Abstract
Regulatory T (T reg) cells are indispensable for preventing autoimmunity. Incumbent to this role is the ability of T reg cells to exert their suppressor function under inflammatory conditions. We found that T reg cell–mediated tolerance is critically dependent on the Dicer-controlled microRNA (miRNA) pathway. Depletion of miRNA within the T reg cell lineage resulted in fatal autoimmunity indistinguishable from that in T reg cell–deficient mice. In disease-free mice lacking Dicer in all T cells or harboring both Dicer-deficient and -sufficient T reg cells, Dicer-deficient T reg cells were suppressive, albeit to a lesser degree, whereas their homeostatic potential was diminished as compared with their Dicer-sufficient counterparts. However, in diseased mice, Dicer-deficient T reg cells completely lost suppressor capacity. Thus, miRNA preserve the T reg cell functional program under inflammatory conditions.
The deleterious effects of autoreactive cells and excessive pathogen-specific immunity are curtailed by multiple mechanisms, of which T reg cell–mediated suppression is particularly prominent. The differentiation of the T reg cell lineage is guided by the X chromosome–encoded transcription factor Foxp3 (1–4). Mutations affecting T reg cell differentiation and homeostasis in humans and mice revealed their indispensable role in preventing systemic autoimmunity (5–8). Likewise, ablation of T reg cells in healthy adult mice leads to a systemic aggressive autoimmune myelolymphoid hyperproliferative syndrome and death within 3 wk (9).
The high level of Foxp3 expression, a hallmark of T reg cells, is needed to establish and maintain a distinct transcriptional program determining metabolic, signaling, and effector features (i.e., suppressive functions), distinguishing these cells from other T cell lineages (10–14). Several thousand genes are differentially expressed in Foxp3+ T reg cells, including a subset of noncoding small regulatory microRNA (miRNA), some of which are directly regulated by Foxp3, suggesting a role for miRNA-mediated regulation of gene expression in T reg cell differentiation, maintenance, or function (unpublished data) (11, 15–17). One of the models of miRNA function in cell physiology postulates that miRNA are essential for “buffering” the gene expression “noise” elicited by the environmental stress (18). Incumbent to the role of T reg cells in suppressing autoimmunity is the ability to exert their suppressor function while operating within microenvironments in which cytokines and other bioactive substances produced by activated cells of the adaptive and innate immune system result in inflammation, a context guiding the effector T (TE) cell differentiation. Thus, we examined a role for miRNA in T reg cell suppressor function under physiological and inflammatory conditions.
The recent observation of a two- to threefold decrease in the proportion of Foxp3+ T reg cells in mice subjected to a T cell–specific deletion of a conditional Dicer allele seems manifest to a role for miRNA in T reg cell differentiation (15). However, a general impairment in thymic differentiation observed upon Dicer deletion at the double-negative or double-positive stage of thymocyte development mediated by lck-Cre or CD4-Cre, respectively (15, 19), obscures the understanding of a specific role for the Dicer-controlled miRNA pathway in T reg cell biology. Furthermore, in addition to the reduced T reg cell subset, diminished T cell numbers and perturbed cytokine production by effector helper T cells may contribute to the colitis reported in aged CD4-Cre Dicerfl/fl mice (15). To overcome these confounding factors, we used a Foxp3Cre knock-in allele to delete a conditional Dicer allele in T reg cells. Although certain small regulatory RNA species other than miRNA were shown to be dependent on Dicer in Drosophila and mice, they have not been found so far in T cells (20–24). Therefore, the observed effects of Dicer deficiency in T reg cells can be most attributed to an impaired miRNA pathway. We found that both the homeostasis and suppressor capacity of T reg cells were markedly reduced in T reg cells under noninflammatory conditions. However, the most striking role for miRNA in T reg cells was revealed under inflammatory conditions, where T reg cells became activated and increased in numbers yet entirely lost their suppressive capacity. This led to the progression of fatal early onset lymphoproliferative autoimmune syndrome indistinguishable from that observed in Foxp3 mutant mice devoid of T reg cells. These data implicate miRNA as key guardians of a stable T reg cell functional program under inflammatory conditions.
RESULTS
Rapid fatal autoimmunity caused by T reg cell–specific ablation of Dicer
To understand the role of the Dicer-controlled miRNA pathway in T reg cell biology, we induced ablation of a conditional Dicerfl allele, and therefore miRNA production, before and after up-regulation of Foxp3 using the CD4-Cre transgene (pan–T cell) and a Foxp3Cre knock-in allele (T reg cell specific), respectively (Fig. 1 A). The latter was generated by insertion of a yellow fluorescent protein (YFP)–Cre fusion protein DNA coding sequence equipped with an internal ribosome entry site into the 3′ untranslated region of the Foxp3 gene (25). Depletion of select miRNA as a result of Foxp3-Cre–mediated Dicer ablation was confirmed by quantitative PCR (qPCR; Fig. 1, B and C). To enable functional analysis of T reg cells, we introduced the previously described Foxp3gfp reporter allele (4) into CD4-Cre–expressing mutant mice, while T reg cells were marked by YFP in Foxp3Cre mice. Although CD4-Cre Dicerfl/fl mice remained healthy during the time of observation (up to 16 wk of age), Foxp3CreDicerfl/fl mice developed highly aggressive autoimmune lesions during the third week of life. Disease manifested in runting, failure to thrive, dermatitis, lymphadenopathy and splenomegaly, and massive lymphocytic tissue infiltration that was particularly severe in the lungs, liver, and skin (Fig. 2, A–C). The severity and precipitous progression of the autoimmune syndrome, which resulted in death by 4 wk of age, were indistinguishable from those observed in mice devoid of T reg cells because of a germline or T cell–specific Foxp3 ablation (3, 4). The observed lymphoproliferation was associated with a significant increase in the CD62Llow CD4 T cell subset, while the Foxp3+ T reg cell population also significantly expanded, albeit to a lesser degree (Fig. 2, D–F; and Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20081062/DC1). This increase in T cell numbers was also accompanied by up-regulation of activation markers, including inducible T cell co-stimulator (ICOS), cytotoxic T lymphocyte antigen 4 (CTLA4), and CD25, in the Foxp3− T cell population (Fig. S2). The lack of severe pathologies in the CD4-Cre Dicerfl/fl strain indicates that a hitherto unappreciated severe cell-intrinsic defect in the activation of naive T (TN) cells and their subsequent differentiation into TE cells masks the devastating consequences of Dicer deficiency in T reg cells.
Figure 1.
