Abstract
Marginal zone (MZ) B cells resemble fetally derived B1 B cells in their innate-like rapid responses to bacterial pathogens, but the basis for this is unknown. We report that the MZ is enriched in “fetal-type” B cell receptors lacking N regions (N−). Mixed bone marrow (BM) chimeras, made with adult terminal deoxynucleotidyl transferase (TdT)+/+ and TdT−/− donor cells, demonstrate preferential repertoire-based selection of N− B cells into the MZ. Reconstitution of irradiated mice with adult TdT+/+ BM reveals that the MZ can replenish N− B cells in adult life via repertoire-based selection and suggest the possibility of a TdT-deficient precursor population in the adult BM. The mixed chimera data also suggest repertoire-based bifurcations into distinct BM and splenic maturation pathways, with mature “recirculating” BM B cells showing a very strong preference for N+ complementarity-determining region (CDR) 3 compared with follicular B cells. Because the T1 and MZ compartments are both the most enriched for N− H-CDR3, we propose a novel direct T1→MZ pathway and identify a potential T1–MZ precursor intermediate. We demonstrate progressive but discontinuous repertoire-based selection throughout B cell development supporting multiple branchpoints and pathways in B cell development. Multiple differentiation routes leading to MZ development may contribute to the reported functional heterogeneity of the MZ compartment.
Immature B cells progress through several identifiable developmental stages in the BM and spleen before becoming mature B cells (1). Although B cell differentiation is thought to be mainly linear, some small subsets of immature and transitional B cells have been proposed to branch from the main pathway and could be the initiating cells for distinct routes of differentiation (2). Because of the stochastic nature of the B cell receptor (BCR) assembly process, a large number of B cell precursors initially generate nonfunctional or autoreactive receptors. Consequently, these cells are vetted for functionality and self-reactivity during BM immature and splenic transitional B cell maturation stages. These tolerance “checkpoints” shape the immature B cell repertoire into a permissible pool of specificities from which mature B cells can develop and, hence, the majority of newly generated B cells never enter the mature B cell pool.
Before final maturation, B cells undergo additional selective cell fate decisions. There are three main categories of mature B cells: B1, follicular (FO), and marginal zone (MZ) B cells. Each subset can be identified based on anatomical localization and differential expression of several surface markers (3–5). Whereas B1 cells mainly reside in the peritoneal cavity, FO B cells, by far the largest B cell population, are found in the spleen and lymph nodes and also circulate throughout the body. In contrast, MZ B cells in the mouse are largely restricted to the marginal zone of the spleen (6, 7). Their location, surrounding the marginal sinus, provides MZ B cells with the ideal opportunity for interactions with blood-borne pathogens. Therefore, along with B1 cells, MZ B cells act as a rapid first line of defense against bacterial pathogens (6).
There is now good evidence that B1 cells represent a separate, largely fetally derived, lineage of B cells (8, 9). In contrast, MZ and FO B cells are thought to arise predominantly in adult life (7). Currently, the different factors involved in these B cell lineage decisions and cell fate choices are not well understood. In addition to Notch2 signaling, which is essential for MZ B cell development (10, 11), there is considerable evidence that shows that the strength or quality of BCR signals is also critical in B cell fate decisions (7, 12, 13).
A fetal versus adult origin has particular relevance for B cells in that the fetal BCR repertoire is considerably different from that produced in adult life (14–17). This partially stems from a predisposition to use certain V genes more commonly in fetal than adult life (18, 19). But more importantly, because of the absence of terminal deoxynucleotidyl transferase (TdT) in fetal life, the fetal repertoire lacks the junctional diversity provided by N nucleotides in heavy chain complementarity-determining regions (CDR) 3 (16, 17). Junctional diversity is further constrained because of the frequent occurrence of homology-directed recombination in the absence of N regions (17, 20). Thus, fetally derived CDR3s are quite different from those generated in the adult. Although the lack of N regions significantly restricts fetal repertoire diversity, it has been suggested that this germline-defined sequence preference is an important evolutionary strategy aimed at generating valuable specificities, such as those involved in anti-bacterial responses (14, 15).
In addition to similarities in the functions of B1 and MZ B cells, there is some data that support a fetal origin for at least some MZ cells. It has been shown that IL7−/− mice, which exhibit a severe block in BM B cell development, possess a small but stable MZ population (21). Also, in mice in which the RAG2 gene was deleted at birth, the MZ compartment grew over time, whereas the FO compartment did not, suggesting the preferential expansion of fetally derived B cells in the MZ (22). It has also been reported that MZ B cells possess shorter CDR3 regions than FO cells (23, 24). As the affinity of a BCR for antigen is a function of the CDRs, these data suggest that repertoire-based selection for shorter CDR3 may contribute to the MZ versus FO B cell fate decision. Also, because CDR3s produced in fetal life are inherently shorter than those produced in adult life because of the absence of N regions (14), we hypothesized that the MZ compartment might contain a significant fetal component that could account for the reported functional similarities between MZ and B1 B cells.
