.Pal U, Dai J, Li X, Neelakanta G, Luo P, et al. A Differential Role for BB0365 in the Persistence of Borrelia burgdorferi in Mice and Ticks. J Infect Dis. 2008;197:148–155. doi: 10.1086/523764. [DOI] [PubMed] [Google Scholar]
- 39.Pal U, Yang X, Chen M, Bockenstedt LK, Anderson JF, et al. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J Clin Invest. 2004;113:220–230. doi: 10.1172/JCI19894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brooks CS, Vuppala SR, Jett AM, Akins DR. Identification of Borrelia burgdorferi outer surface proteins. Infect Immun. 2006;74:296–304. doi: 10.1128/IAI.74.1.296-304.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolz DD, Sundsbak RS, Ma Y, Akira S, Kirschning CJ, et al. MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J Immunol. 2004;173:2003–2010. doi: 10.4049/jimmunol.173.3.2003. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Ma Y, Weis JH, Zachary JF, Kirschning CJ, et al. Relative contributions of innate and acquired host responses to bacterial control and arthritis development in Lyme disease. Infect Immun. 2005;73:657–660. doi: 10.1128/IAI.73.1.657-660.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X, Pal U, Ramamoorthi N, Liu X, Desrosiers DC, et al. The Lyme disease agent Borrelia burgdorferi requires BB0690, a Dps homologue, to persist within ticks. Mol Microbiol. 2007 doi: 10.1111/j.1365-2958.2006.05550.x. [DOI] [PubMed] [Google Scholar]
- 44.Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med. 2004;199:641–648. doi: 10.1084/jem.20031960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alitalo A, Meri T, Lankinen H, Seppala I, Lahdenne P, et al. Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J Immunol. 2002;169:3847–3853. doi: 10.4049/jimmunol.169.7.3847. [DOI] [PubMed] [Google Scholar]
- 46.Hanson MS, Cassatt DR, Guo BP, Patel NK, McCarthy MP, et al. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect Immun. 1998;66:2143–2153. doi: 10.1128/iai.66.5.2143-2153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cassatt DR, Patel NK, Ulbrandt ND, Hanson MS. DbpA, but not OspA, is expressed by Borrelia burgdorferi during spirochetemia and is a target for protective antibodies. Infect Immun. 1998;66:5379–5387. doi: 10.1128/iai.66.11.5379-5387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Probert WS, Lefebvre RB. Protection of C3H/HeN mice from challenge with Borrelia burgdorferi through active immunization with OspA, OspB or OspC but not OspD or the 83-kilodalton antigen. Infect Immun. 1994;62:1920–1926. doi: 10.1128/iai.62.5.1920-1926.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fikrig E, Barthold SW, Kantor FS, Flavell RA. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990;250:553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- 50.Feng S, Hodzic E, Barthold SW. Lyme arthritis resolution with antiserum to a 37-kilodalton Borrelia burgdorferi protein. Infect Immun. 2000;68:4169–4173. doi: 10.1128/iai.68.7.4169-4173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng S, Hodzic E, Freet K, Barthold SW. Immunogenicity of Borrelia burgdorferi arthritis-related protein. Infect Immun. 2003;71:7211–7214. doi: 10.1128/IAI.71.12.7211-7214.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang FT, Nelson FK, Fikrig E. Molecular adaptation of Borrelia burgdorferi in the murine host. J Exp Med. 2002;196:275–280. doi: 10.1084/jem.20020770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iyer R, Kalu O, Purser J, Norris S, Stevenson B, et al. Linear and circular plasmid content in Borrelia burgdorferi clinical isolates. Infect Immun. 2003;71:3699–3706. doi: 10.1128/IAI.71.7.3699-3706.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Labandeira-Rey M, Skare JT. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28–1. Infect Immun. 2001;69:446–455. doi: 10.1128/IAI.69.1.446-455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDowell JV, Sung SY, Labandeira-Rey M, Skare JT, Marconi RT. Analysis of mechanisms associated with loss of infectivity of clonal populations of Borrelia burgdorferi B31MI. Infect Immun. 2001;69:3670–3677. doi: 10.1128/IAI.69.6.3670-3677.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Purser JE, Norris SJ. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci U S A. 2000;97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woodman ME, Cooley AE, Miller JC, Lazarus JJ, Tucker K, et al. Borrelia burgdorferi binding of host complement regulator factor H is not required for efficient mammalian infection. Infect Immun. 2007;75:3131–3139. doi: 10.1128/IAI.01923-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gilmore RD, Jr, Howison RR, Schmit VL, Nowalk AJ, Clifton DR, et al. Temporal expression analysis of the Borrelia burgdorferi paralogous gene family 54 genes BBA64, BBA65, and BBA66 during persistent infection in mice. Infect Immun. 2007;75:2753–2764. doi: 10.1128/IAI.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maruskova M, Esteve-Gassent MD, Sexton VL, Seshu J. Role of the BBA64 locus of Borrelia burgdorferi in early stages of infectivity in a murine model of Lyme disease. Infect Immun. 2008;76:391–402. doi: 10.1128/IAI.01118-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li X, Neelakanta G, Liu X, Beck DS, Kantor FS, et al. Role of outer surface protein D in the Borrelia burgdorferi life cycle. Infect Immun. 2007;75:4237–4244. doi: 10.1128/IAI.00632-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stewart PE, Bestor A, Cullen JN, Rosa PA. A tightly regulated surface protein of Borrelia burgdorferi is not essential to the mouse-tick infectious cycle. Infect Immun. 2008;76:1970–1978. doi: 10.1128/IAI.00714-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(0.05 MB DOC)