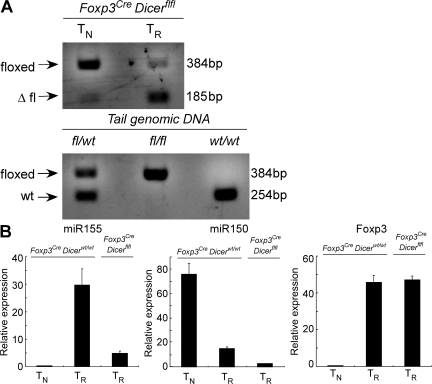
Deletion of a conditional Dicer allele and the resulting miRNA paucity in Foxp3+ CD4+ T cells from Foxp3CreDicerfl/fl mice. (A) An efficient Cre-mediated excision of the Dicerfl/fl allele in CD4+Foxp3+ cells, but not in CD4+Foxp3− cells, in Foxp3CreDicerfl/fl mice. The excised “floxed” allele produced a predominant 185-bp PCR product, whereas CD4+Foxp3− cells retained the intact floxed allele producing a 384-bp PCR product. In non–T reg (TR) cells (CD4+Foxp3−), we observed a weak signal corresponding to a deleted Dicerfl allele, in agreement with our recent finding that Cre-mediated recombination of a single floxed allele might occur in 1–5% of Foxp3− T cells in Foxp3Cre mice, whereas the deletion of both floxed alleles is likely negligible (reference 25). (B) Real-time PCR analysis of miR155, miR150, and Foxp3 mRNA expression in CD4+Foxp3−CD62Lhi TN cells and CD4+YFP+Foxp3+ T reg (TR) cells purified from Foxp3CreDicerfl/wt and Foxp3Cre/wtDicerfl/fl mice. The data are shown as the mean and standard deviation representing three independent experiments. miR155 was selected as an example of an miRNA with an increased expression in Foxp3+ cells compared with Foxp3−CD4+ T cells, and miR150 was selected as an example of an miRNA with a reduced expression in Foxp3+ cells.
Figure 2.
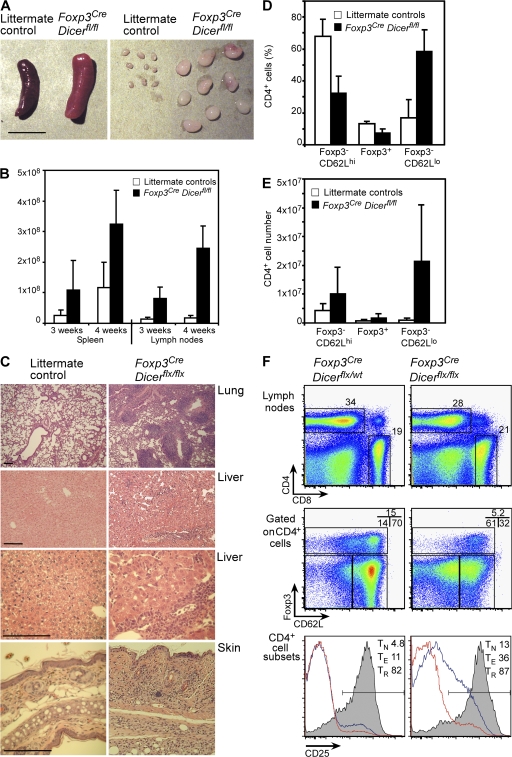
Fatal early onset autoimmunity in mice with T reg cell–specific Dicer deficiency. (A and B) Lymphadenopathy and splenomegaly in Foxp3CreDicerfl/fl mice. A 4-fold increase in spleen (P < 0.05) and a 6-fold increase in lymph node (P < 0.0005) cellularity in 3-wk-old mutant mice (n = 8 per group) occurred; a 3-fold increase in spleen (P < 0.005) and a 14-fold increase in lymph node (P < 0.0001) cellularity in 4-wk-old mutant mice (n = 3–15 per group) occurred compared with the littermate controls. Bar, 1 cm. (C) Histopathology of lung, liver, and skin in Foxp3CreDicerfl/fl mice. Hematoxylin and eosin–stained tissue sections of 3-wk-old Foxp3CreDicerfl/fl mice showed massive lymphocytic infiltrates and disrupted tissue architecture compared with Foxp3CreDicerwt/fl littermates. Sections shown are representative of three individual mice per group. Bars, 100 μm. (D) The proportion of naive Foxp3−CD62Lhi (TN), Foxp3+ T reg, and effector Foxp3−CD62Llow (TE) cells within the lymph node CD4+ T cell population in Foxp3CreDicerfl/fl mice and littermate controls. (E) Absolute numbers of CD62Lhi TN, CD62LlowTE, and Foxp3+ T reg lymph node cells. (F) Analysis of lymph node T cell subsets in Foxp3CreDicerfl/fl and control Foxp3CreDicerwt/fl mice for CD4, CD8, Foxp3, CD62L, and CD25 expression (percentages of cells in the indicated gates are shown). CD25 histograms include TN (red line), TE (blue line), and T reg (shaded) populations (n = 11–18). Values shown are means ± standard deviation.
Immune dysregulation observed upon T reg cell–specific ablation of Dicer
We next sought to examine whether the loss of Dicer in T reg cells resulted in the increased T cell cytokine and IgE production characteristic of Foxp3-deficient mice and human patients lacking T reg cells (3, 26, 27). Indeed, both Foxp3creDicerfl/fl and Foxp3ko mice exhibited a sharply augmented Th1, Th2, and Th17 cell differentiation based on the increased proportion and absolute numbers of IL-2–, IL-4–, IL-5–, IFN-γ–, and IL-17–producing CD4+ T cells and IFN-γ–producing CD8+ T cells (Fig. 3, A and B; and Table I). One notable exception was TNF-α, where the production by CD4 T cells was not changed in Foxp3creDicerfl/fl mice yet was somewhat diminished in Foxp3ko mice. In contrast to T reg cell–restricted Dicer deficiency, cytokine secretion levels in CD4-Cre Dicerfl/fl mice with pan–T cell Dicer deficiency remained largely intact (Fig. 3, A and B; and Table I). Similarly, cytokine secretion profiles in heterozygous female Foxp3cre/wtDicerfl/fl mice were comparable to those observed in the littermate controls, demonstrating that the presence of Dicer-sufficient T reg cells was able to maintain immunological tolerance (Fig. S3 and Table S1, available at http://www.jem.org/cgi/content/full/jem.20081062/DC1).
Figure 3.