Therefore, we examined the IgH repertoires of MZ and FO populations and showed that the MZ compartment is enriched in N region–lacking H-CDR3s (N−). Furthermore, using a competitive BM reconstitution approach with Ly5.1 TdT+/+ and Ly5.2 TdT−/− donor cells, we demonstrated by flow cytometry that the MZ compartment has an inherent selective preference for the N− fetal-type repertoire. This N− preference is retained in adult life and allows the MZ to replenish N− specificities through the selection of rare N− cells produced in adult life. Repertoire-based selection is apparent throughout B cell development in chimeric mice, and our findings lead us to suggest a repertoire-based bifurcation into distinct BM and splenic B cell maturation pathways. Furthermore, we provide evidence for an early differentiation branchpoint for MZ cells from the T1 stage of splenic B cell development through an intermediate T1–MZ precursor (MZP) stage.
RESULTS
MZ B cells possess fewer N regions than FO B cells
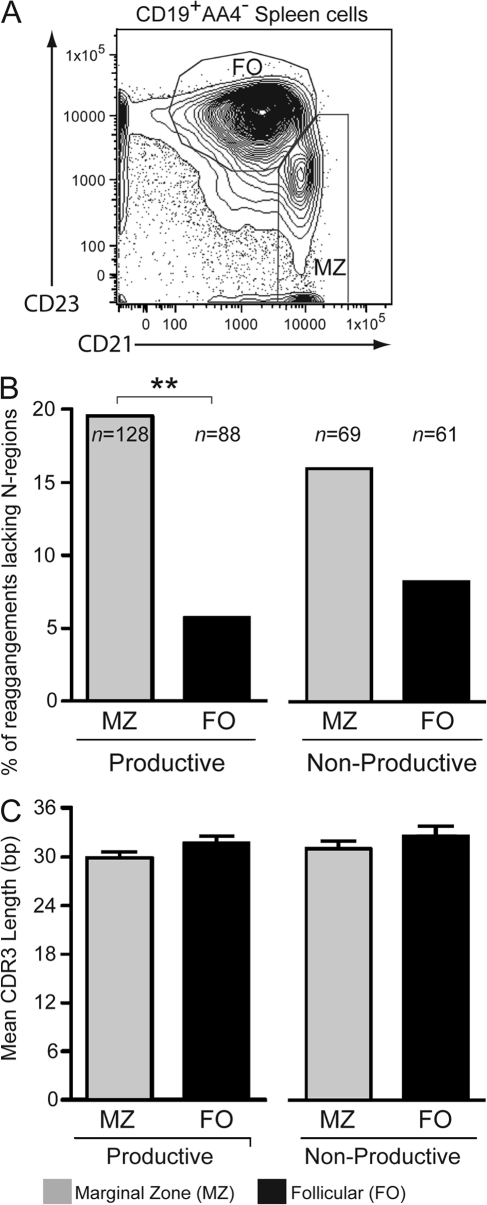
To test our hypothesis that some MZ B cells may lack N regions, we amplified, cloned, and sequenced VH7183 rearrangements from DNA of sorted splenic FO and MZ B cells from C57BL/6 mice. The parameters used for the sorting of MZ and FO populations are depicted in Fig. 1 A. Consistent with our hypothesis, nearly 20% of MZ sequences lacked N regions (Fig. 1 B). In contrast, N-region nucleotides were almost always found in the CDR3 junctions of FO B cells. As essentially all fetal/neonatal B cells lack N regions (16, 17), whereas only 1.4% of adult BM pre–B cells lack N regions (unpublished data) (14), the absence of N nucleotides suggests the preferential accumulation of fetally derived cells in the MZ compartment. The fact that the level of N− H-CDR3 in the nonproductive sequences is similar to that in the productive sequences strongly suggests a fetal origin for the majority of these N− MZ cells.
Figure 1.
The MZ B cell compartment is enriched with N− fetal-type sequences. (A) The FACS gates used to sort MZ and FO B cells. (B) The proportion of VH7183 CDR3 rearrangements lacking H-CDR3 N nucleotides in MZ and FO compartments in B6 mice. Statistically significant differences are indicated (**, P < 0.01). (C) The mean CDR3 length (±SEM) of sequences in B.
The VH7183 MZ CDR3s in our study were, on average, slightly shorter than those of FO B cells; however, the difference did not achieve statistical significance (P = 0.12; Fig. 1 C). Because TdT deficiency significantly restricts H-CDR3 length (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20080559/DC1), we examined the CDR3 length of N− and N+ MZ sequences separately. The N− MZ sequences were, on average, 8 bp shorter than N+ MZ sequences (P < 0.001; unpublished data), and their removal from our pool of MZ sequences leaves the mean MZ CDR3 length only 0.3 bp shorter than that of the FO population.
Because of its pivotal role in B cell survival and homeostasis and the greater sensitivity of MZ B cells to its presence, we examined whether BAFF overexpression in C57BL/6 BAFF transgenic mice might preferentially promote the survival of N− B cells in the MZ. However, we found that the levels of N− CDR3s in MZ B cells from these mice were the same as in wild-type mice (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20080559/DC1). There were no significant differences between C57BL/6 MZ and FO cells with respect to VH and DH gene usage, CDR3 amino acid usage, or hydrophobicity (Fig. S3 and S4), but MZ B cells had lower levels of somatic hypermutation than FO cells (P < 0.01; Fig. S5). Thus, the enrichment in the MZ with N− cells is the most striking difference between the FO and MZ compartments.
Examining repertoire-based selection by a TdT+/+/TdT−/− BM chimera approach
To clarify whether N− MZ cells represented a previously unidentified fetal B cell lineage or if CDR3 repertoire-based selection was responsible for the enrichment of these cells in the MZ, we used a novel approach wherein we competitively reconstituted irradiated C57BL/6 mice with TdT+/+ and TdT−/− donor BM cells from adult mice. Repertoire-based selection for N+ and N− BCRs could then be clearly identified by skewing of the original reconstitution ratio. These studies also allowed us to examine the role of repertoire not only for mature cell fate decisions but also at each of the earlier developmental stages during B cell differentiation in these nontransgenic polyclonal mice.
CD45 (i.e., Ly5) allelic markers were used to distinguish TdT+/+ from TdT−/− donor-derived cells by flow cytometry. The surface markers and gating strategies used for the identification of BM and splenic B cell subsets are shown in Figs. S6–10 (available at http://www.jem.org/cgi/content/full/jem.20080559/DC1). With the exception of peritoneal B1 cells, which are known to be relatively radio resistant (25), autoreconstitution of lethally irradiated TdT−/− hosts with endogenous B cells did not occur.
BM B cells favor the TdT+/+ and Igκ repertoires
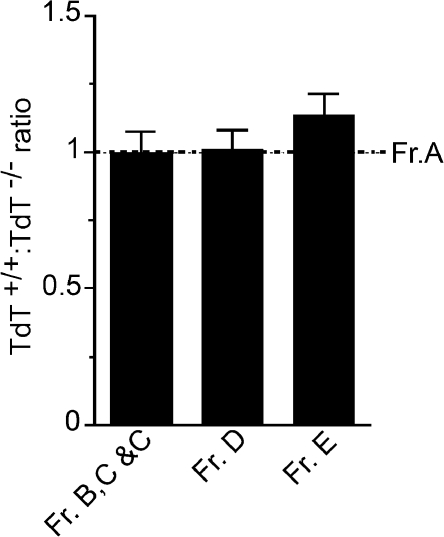
During the early B cell developmental stages in the BM (Hardy pro-B fraction [Fr.] A, Fr. B+C, and pre-B Fr.D) the ratio of TdT+/+/TdT−/− cells remained constant (Fig. 2) and this baseline is marked in all figures. The chimeras were analyzed for all splenic and BM subsets and clear differences were apparent between many subsets.
Figure 2.
TdT+/+/TdT−/− reconstitution ratios remain constant in surface Ig− early BM B cell subsets. Lethally irradiated C57BL/6 TdT−/− mice received equal proportions of B220-depleted Ly5.1 TdT+/+ or Ly5.2 TdT−/− adult donor BM cells and were allowed to reconstitute for 5 wk before analysis. The TdT+/+/TdT−/− ratio in early BM B cell developmental stages is shown. Data were normalized with respect to the Fr. A compartment (dotted line; n = 21 mice). Error bars represent SEM.
Fr. E immature B cells, the first stage to express surface Ig, were slightly skewed in favor of the TdT+/+ repertoire with respect to the preceding pre-B cell stage (Fig. 2). It has recently been reported that a small population of cells resembling splenic transitional T2 cells (i.e., AA4+CD23+) are present in the BM and represent ∼15% of the traditional Fr. E compartment (26). Resolution of the Fr. E compartment into CD23− (Fr. E) and CD23+ (BM T2) subsets revealed that this CD23+ subset was highly enriched in TdT+/+ cells (Fig. 3 A) compared with the bulk of immature B cells. These data demonstrate repertoire-based selection of TdT+/+ cells into the BM transitional compartment.