Augmented cytokine production and elevated serum levels of IgE in mice harboring Dicer-deficient T reg cells. (A and B) Flow cytometric analysis of IL-2 and IFN-γ production by splenic T cells in Foxp3creDicerfl/fl and control mice induced upon 5 h of in vitro stimulation with 1 μg/ml CD3 and 1 μg/ml CD28 antibodies. The results are representative of three independent experiments with two to four mice per group (percentages of cells in the indicated gates are shown). (C) Quantification of serum Ig isotype levels in Dicerwt/wt (n = 6), Foxp3KO (n = 4), CD4-Cre Dicerfl/fl (n = 4), and Foxp3CreDicerfl/fl (n = 5) mice using ELISA. Foxp3CreDicerfl/fl and Foxp3KO mice exhibited ∼10-fold elevation in serum IgM and IgG1 and even higher elevation in serum IgE. ND, not detected. In these experiments, the control group included serum samples from both B6 mice as a control for Foxp3ko and from Dicer-sufficient littermate control mice. The two control groups did not show significant differences in Ig isotype levels in two independent experiments (B6, n = 5; Dicer littermate controls, n = 13). Values shown are means ± standard deviation.
Table I.
Mice with a T reg cell–specific Dicer deficiency exhibit sharply increased CD4 T cell cytokine production
| Foxp3creDicerwt/wt | Foxp3KO | CD4-Cre Dicerfl/fl | Foxp3creDicerfl/fl | |
|---|---|---|---|---|
| IFN-γ | 1.99 ± 2.47 | 4.64 ± 0.31 | 1.36 ± 1.67 | 10.39 ± 5.93 |
| TNF-α | 26.53 ± 4.26 | 10.61 ± 0.46 | 32.18 ± 4.13 | 23.94 ± 4.44 |
| IL-2 | 1.01 ± 0.6 | 3.9 ± 0.5 | 0.48 ± 0.17 | 7.77 ± 1.81 |
| IL-4 | 0.38 ± 0.28 | 2.65 ± 0.69 | 2.31 ± 1.86 | 3.85 ± 1.7 |
| IL-5 | 0.9 ± 0.48 | 2.48 ± 1.08 | 0.75 ± 0.19 | 3.54 ± 1.06 |
| IL-17 | 0.49 ± 0.25 | 1.2 ± 0.03 | 0.8 ± 0.64 | 1.81 ± 0.42 |
Splenocytes isolated from mice of the indicated genotypes were stimulated in vitro with 1 μg/ml CD3 and 1 μg/ml CD28 antibodies for 5 h and assessed for cytokine production by intracellular staining. Values shown represent the mean and standard deviation of the percentage of CD4+Foxp3− cells producing the corresponding cytokines (n = 4–7).
In addition to the regulation of T cell proliferation and cytokine production, T reg cell–mediated suppression has been implicated in restraining B cell responses, as both Foxp3-deficient mice and human patients exhibit hyper-IgE syndrome (27, 28). Therefore, to further compare the autoimmune syndrome commencing in Foxp3creDicerfl/fl with that in Foxp3ko mice, we measured serum Ig isotype levels in these and control mice. In full agreement with the observed augmentation of Th2 cytokine secretion, we found significant increases in serum IgM and IgG1 levels, as well as sharply augmented serum IgE levels reaching ∼100 μg/ml in both Foxp3KO and Foxp3creDicerfl/fl mice, while remaining at undetectable levels in littermate controls. Consistent with the lack of obvious signs of T cell activation and unaltered cytokine production, serum Ig levels were unchanged in CD4-Cre Dicerfl/fl mice (Fig. 3 C). These data suggest that the immune lesions observed in the presence of Dicer-deficient T reg cells, like those in Foxp3-deficient mice, are associated with the comparable excessive differentiation of Th1, Th2, and Th17 cell lineages and increased Ig production.
Compromised homeostasis in Dicer-deficient T reg cells
Rapid progression and severity of clinical manifestations and tissue lesions, the extent of T cell activation, and elevated IgE and IgG1 production indistinguishable from Foxp3ko mice lacking T reg cells were indicative of a severely compromised T reg cell compartment in Foxp3CreDicerfl/fl mice. Deletion of Dicer before Foxp3 up-regulation resulted in a diminished T reg cell subset (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20081062/DC1) (15). In our study, we found that female Foxp3Cre/wt heterozygous mice, harboring a Foxp3Cre Dicer-deficient T reg cell population and a Foxp3wt Dicer-sufficient T reg cell population caused by X chromosome inactivation, were healthy and showed no alterations in percentages or numbers of total Foxp3+ T reg, naive CD62Lhi (TN cells), and “antigen-experienced” CD62Llo CD4 T cells (TE cells; Fig. 4 A). Of the Foxp3+ T reg cell population in female heterozygotes, a comparable proportion of Foxp3Cre-expressing Dicer-deficient and -sufficient Foxp3+ T reg cells were found in the thymus (Fig. 4, B and C). However, these mice exhibited a significant relative reduction in Dicer-deficient T reg cells in the periphery (Fig. 4, B and D). Diminished competitive fitness of Dicer-deficient T reg cells was consistent with previously observed reduced proliferative activity and increased apoptosis in the bulk Dicer-deficient T cell population (19). To further examine the impaired homeostasis of Dicer-deficient T reg cells in noninflammatory settings, total lymphocyte populations were isolated from female Foxp3Cre/wtDicerfl/fl mice or control Foxp3Cre/wtDicerfl/wt mice, and the proliferative activity and apoptosis were assessed in freshly isolated and in vitro–stimulated T reg cells using Ki67 and active caspase-3 (FITC-tagged VAD-FMK) staining, respectively. We found similar frequencies of Ki67+ and VAD-FMK+ cells within ex vivo–isolated T reg cell subsets regardless of the presence or absence of Dicer. Upon TCR stimulation, however, the proportion of proliferating Ki67+ Dicer-deficient T reg cells was decreased, whereas the proportion of apoptotic VAD-FMK+ Dicer-deficient T reg cells was increased in comparison to their Dicer-sufficient counterparts (Fig. 4, E and F). As a result, the ratio of Dicer-deficient (YFP+) to Dicer-sufficient (YFP−) T reg cells in Foxp3Cre/wtDicerfl/fl cultures progressively diminished over time in comparison to cells from Foxp3Cre/wtDicerfl/wt mice (Fig. S5). Thus, impaired peripheral homeostasis can account for the decreased proportion of Dicer-deficient T reg cells cohabiting with wild-type T reg cells in the healthy heterozygous Foxp3Cre/wtDicerfl/fl female mice. However, the homeostatic insufficiency of Dicer-deficient T reg cells alone is unlikely to account for the severe pathology exhibited by male Foxp3CreDicerfl/fl mice, as comparably deficient T reg cell proliferation and diminished T reg cell numbers were observed upon the loss of a single miRNA, miR155, in the absence of frank immune dysregulation (unpublished data).
Figure 4.