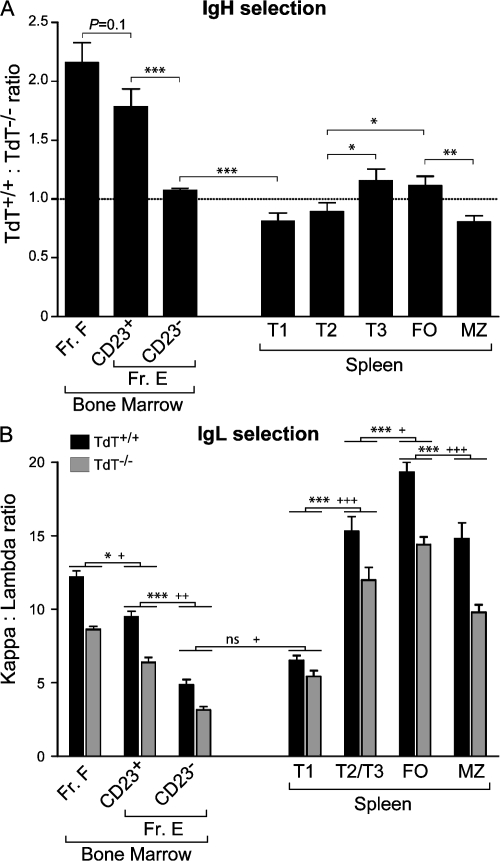
Figure 3.
IgH and IgL selection in TdT+/+/TdT−/− chimeric mice. Mice were reconstituted with equal proportions of B220− donor BM TdT+/+ and TdT−/− cells. (A) The TdT+/+/TdT−/− ratio in BM and spleen B cell compartments of chimeric mice. Data were normalized with respect to the combined Fr. A–D compartment (dotted line; n = 21 mice). Data represent the mean ± SEM. Statistically significant differences are indicated (*, P < 0.05; **, P < 0.01; ***, P < 0.001). (B) IgL usage in BM and spleen B cell compartments of chimeric mice (n = 12–20 mice). Data represent the mean ± SEM. With the exception of the T1 compartment, TdT−/− cells had significantly higher frequencies of Igλ+ cells than TdT+/+ cells. Statistically significant differences in light chain usage between TdT+/+ cells in each subset are indicated (*, P < 0.05; ***, P < 0.001), and between TdT−/− cells, statistical significance is shown (+, P < 0.05; ++, P < 0.01; +++, P < 0.001).
The IgH CDR3 selection pattern is paralleled by a corresponding selection in favor of Igκ usage in the BM transitional cells of these mice (Fig. 3 B). This is consistent with a previous paper showing selection in favor of Igκ in BM transitional cells of intact mice (26). Interestingly, Fr. E TdT−/− cells more frequently used Igλ chains than Igκ chains when compared with TdT+/+ cells (Fig. 3 B). Furthermore, with the exception of the splenic T1 compartment, TdT−/− B cells in chimeric mice had significantly higher levels of Igλ+ cells than TdT+/+ cells within each compartment.
BM Fr. F B cells have been considered to be recirculating FO-derived B cells. Therefore, although the Fr. F compartment is similar to BM transitional cells with respect to IgH and IgL selective preferences, it is striking that the composition of this compartment is much more skewed toward TdT+/+ (P < 0.001) and Igλ+ (P < 0.001) cells when compared with the splenic FO compartment (Fig. 3, A and B). This suggests that any recirculation that does occur between these compartments is not sufficient to homogenize the repertoires in these two distinct locations.
Entry into the splenic transitional pathway is skewed toward the TdT−/− repertoire, but subsequent transitional development favors TdT+/+ and Igκ+ cells
The transition from BM Fr. E (AA4+CD23−) to the first splenic transitional stage (T1) was marked by pronounced enrichment in TdT−/− cells (Fig. 3 A).This may be attributable in part to the preferential retention of TdT+/+ cells in the BM CD23+ transitional compartment. In contrast to this BM-to-spleen T1 transition, progression through the splenic T2 and T3 stages is characterized by an increased proportion of TdT+/+ cells (Fig. 3 A). As in the BM transitional compartment, this is mirrored by an increased frequency of Igκ+ cells (Fig. 3 B). However, significant differences are apparent between the BM transitional and splenic T2/T3 compartments as the BM transitional cells are more highly enriched in TdT+/+ (P < 0.001) and Igλ+ cells (P < 0.001; Fig. 3, A and B). These data support the idea that these compartments may represent distinct pathways and also demonstrate that repertoire plays an important role in this divergence.
The MZ compartment preferentially selects for N− fetal-type specificities
The initial purpose in carrying out these chimera studies was to determine whether the enrichment of the MZ with N− cells (Fig. 1 B) was caused by repertoire-based selection of these specificities. Our data demonstrate that the MZ compartment preferentially enriches for an N− repertoire (Fig. 3 A). This is consistent with our sequence data (Fig. 1 B) and demonstrates that N− fetal-type specificities, with their inherently shorter CDR3s, are selected into the MZ based on their BCR. This relative preference for N− BCRs contrasts with FO B cells (Fig. 3 A) and other mature B cells in the BM (i.e., Fr. F; Fig. 3 A), which are enriched for the N+ repertoire. The reconstitution ratio among peritoneal B2 B cells resembled that in the splenic FO compartment (unpublished data). Thus, MZ B cells are unique among mature B2 B cells in their preference for N− CDR3s.