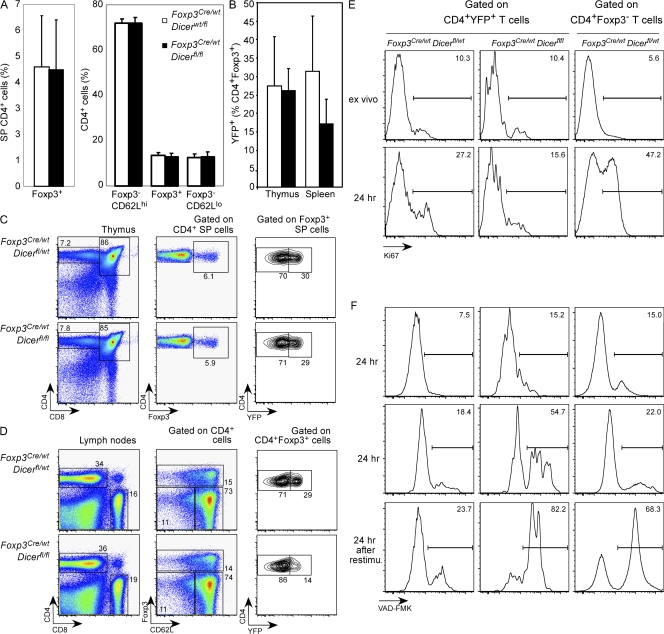
Reduced homeostatic potential of Dicer-deficient T reg cells. In female Foxp3Cre/wt heterozygous mice, random X chromosome inactivation results in two distinct subsets of T reg cells: a YFP− population expressing the wild-type Foxp3 protein and lacking in Cre activity, and a YFP+ population expressing Foxp3Cre. (A and B) Comparable size of thymic and peripheral YFP+Foxp3+ T reg cell subsets, as well as peripheral CD62LhiFoxp3− TN, Foxp3+ T reg, and CD62LlowFoxp3− TE subsets in Foxp3Cre/wtDicerfl/fl and Foxp3Cre/wtDicerfl/wt mice (n = 7–8 per group). Values shown are means ± standard deviation. (C) Flow cytometric analysis of CD4, CD8, and Foxp3 expression by CD4 single-positive thymocytes, and CD4 and YFP expression by Foxp3+ single-positive thymocytes. (D) Flow cytometric analysis of CD62L and Foxp3 expression by peripheral CD4+ cells, and CD4 and YFP expression by peripheral Foxp3+ cells (n = 6 per group). (E) Dicer-deficient T reg cells (YFP+) from healthy Foxp3Cre/wtDicerfl/fl or control Foxp3Cre/wtDicerfl/wt mice were stained for Ki67 upon ex vivo isolation or after 24 h of in vitro stimulation in the presence of CD3 and CD28 antibodies and IL-2. (F) Apoptosis was measured by staining for active caspase-3 using FITC-VAD-FMK after in vitro stimulation for the indicated periods of time. Some activated cells were restimulated with anti-CD3 for an additional 24 h before the apoptosis analysis. Percentages of cells in the indicated gates are shown in C–F.
Complete failure of suppressor function in Dicer-deficient T reg cells under inflammatory conditions
Although the inferior homeostatic properties of Dicer-deficient T reg cells revealed in the absence of inflammation may contribute to their reduced proportion in sick Foxp3CreDicerfl/fl male mice, the aforementioned significant increase in the absolute numbers of Dicer-deficient Foxp3+ T reg cells in these mice indicated that Dicer-deficient T reg cells are capable of expansion and activation (see the following section), and suggested that the critical tolerance breakdown in Foxp3CreDicerfl/fl mice might result, at least in part, from the crippling of T reg cell function. To examine the suppressor function of these T reg cell subsets in a semiquantitative manner, we performed in vitro suppression assays. T reg cells isolated from Foxp3CreDicerwt/wt or Foxp3CreDicerfl/wt littermates served as a control in these experiments. We found that FACS-purified Dicer-deficient T reg cells from healthy female Foxp3Cre/wtDicerfl/fl mice were “anergic” (i.e., unable to proliferate in response to TCR stimulation), retaining this distinguishing feature of wild-type T reg cells. More importantly, these cells were still functional, albeit markedly (approximately fourfold) less efficient on a per cell basis compared with the Dicer-sufficient T reg cells isolated from their wild-type counterparts (Fig. 5 A). In agreement with these results, T reg cells isolated from CD4-Cre Dicerfl/fl mice also exhibited a similarly reduced suppressive capacity when co-cultured with wild-type naive CD4 T cells (Fig. 5 B). Naive CD4 T cells from CD4-Cre Dicerfl/fl mice exhibited a reduced proliferative response (Fig. S6, available at http://www.jem.org/cgi/content/full/jem.20081062/DC1) (19) and altered cytokine production upon in vitro stimulation under Th1, Th2, and Th17 skewing conditions (15, 19), making it likely that the diminished suppressive capacity of T reg cells is still sufficient to keep in check TE cells in CD4-Cre Dicerfl/fl mice. Remarkably, Dicer-deficient Foxp3+ T cells FACS purified from Foxp3CreDicerfl/fl littermates were completely devoid of suppressor activity and instead showed a robust in vitro proliferative response consistent with the identical autoimmune syndrome in these and Foxp3-deficient mice (Fig. 5, A and B). The loss of the typical anergic phenotype of Dicer-deficient T reg cells isolated from the inflammatory environment has two important implications: first, it demonstrates that the loss of suppressive function cannot be caused by T reg cell failure to survive in vitro; and second, it indicates that two principal T reg cell characteristics, suppressive capacity and in vitro anergy, are lost in an inflammatory environment.
Figure 5.
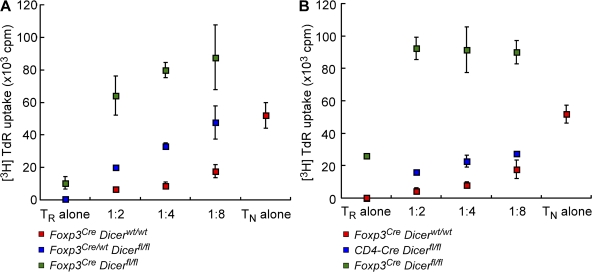
A complete loss of suppressive capacity of Dicer-deficient T reg cells in diseased mice. (A) Dicer-deficient T reg (TR) cells (YFP+) isolated from healthy control Foxp3Cre/wtDicerfl/fl mice and autoimmune Foxp3CreDicerfl/fl mice, as well as wild-type Foxp3CreDicerwt/wt littermate controls, were co-cultured with responder CD4 T cells at the indicated ratios for 72 h in the presence of 1 μg/ml CD3 antibody and irradiated T cell–depleted splenocytes. (B) Dicer-deficient T reg cells (GFP+) isolated from CD4-Cre Dicerfl/fl mice were examined in comparison with T reg cells from sick Foxp3creDicerfl/fl mice and wild-type Foxp3CreDicerwt/wt littermate controls. Values shown are means ± standard deviation.
Diminished expression of multiple suppressor molecules in Dicer-deficient T reg cells
Recent papers have demonstrated that Foxp3 ablation or reduced expression in mature T reg cells results in a complete or partial loss of suppressor function, providing a potential clue for the loss of suppressor function described above (12, 14). However, we found comparable Foxp3 mRNA and protein levels in the T reg cells of each genotype (Fig. 1 D and Fig. 6 A), suggesting that the loss of suppressive capacity was not caused by changes in Foxp3 itself, but rather by low expression of putative suppressor effector molecules, such as CTLA4, subject to miRNA-dependent regulation. In agreement with the marked impairment in suppressor capacity of Dicer-deficient T reg cells in disease-free Foxp3Cre/wtDicerfl/fl mice, we found decreased expression of some putative suppressor molecules, including CTLA4, IL-10, EBV-induced gene 3 (Ebi3), and granzyme B (Fig. 6, A and B), whereas others, such as TGF-β, remained unaffected (Fig. 6 B). Dicer-deficient T reg cell surface levels of CTLA4 and other surface markers were similar in CD4-Cre Dicerfl/fl mice to those observed in Foxp3Cre/wtDicerfl/fl mice (Fig. S7, available at http://www.jem.org/cgi/content/full/jem.20081062/DC1).
Figure 6.
Altered phenotypic features and diminished expression of putative T reg cell suppressor molecules in the absence of the Dicer-dependent miRNA pathway. (A) Expression of Foxp3, CD25, IL-7Rα, GITR, CTLA4, ICOS, and CD103 on Dicer-deficient T reg (TR) cells, wild-type littermate YFP+ T reg cells (Foxp3Cre/wtDicerfllwt), YFP+ Foxp3Cre/wtDicerfl/fl T reg cells, and YFP+ Foxp3CreDicerfl/fl T reg cells compared with TN cells from wild-type littermates. (B) Expression of IL-10, Ebi3, TGF-β, and granzyme B mRNA in Dicer-deficient T reg (TR) cells (CD4+YFP+; Foxp3Cre/wtDicerfl/fl), wild-type littermate TN cells (CD4+CD25−CD62Lhi), and T reg cells (CD4+YFP+; Foxp3Cre/wtDicerfllwt). Values shown are means ± standard deviation. (C) Ly5.1+ T reg cells were transferred to Foxp3CreDicerfl/fl mice. 5 d after transfer, mice were killed and analyzed by flow cytometry. (D) Resident Dicer-deficient and transferred Dicer-sufficient T reg cells were gated by CD4, Foxp3, and Ly5.1 from total lymphocytes isolated from peripheral lymph nodes (the percentage of cells in the indicated gate is shown). (E) Expression of Ki67, CD25, CD62L, CD73, GITR, CTLA4, and ICOS on recipient Dicer-deficient T reg (TR) cells, transferred wild-type T reg cells (Ly5.1+Dicerwtlwt), and Foxp3− TE cells.
However, in apparent contradiction to the aforementioned reasoning, CTLA4 as well as ICOS were increased in diseased Foxp3CreDicerfl/fl mice to levels approaching those observed in Dicer-sufficient T reg cells in wild-type mice (Fig. 6 A). However, expression of these molecules is also significantly augmented in wild-type T reg cells under inflammatory conditions. Therefore, to examine the expression of several characteristic cell-surface molecules, including those implicated in T reg cell effector function, by Dicer-deficient and -sufficient T reg cells in the same inflammatory environment, we adoptively transferred wild-type Ly5.1+ T reg cells into Foxp3CreDicerfl/fl mice (Fig. 6 C). These experiments also tested the competitive fitness of Dicer-deficient T reg cells in diseased mice. Unexpectedly, on day 5 after transfer, we found that transferred wild-type T reg cells represented only 1% of the Foxp3+ T cell population in recipient mice and, therefore, failed to rescue the disease (Fig. 6 D). Thus, despite a decreased proportion of Ki67+ cells in sick mice (Fig. 6 E), the Dicer-deficient Foxp3+ cell subset keeps the T reg cell niche fully occupied and, thus, represents a “niche-filling” nonfunctional T reg cell population. This provides further evidence that the T reg cell deficiency underlying the fatal pathology is not solely caused by a homeostatic insufficiency. Consistent with their functional deficiency, these cells expressed significantly lower levels of CTLA4, ICOS, and CD73 compared with their wild-type counterparts, whereas levels of CD25, CD62L, IL-7Rα, and glucocorticoid-induced TNFR (GITR) were comparable (Fig. 6 E). CTLA4-induced cross-linking of B7 on APCs and activated T cells and CD73-dependent adenosine generation were both proposed to serve as effector mechanisms of T reg cell–mediated suppression (29, 30). Furthermore, ablation of CTLA4 in T reg cells leads to fatal autoimmunity at 6–8 wk of age (unpublished data). Thus, in disease-free mice, multiple effector modalities were impaired in T reg cells in the absence of Dicer, consistent with their reduced ability to suppress.
DISCUSSION
Previous studies showed that ablation of Dicer in developing thymocytes interfered with the differentiation of Foxp3+ T reg cells, as well as with in vitro differentiation of Th1 and Th17 TE cells (15, 19). Our investigation of the functional consequences of miRNA depletion in T reg cells, using the deletion of a conditional Dicer allele subsequent to Foxp3 up-regulation, and, therefore, T reg lineage commitment, revealed a multifaceted role for miRNA in T reg cell biology. Consistent with the described role for the miRNA pathway in conventional T cells and B cells, miRNA-mediated regulation of gene expression significantly contributed to the resistance of T reg cells to activation-induced apoptosis. In B cells, up-regulation of the proapoptotic protein Bim was shown to be responsible, at least in part, for this effect (31). We also observed an increase in Bim mRNA in Dicer-deficient T reg cells (unpublished data). Together with the increased apoptosis, inferior proliferative potential can explain the overall impairment in the homeostasis of Dicer-deficient T reg cells compared with their Dicer-sufficient counterparts. In addition to these expected outcomes of miRNA depletion in T reg cells, we found a marked impairment in the suppressor function of Dicer-deficient T reg cells isolated from healthy mice. The latter was accompanied by a decrease in the expression of several, but not all, suppressor–effector genes, which were recently implicated in the execution of T reg cell suppressor function. In this regard, the deleterious effect of the loss of Dicer on in vitro suppressor function was somewhat greater on a per cell basis than that observed because of a complete lack of one known mechanism, Ebi3 (32). Thus, diminution in multiple effector modalities likely contributes to the observed functional insufficiency of Dicer-deficient T reg cells under physiological conditions. Although not formally excluded, possible low Dicer activity in T reg cells in healthy Foxp3Cre/wtDicerfl/fl females appears unlikely to account for the remaining suppressor activity in these cells, as comparable activity was found in T reg cells isolated from CD4-Cre Dicerfl/fl mice (Fig. 5). Previous studies showed that CD4-Cre–mediated deletion of Dicer in T cells depleted miRNA essentially to completion (19).