Differential selection for the N+ and N− repertoires within the FO I and FO II subsets
Two functionally distinct FO B cell compartments have recently been reported that are differentiated based on levels of IgM expression (27). FO I cells (IgMlow), which encompass the majority of mature FO cells, are thought to be Btk dependent, and strong BCR signals facilitate their development (7, 27). In contrast, FO II cells (IgMhigh) have been proposed to represent an earlier stage capable of differentiating into either FO I or MZ cells depending on the BCR and intracellular signals they receive. Like MZ cells, FO II cells are proposed to develop in the absence of Btk.
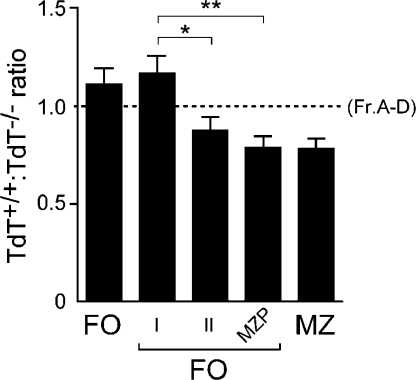
We therefore subdivided our FO compartment into FO I and FO II subsets (Fig. S10). The N+ repertoire is preferentially enriched in the FO I compartment, whereas the FO II population is skewed in favor of the N− repertoire (Fig. 4). This lower frequency of N+ CDR3s is consistent with the hypothesis that these FO II cells may give rise to MZ cells. As our FO gate includes some CD23+CD21Hi cells, a small proportion of the FO-gated cells possessed an MZP phenotype, and these cells exhibited a similar selection ratio to that of the MZ compartment. These data show that H-CDR3 repertoire-based selection may contribute to this proposed mature cell fate branchpoint and that the TdT−/− repertoire is disfavored by the FO compartment. This hypothesis is supported by our finding that intact TdT−/− mice have smaller FO compartments than age-matched TdT+/+ mice (Fig. S11, available at http://www.jem.org/cgi/content/full/jem.20080559/DC1).
Figure 4.
The TdT+/+/TdT−/− ratio differs in splenic FO I and FO II subsets. Data were normalized with respect to the combined Fr. A–D compartment (dotted line; n = 21 mice). *, P < 0.05; **, P < 0.01. Error bars represent SEM. The FACS gating strategy is shown in Fig. S10 (available at http://www.jem.org/cgi/content/full/jem.20080559/DC1).
TdT−/− cells traverse the splenic transitional stages more efficiently
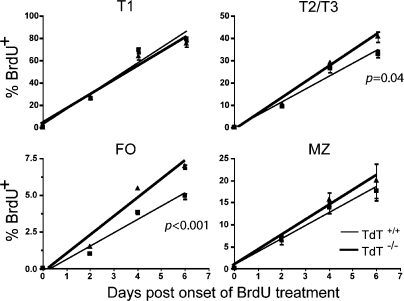
By using continuous BrdU labeling, it was apparent that BrdU+ cells accumulated at a similar rate within the TdT+/+ and TdT−/− T1 compartments of chimeric mice. However, subsequently, BrdU+ TdT−/− cells accumulated more rapidly in the T2/T3 and FO compartments of chimeric mice than did TdT+/+ cells (Fig. 5). Therefore, although proportionally more TdT+/+ cells proceed from the T1 to the T2, T3, and FO compartments in these chimeric mice (Fig. 3 A), those TdT−/− cells that do become FO B cells manage to transit the splenic transitional stages more efficiently than TdT+/+ cells.
Figure 5.
BrdU labeling kinetics in splenic B cells of chimeric mice. Continuous BrdU labeling was performed in mice in the final 6 d of a 5-wk reconstitution period (n = 2 mice per time point). Data were compiled by first identifying the TdT+/+ or TdT−/− cells in each splenic B cell population using the Ly5 congenic marker and then determining the proportions of BrdU+ cells in each TdT+/+ and TdT−/− subset separately. Data represent the mean ± SEM. Statistical significance is shown.
The MZ replenishes N− specificities by the selection of rare N− cells produced in adult life
Although only 1.4% of adult BM pre-B cells lack N regions (unpublished data) (14), our chimera data suggests a possible enrichment point later in development for such specificities during the BM Fr. E/splenic T1 transition. Therefore, with a view to clarifying whether rare N− cells produced in adult life can provide an ongoing source for the replenishment of the MZ with N− specificities, 6-wk-old TdT+/+ mice were reconstituted with B220− BM precursors from TdT+/+ littermate donors. Consequently, B cell production in these reconstituted mice was solely from precursors present in adult mice. After 5 wk of reconstitution, 8% of VH7183 MZ sequences lacked CDR3 N regions, whereas all FO sequences possessed N regions (Table I). Furthermore, similar to our data in intact mice, the level of N− H-CDR3 in nonproductive sequences resembled that in the productive sequences. With the caveat that we have limited numbers of nonproductive sequences, this suggests that the N− H-CDR3 produced in adult life may derive from TdT-deficient precursors. These data indicate that the N− MZ sequences present in similarly aged intact mice (Fig. 1 B) are likely of mixed fetal and adult origin.
Table I.
The MZ can replenish N− H-CDR3 from adult BM precursors
| Productive
|
Nonproductive
|
|||||
|---|---|---|---|---|---|---|
| Total H-CDR3 sequences | Number of N− H-CDR3 sequences |
Percentage N− | Total H-CDR3 sequences |
Number of N− H- CDR3 sequences |
Percentage N− | |
| MZ | 73 | 6 | 8 | 27 | 2 | 7 |
| FO | 43 | 0 | 0 | 17 | 0 | 0 |
Recipient mice (n = 2) were lethally irradiated, as described in Materials and methods, and reconstituted with B220-depleted BM precursors from littermate donors. After 5 wk of reconstitution, MZ and FO B cells were sorted as previously described. MZ and FO DNA was PCR amplified (four to eight independent PCRs), cloned, and sequenced, and the N− H-CDR3 were analyzed.
The T1 compartment harbors MZ precursor-like cells
Current models for peripheral B cell development suggest that mature MZ cells arise from CD21HiCD23+ MZ precursors, which are themselves derived from T2 or FO II cells (27, 28). However, considering the similarity in TdT+/+/TdT−/− reconstitution ratios between the T1 and MZ compartments, we examined the possibility that T1 cells can directly give rise to MZ cells through an identifiable intermediate developmental stage. As high expression of CD21 is a hallmark of MZ cells, we first examined whether any CD21Hi-expressing cells were present in the T1 compartment. We found that a discrete subpopulation of cells, accounting for 1–5% of the gated T1 compartment, did express high levels of CD21 (Fig. 6 A). These CD21Hi T1 cells, or T1-MZP cells, more closely resembled MZ cells than other B cells, including AA4− MZPs, with respect to several other phenotypic markers including IgM, IgD, CD19, CD23, CD1d, CD36, CD11a (integrin αL chain), and CD180 (RP105; Fig. 6 B). AA4 expression, which clearly marks immature/transitional B cells (29), was comparable to the levels expressed by the transitional T2 and T3 stages.
Figure 6.
The T1 compartment contains a discrete population of T1-MZP cells. (A) Splenic B cell populations were identified using the Allman and Hardy criteria (29). A discrete IgMHiCD21Hi population of cells is present within the T1 compartment. (B) B cell compartments were compared for their expression of several surface markers, including some that are expressed at high levels by the MZ compartment. These analyses were performed in intact B6 TdT+/+ mice (n = 5 mice). (C) TNP-Fluorescein-Ficoll binding by splenic B cells was assessed by flow cytometry (n = 3 mice). Data represent the mean ± SEM.
A functional characteristic of MZ B cells is their ability to trap T-independent antigen (30). Therefore, to determine whether T1-MZP cells resemble MZ cells in this respect, we injected C57BL/6 mice i.v. with fluorescein-labeled TNP-Ficoll. 1 h later, spleens were harvested from these animals and the degree of TNP-Ficoll retention by individual B cells was reflected by their fluorescence as measured by flow cytometry. Our data show that T1-MZP cells can trap the T-independent antigen TNP-Ficoll as efficiently as mature MZ cells (Fig. 6 C). Therefore, considering the phenotypic and functional similarities between T1-MZP and MZ cells and the similar N− H-CDR3 selective preferences of T1 and MZ cells, it seems likely that the T1→T1-MZP→MZ route represents a significant route for development of a subset of MZ B cells.
DISCUSSION
A key finding of our study is that MZ and FO B cell compartments maintain a preference for distinctly different BCR repertoires. Although our comparison of FO and MZ VH7183 and DH gene usage suggests that, broadly speaking, the MZ IgH repertoire is quite diverse and employs similar VH and DH genes, our sequence analysis revealed that a subset of MZ cells do, in fact, possess a restricted, fetal-type repertoire characterized by the absence of N-region additions in the H-CDR3. Our mixed BM chimera approach using adult BM from TdT+/+ and TdT−/− mice demonstrated that the enrichment of these cells is caused by a selective preference in the MZ for B cells with BCRs lacking N nucleotides in their H-CDR3 junctions. In addition, our chimera data show significant differences in the proportions of TdT+/+ and TdT−/− cells among B cell subsets in the spleen and BM. These data challenge the simple linear pathway of B cell differentiation and suggest multiple pathways of differentiation caused by repertoire-based selection.