The most striking finding, however, was the observed necessity for miRNA for T reg cell–mediated tolerance in diseased mice. This need likely stems from the aforementioned discernable functions of miRNA-dependent gene regulation in T reg cell homeostasis and suppressive function under physiological conditions, eventually leading to the inflammatory condition. In this regard, an impediment in T reg cell homeostasis quantitatively similar to that of Dicer-deficient T reg cells was observed in lymphoreplete mice upon the loss of a single miRNA, miR155, without a marked impairment in suppressor capacity (unpublished data). Furthermore, the robust in vitro proliferation of Dicer-deficient T reg cells isolated from diseased mice, together with the significant increase in Foxp3+ T cell numbers and their markedly augmented activation status, argue against the possibility that impaired T reg cell homeostasis solely accounts for the loss of T reg cell–mediated suppressive function under inflammatory settings.
Nevertheless, it is likely that at an early point of disease progression, before a major flare up of inflammation, inferior proliferative capacity and increased cell death contribute to the failure of T reg cell–mediated tolerance in Foxp3CreDicerfl/fl mice, along with significantly impaired suppressor function. At later stages of disease, however, a complete loss of suppressor activity by expanded Dicer-deficient Foxp3+ cells can fully account for the rapid progression of fatal lesions matching those in mice lacking T reg cells. In this regard, we recently found a similarly diminished in vitro suppressor capacity and impaired homeostasis of Foxp3+ T reg cells deficient in NFAT activation, STAT5, or CD122 in disease-free mice cohabited by a subset of mutant and wild-type T reg cells. However, unlike Dicer-deficient T reg cells, all mutant T reg cells significantly increased their suppressor capacity in diseased mice (unpublished data). Thus, the essential stabilizing effect of the Dicer-controlled miRNA pathway on establishing T reg cell homeostasis and maintaining suppressor function appears distinct from the role of several key transcription factors, facilitating a fully competent suppressor function in T reg cells in the partnership with Foxp3.
It seems likely that the loss of multiple miRNA species contributed to proliferative inferiority, increased apoptosis, weakened suppressor function under physiological conditions, and loss of lineage integrity (i.e., suppressor function and in vitro anergy) under inflammatory conditions. Support for this notion comes from the aforementioned observation that miR155-deficient T reg cells exhibited impaired homeostasis and proliferative potential, but were comparable in their susceptibilities to apoptosis. Furthermore, the in vitro and in vivo suppressive functions of miR155-deficient T reg cells were largely intact, again demonstrating that distinct miRNA species control different facets of the Dicer-dependent T reg cell behavior (unpublished data).
Collectively, our results suggest that miRNA-mediated regulation of gene expression is important for the establishment of Foxp3-dependent T reg cell homeostasis and a suppressor program. In addition, the miRNA pathway might have a role in the sustenance of this program in the face of inflammation. This finding opens up an avenue for the future identification and mechanistic analyses of specific miRNA involved in this process, and points to targeted manipulation of the miRNA pathway as a potential means of affecting regulatory T cell suppressor function in experimental and clinical settings.
MATERIALS AND METHODS
Mice.
Foxp3KO, Foxp3fl, Foxp3GFP, CD4-Cre, Foxp3YFPCre, and Dicerfl mice were all backcrossed to the C57BL/6 background (3, 4, 25, 33, 34). Experimental mice were age matched and housed under specific pathogen–free conditions. Disease development was monitored by frequent visual observation and histological analysis of the tissues using hematoxylin and eosin staining (Histology Consultation Services). All mice were used in accordance with guidelines from the University of Washington Institutional Animal Care Committee.
In vitro proliferation, apoptosis, and suppression assays.
2 × 106 lymphocytes isolated from Foxp3Cre/wtDicerfl/fl and Foxp3Cre/wtDicerfl/wt mice were cultured in a 24-well plate in the presence of 1 μg/ml CD3 (2C11) and 1 μg/ml CD28 (37.51) antibodies and 100 U/ml of human IL-2 at 37°C for the times indicated in the figures. For the proliferation studies, Ki67 staining was performed as described in Flow cytometry, cytokine secretion assays… For the apoptosis studies, FITC-VAD-FMK (Promega) was used to measure caspase-3 activity according to the manufacturer's instructions. Total lymphocytes were stimulated as described. For some cases, anti-CD3/anti-CD28–activated cells were washed and restimulated with 1 μg/ml CD3 antibody for an additional 24 h before the apoptosis analysis.
In vitro suppression assays were performed on a co-culture of 2 × 104 CD4+YFP−CD62Lhi T cells with the numbers of CD4+YFP+CD62Lhi T reg cells or CD4+GFP+CD62Lhi cells indicated in the figures FACS purified to >90% purity from Foxp3CreDicerfl/fl or CD4-Cre Dicerfl/fl mice, respectively, or littermate control mice, in the presence of irradiated (2,000 rad) T cell–depleted splenocytes (105 per well) and 1 μg/ml CD3 antibody in round-bottom plates for 72 h at 37°C. T cell proliferation was assessed by [3H]TdR incorporation (counts per minute) in triplicate wells during the last 8 h of culture.
Cell isolation and adoptive transfer.
Ly5.1+CD4+CD25+ T reg cells were isolated by magnetic separation with MACS (Miltenyi Biotec) according to the manufacturer's instructions. 106 isolated T reg cells were then transferred into Foxp3CreDicerfl/fl mice by tail vein injection. Mice were killed 5 d after the transfer, and Foxp3+ T reg cell subsets were analyzed by flow cytometry.
Flow cytometry, cytokine secretion assays, and serum Ig isotype analysis.
Cell-surface staining and flow cytometric analysis of CD4, CD8, CD62L, CD73, GITR, IL-7Rα, CD25, ICOS, and CD103 expression were performed as previously described (4). Intracellular staining with anti-Foxp3 (eBioscience), GFP/YFP (Invitrogen), Ki67, CTLA4, and cytokine-specific antibodies (BD Biosciences) was performed after fixation and permeabilization using the reagents from the Foxp3 staining kit (eBioscience).
To measure T cell cytokine production, 2 × 106 splenocytes were stimulated in 24-well plates with 1 μg/ml CD3 and 1 μg/ml CD28 antibodies in the presence of GolgiPlug (BD Biosciences) for 5 h at 37°C. Cells were stained for CD4, CD8, Foxp3, and the corresponding cytokines, according to the manufacturer's protocol.
Ig isotype levels in titrated serum samples were measured using a standard Ig isotype–specific sandwich ELISA against a standard curve using the SBA Clonotyping System/HRP (SouthernBiotech), according to the manufacturer's instructions.
Antibodies.
Extracellular staining was performed using CD4-PerCP (RM4-5; BD Biosciences), CD8-PE (53-6.7; BD Biosciences), CD62L-PE-Cy7 (MEL-14; eBioscience), CD25-PE (PC61; eBioscience), IL-7Rα–PE (A7R34; eBioscience), GITR-PE (DTA-1; eBioscience), ICOS-PE (7E.1769; eBioscience), and CD103-PE (3E7; eBioscience). Intracellular staining for Foxp3-allophycocyanin (FJK-16; eBioscience), anti-GFP–Alexa Fluor 488 (GSN149 cross-reactive to YFP; Sigma-Aldrich), CTLA4-PE (UC10-4F10-11; BD Biosciences), IL-2–PE (JES6-5H4; eBioscience), IL-4–PE (11B11; eBioscience), IL-5–PE (TRFK4; BD Biosciences), IL-17–PE (TC11-18H10.1; BD Biosciences), IFN-γ–PE (XMG1.2; eBioscience), Ki67-PE (B56; eBioscience), and TNF-α–PE (MP6-XT22; eBioscience) were performed after fixation and permeabilization using the Foxp3 fix/perm kit (eBioscience).
PCR.
Genomic DNA was prepared from CD4+YFP+ and CD4+YFP−CD62Lhi sorted cells (purified to >90% purity) from Foxp3CreDicerfl/fl mice using the Wizard SV Genomic DNA purification system (Promega), according to the manufacturer's instructions. miRNA was prepared from CD4+YFP+ and CD4+YFP−CD62Lhi sorted cells (purified to >90% purity) from Foxp3CreDicerfl/fl and Foxp3CreDicerwt/wt mice using the miRNeasy kit (QIAGEN) or TRIzol (Invitrogen). First-strand complementary DNA was synthesized using the NCode miRNA First-Strand cDNA kit (Invitrogen). Levels of miRNA were measured by qPCR using an miRNA specific forward primer and a universal reverse primer. Ubiquitously expressed U6 small nuclear RNA was used for normalization. The following primer sequences were used for the identification of wild-type, floxed, and deleted Dicer alleles: 5′-ggttacatggctagactcaaagc-3′, 5′-aggtgcctttcgtttaggaac-3′, and 5′-aaagcagaactctaatgcccc-3′. Primers for qPCR were as follows: 5′-tctcccaacccttgtaccagtg-3′ (miR150), 5′-ttaatgctaattgtgataggggt-3′ (miR155), 5′-caaattcgtgaagcgttccata-3′ (U6), 5′-tcccacgctcgggtacac-3′ (Foxp3 forward), 5′-ccacttgcagactccatttgc-3′ (Foxp3 reverse), 5′-ggttgccaagccttatcgga-3′ (IL-10 forward), 5′-acctgctccactgccttgct-3′ (IL-10 reverse), 5′-cggtgccctacatgctaaat-3′ (Ebi3 forward), 5′-gcggagtcggtacttgagag-3′ (Ebi3 reverse), 5′-ttgcttcagctccacagaga-3′ (Ebi3 forward), 5′-ttgcttcagctccacagaga-3′ (TGF-β1 forward), 5′-tggttgtagagggcaaggac-3′ (TGF-β1 reverse), 5′-ctccacgtgctttcaccaaa-3′ (granzyme B forward), and 5′-aggatccatgttgcttctgtagttag-3′ (granzyme B reverse).
Online supplemental material.
Fig. S1 shows that activated splenic T cells are expanded in Foxp3CreDicerfl/fl mice. Fig. S2 shows that TE cells from Foxp3CreDicerfl/fl mice have up-regulated activation markers. Fig. S3 and Table S1 show that TE cells from Foxp3Cre/wtDicerfl/fl female mice do not have elevated cytokine expression. Fig. S4 demonstrates the reduced production of Foxp3+ T cells in CD4-Cre Dicerfl/fl mice. Fig. S5 shows impaired in vitro survival of Dicer-deficient T reg cells. Fig. S6 shows reduced in vitro proliferation of Dicer-deficient TN cells. Fig. S7 shows the altered phenotype of Dicer-deficient T reg cells from CD4-Cre Dicerfl/fl mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20081062/DC1.
Acknowledgments
We thank G. Loeb and L. Makaroff for their help in key experiments; P. deRoos, L. Karpik, and K. Forbush for superb technical assistance; Y. Rubtsov for sharing unpublished data; and all members of our laboratory for discussions.
This work was supported by grants from the National Institutes of Health (to A.Y. Rudensky). A. Liston is a National Health and Medical Research Council and Menzies Foundation Fellow. L.-F. Lu is a Leukemia and Lymphoma Society Fellow. A.Y. Rudensky is a Howard Hughes Medical Institute investigator.
The authors declare that they have no conflicting financial interests.
Abbreviations used: CTLA4, cytotoxic T lymphocyte antigen 4; Ebi3, EBV-induced gene 3; GITR, glucocorticoid-induced TNFR; ICOS, inducible T cell co-stimulator; miRNA, microRNA; qPCR, quantitative PCR; TE, effector T; TN, naive T; YFP, yellow fluorescent protein.
A. Liston and L.-F. Lu contributed equally to this paper.
D. O'Carroll's present address is European Molecular Biology Laboratory, Mouse Biology Unit, 00015 Monterotondo Scalo Rome, Italy.