Two recent studies suggest that immature/transitional phenotype cells present in the BM represent a distinct pathway from that in the spleen and that these immature cells have the potential to mature in situ into mature BM B cells (26, 31). Our chimera data provide strong support for this hypothesis as well as a repertoire-based rationale for this bifurcation of the transitional cells into a splenic or BM developmental pathway. As the BM transitional and mature Fr. F compartments have a much greater preference for TdT+/+ B cells than any splenic subsets, this suggests to us that the BM transitional pathway may primarily culminate in situ into mature Fr. F cells. The reported Btk dependence of BM transitional cells and Btk independence of splenic FO II cells also supports a largely distinct nature for these pathways (26, 27).
It has been reported that mature B cells in the BM reside in an extravascular perisinusoidal niche (32). Similar to the MZ, this location should provide these cells with ample opportunity for interaction with blood-borne pathogens. Indeed, it has been shown that humoral immune responses directed against blood-borne pathogens can be initiated in situ in the BM (32). Assuming that the BM and the MZ are both important sites for monitoring of blood-borne infections, our data clearly indicate that the BCR repertoires available in these distinct locations to fulfill this role are quite different.
The splenic FO and BM Fr. F compartments are both considered to form part of the recirculating pool of B cells in the body. Recent parabiotic studies strongly support the notion that mature BM B cells can recirculate (32). Therefore, it is surprising that increased BCR signaling, such as in Aiolos and other mutant mice (7, 33), results in the loss of mature BM B cells but the preservation of splenic FO cells. This suggests that the BCR signaling requirements for entering into and being retained in these compartments are qualitatively different. Furthermore, if recirculation of cells between the BM and spleen was both frequent and thorough, one might expect the reconstitution ratios between these compartments in chimeric mice to more closely resemble each other. Hence, the highly significant differences in reconstitution ratios of TdT+/+/TdT−/− B cells between the Fr. F and FO compartments are consistent with there being a selective repertoire-based retention of some mature B cells in the BM. In contrast, the similarity between reconstitution preferences of the FO and peritoneal cavity B2 compartments supports there being greater homogeneity in the repertoire preferences and/or the recirculation between these locations.
A signal-strength hypothesis has been proposed based on the analysis of mice that are deficient in a variety of signaling molecules (7). Broadly speaking, this suggests that, within a permissible range, weaker signals promote MZ differentiation, whereas stronger BCR signals favor FO and B1 cell fates, although one study suggests that the FO cell fate is the default cell fate in the absence of BCR signals (12). Selection based on signal strength would clearly result in the differential assortment of specificities into the mature B cell compartments. When considering our data in the context of a signal-strength model, it is perhaps of some import that the splenic T3 compartment was enriched in TdT+/+ cells. It has been proposed that this compartment represents a reservoir for anergic and autoreactive B cells and not an intermediate developmental stage that gives rise to mature B cells (34, 35). Therefore, our data may reflect an inherently higher degree of self-reactivity in the TdT+/+ repertoire. Consistent with this, autoimmune-prone mice exhibit decreased disease severity and mortality when TdT deficiency is introduced, which provides a compelling argument for reduced harmful autoreactivity in the TdT−/− repertoire (36–38). It has been suggested that the fetal repertoire is enriched in auto- or polyreactive specificities (39); however, it may be that such polyreactivity is beneficial as opposed to pathogenic. It has been suggested that shorter CDR3s will leave more space in the binding site for antigen to enter and to contact the CDRs 1 and 2 (40), thereby perhaps increasing CDR3 promiscuity. Furthermore, mouse DH genes predominantly use reading frame 1 (RF1) but N− CDR3s are even more biased in their RF1 usage. Therefore, it may be that skewed RF usage combined with their shorter CDR3s gives N− cells the selective suitability for being selected into the MZ compartment.
From the immature B stage onwards, TdT−/− B cells in our chimeric mice more frequently used Igλ. This might suggest that the TdT−/− repertoire requires more receptor editing than the TdT+/+ repertoire because increased Igλ usage is a hallmark of receptor editing. However, the transit time through the pre-B compartment is the same for TdT−/− and TdT+/+ cells in intact mice (unpublished data), arguing that there is not increased receptor editing in TdT−/− cells. It has been suggested that there is a direct correlation between CDR3 length and the potential for interaction between individual heavy and light chains (41). Therefore, it may be that some N− H-CDR3 have an inherently better “fit” with Igλ chains. Indeed, there have been several reports where IgH using V genes common in fetal life can only poorly associate with SLC (42, 43).