References
- 1.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061. [DOI] [PubMed] [Google Scholar]
- 2.Khattri, R., T. Cox, S.A. Yasayko, and F. Ramsdell. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337–342. [DOI] [PubMed] [Google Scholar]
- 3.Fontenot, J.D., M.A. Gavin, and A.Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336. [DOI] [PubMed] [Google Scholar]
- 4.Fontenot, J.D., J.P. Rasmussen, L.M. Williams, J.L. Dooley, A.G. Farr, and A.Y. Rudensky. 2005. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 22:329–341. [DOI] [PubMed] [Google Scholar]
- 5.Brunkow, M.E., E.W. Jeffery, K.A. Hjerrild, B. Paeper, L.B. Clark, S.A. Yasayko, J.E. Wilkinson, D. Galas, S.F. Ziegler, and F. Ramsdell. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27:68–73. [DOI] [PubMed] [Google Scholar]
- 6.Wildin, R.S., F. Ramsdell, J. Peake, F. Faravelli, J.L. Casanova, N. Buist, E. Levy-Lahad, M. Mazzella, O. Goulet, L. Perroni, et al. 2001. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 27:18–20. [DOI] [PubMed] [Google Scholar]
- 7.Chatila, T.A., F. Blaeser, N. Ho, H.M. Lederman, C. Voulgaropoulos, C. Helms, and A.M. Bowcock. 2000. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J. Clin. Invest. 106:R75–R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torgerson, T.R., and H.D. Ochs. 2007. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked: forkhead box protein 3 mutations and lack of regulatory T cells. J. Allergy Clin. Immunol. 120:744–750. [DOI] [PubMed] [Google Scholar]
- 9.Kim, J.M., J.P. Rasmussen, and A.Y. Rudensky. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8:191–197. [DOI] [PubMed] [Google Scholar]
- 10.Fontenot, J.D., J.P. Rasmussen, M.A. Gavin, and A.Y. Rudensky. 2005. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6:1142–1151. [DOI] [PubMed] [Google Scholar]
- 11.Gavin, M.A., J.P. Rasmussen, J.D. Fontenot, V. Vasta, V.C. Manganiello, J.A. Beavo, and A.Y. Rudensky. 2007. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 445:771–775. [DOI] [PubMed] [Google Scholar]
- 12.Wan, Y.Y., and R.A. Flavell. 2007. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 445:766–770. [DOI] [PubMed] [Google Scholar]
- 13.Lin, W., D. Haribhai, L.M. Relland, N. Truong, M.R. Carlson, C.B. Williams, and T.A. Chatila. 2007. Regulatory T cell development in the absence of functional Foxp3. Nat. Immunol. 8:359–368. [DOI] [PubMed] [Google Scholar]
- 14.Williams, L.M., and A.Y. Rudensky. 2007. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat. Immunol. 8:277–284. [DOI] [PubMed] [Google Scholar]
- 15.Cobb, B.S., A. Hertweck, J. Smith, E. O'Connor, D. Graf, T. Cook, S.T. Smale, S. Sakaguchi, F.J. Livesey, A.G. Fisher, and M. Merkenschlager. 2006. A role for Dicer in immune regulation. J. Exp. Med. 203:2519–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng, Y., and A.Y. Rudensky. 2007. Foxp3 in control of the regulatory T cell lineage. Nat. Immunol. 8:457–462. [DOI] [PubMed] [Google Scholar]
- 17.Marson, A., K. Kretschmer, G.M. Frampton, E.S. Jacobsen, J.K. Polansky, K.D. MacIsaac, S.S. Levine, E. Fraenkel, H. von Boehmer, and R.A. Young. 2007. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 445:931–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hornstein, E., and N. Shomron. 2006. Canalization of development by microRNAs. Nat. Genet. 38:S20–S24. [DOI] [PubMed] [Google Scholar]
- 19.Muljo, S.A., K.M. Ansel, C. Kanellopoulou, D.M. Livingston, A. Rao, and K. Rajewsky. 2005. Aberrant T cell differentiation in the absence of Dicer. J. Exp. Med. 202:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Czech, B., C.D. Malone, R. Zhou, A. Stark, C. Schlingeheyde, M. Dus, N. Perrimon, M. Kellis, J.A. Wohlschlegel, R. Sachidanandam, et al. 2008. An endogenous small interfering RNA pathway in Drosophila. Nature. 453:798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okamura, K., W.J. Chung, J.G. Ruby, H. Guo, D.P. Bartel, and E.C. Lai. 2008. The Drosophila hairpin RNA pathway generates endogenous short interfering RNAs. Nature. 453:803–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghildiyal, M., H. Seitz, M.D. Horwich, C. Li, T. Du, S. Lee, J. Xu, E.L. Kittler, M.L. Zapp, Z. Weng, and P.D. Zamore. 2008. Endogenous siRNAs derived from transposons and mRNAs in Drosophila somatic cells. Science. 320:1077–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tam, O.H., A.A. Aravin, P. Stein, A. Girard, E.P. Murchison, S. Cheloufi, E. Hodges, M. Anger, R. Sachidanandam, R.M. Schultz, and G.J. Hannon. 2008. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature. 453:534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe, T., Y. Totoki, A. Toyoda, M. Kaneda, S. Kuramochi-Miyagawa, Y. Obata, H. Chiba, Y. Kohara, T. Kono, T. Nakano, et al. 2008. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature. 453:539–543. [DOI] [PubMed] [Google Scholar]
- 25.Rubtsov, Y.P., J.P. Rasmussen, E.Y. Chi, J. Fontenot, L. Castell, X. Ye, P. Treuting, L. Siewe, A. Roers, W.R.J. Henderson, et al. 2008. IL-10 produced by regulatory T cells contributes to their suppressor function by limiting inflammation at environmental interfaces. Immunity. 28:546–558. [DOI] [PubMed] [Google Scholar]
- 26.Singh, N., P.R. Chandler, Y. Seki, B. Baban, M. Takezaki, D.J. Kahler, D.H. Munn, C.P. Larsen, A.L. Mellor, and M. Iwashima. 2007. Role of CD28 in fatal autoimmune disorder in scurfy mice. Blood. 110:1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wildin, R.S., S. Smyk-Pearson, and A.H. Filipovich. 2002. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J. Med. Genet. 39:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim, H.W., P. Hillsamer, A.H. Banham, and C.H. Kim. 2005. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J. Immunol. 175:4180–4183. [DOI] [PubMed] [Google Scholar]
- 29.Kobie, J.J., P.R. Shah, L. Yang, J.A. Rebhahn, D.J. Fowell, and T.R. Mosmann. 2006. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J. Immunol. 177:6780–6786. [DOI] [PubMed] [Google Scholar]
- 30.Paust, S., L. Lu, N. McCarty, and H. Cantor. 2004. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc. Natl. Acad. Sci. USA. 101:10398–10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koralov, S.B., S.A. Muljo, G.R. Galler, A. Krek, T. Chakraborty, C. Kanellopoulou, K. Jensen, B.S. Cobb, M. Merkenschlager, N. Rajewsky, and K. Rajewsky. 2008. Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell. 132:860–874. [DOI] [PubMed] [Google Scholar]
- 32.Collison, L.W., C.J. Workman, T.T. Kuo, K. Boyd, Y. Wang, K.M. Vignali, R. Cross, D. Sehy, R.S. Blumberg, and D.A. Vignali. 2007. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 450:566–569. [DOI] [PubMed] [Google Scholar]
- 33.Wolfer, A., T. Bakker, A. Wilson, M. Nicolas, V. Ioannidis, D.R. Littman, P.P. Lee, C.B. Wilson, W. Held, H.R. MacDonald, and F. Radtke. 2001. Inactivation of Notch 1 in immature thymocytes does not perturb CD4 or CD8T cell development. Nat. Immunol. 2:235–241. [DOI] [PubMed] [Google Scholar]
- 34.Yi, R., D. O'Carroll, H.A. Pasolli, Z. Zhang, F.S. Dietrich, A. Tarakhovsky, and E. Fuchs. 2006. Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nat. Genet. 38:356–362. [DOI] [PubMed] [Google Scholar]