Because the proportions of N− H-CDR3 were similar for both productive and nonproductive sequences in intact mice as well as in adult mice reconstituted with adult BM, this suggests that these N− H-CDR3 are likely to arise from TdT-deficient precursors present within the adult BM. Although a portion of N− MZ CDR3 in intact mice may genuinely derive from the fetal period, the source of the N− B cells produced in the adult mouse is uncertain. These N− B cells from the BM may simply arise by chance and then be selected into the MZ by virtue of their N− BCR. Alternatively, the possibility exists that adult BM may contain an as yet unidentified discrete TdT-deficient B cell precursor population, the progeny of which are preferentially selected into the MZ. Because we did not deplete our donor BM cells of CD19+ cells, a possible source of N− precursor cells could be the recently reported CD19+B220− B1 precursor population (9). However, this precursor population did not appear to give rise to MZ cells in transfer studies (9), and it is unknown whether this small population in the adult BM expresses TdT. Interestingly, the extent of enrichment of both N− specificities and Igλ usage that we observed in the MZ is very similar to that reported for B1 cells (44, 45). In addition, the MZ and B1 compartments are both enriched for antibacterial and anticarbohydrate specificities and are thought to act as a first line of defense against invading pathogens (6). Therefore, it is intriguing to speculate that this “fetal-type” subset of MZ cells contributes to the reported functional similarities between B1 and MZ cells. Furthermore, we propose that a fraction of B1 and MZ N− cells may be replenished from N− precursors in the adult BM.
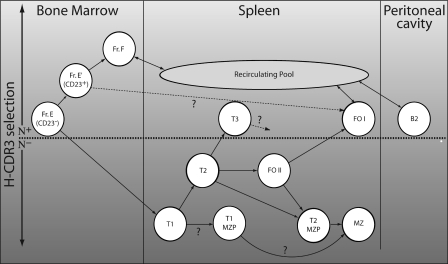
A splenic precursor population for MZ cells was first tentatively identified because of its absence in several strains of knockout mice lacking MZ B cells (11, 46). Subsequent reports support the hypothesis that this population gives rise to MZ cells (7, 28). It was termed T2-MZP because of its similarity to T2 cells. However, considering the similar IgH selective preferences exhibited by the T1 and MZ compartments in our chimeric mice along with the similarities between MZ and T1 cells in surface phenotype (both are IgMHiIgDLoCD23−), we considered that a more direct pathway of differentiation from the T1 to the MZ compartment was also likely. By inspecting the T1 compartment for CD21Hi cells, we have identified a discrete subpopulation which is a likely intermediate population along a direct T1→MZ differentiation pathway. We designate these as T1-MZP cells. Although there is strong evidence that CD21HiCD23+ splenic B cells have MZ precursor potential, we propose that some MZ differentiation can also start even earlier, at the T1 stage of splenic B cell development, and that these cells may bypass the T2 (CD23+) stage altogether, resulting in heterogeneity in the origin, repertoire, and, possibly, function within the MZ compartment. This is supported by the phenotypic similarities between T1-MZP and MZ cells and the similarities in IgH selective preferences between the T1 and MZ compartments of our chimeric mice. We have integrated the findings of this study with the work of others and present a model for repertoire-based B cell development in Fig. 7.
Figure 7.
Model for B cell development incorporating H-CDR3 selective preferences. This schematic merges our repertoire data with other models of B cell development (5, 26, 27, 31). We have included the additional dimension of H-CDR3 repertoire-based selection along the y-axis of this figure.
In summary, this study uses a novel approach to study the role of BCR repertoire in B cell development and cell fate decisions in polyclonal mice. The progressive skewing of the repertoire observed at each developmental step confirms that BCR–ligand interactions, and not just constitutive BCR signals, are important throughout immature and mature B cell development. Furthermore, this approach has allowed us to reconsider specific subset relationships in the BM and spleen, leading us to propose a repertoire-based bifurcation into BM and splenic development pathways and also a novel T1→MZ differentiation pathway. We demonstrate that MZ and FO B subsets in the spleen and mature B cells in the BM each have distinct repertoire profiles, with the MZ exhibiting a unique preference for the restricted N− fetal-type repertoire.
MATERIALS AND METHODS
Mice.
All mice used in this study were bred and maintained at The Scripps Research Institute, and these animal studies were approved by The Scripps Research Institute Institutional Animal Care and Use Committee. TdT−/− and BAFF transgenic mice were backcrossed to C57BL/6 for 10 generations (47). Unless otherwise indicated, mice used in this study were aged between 6 and 10 wk.
Flow cytometry and cell sorting.
Fcγ-receptors were blocked with anti-CD16/32 (BioLegend), and then cells were stained with different combinations of the following antibodies: CD19-PacificBlue/-PECy7, CD23–Alexa Fluor 488/-PE, B220-PECy7, CD180-PE, and IgD-PE (clone 11-26c.2a; BioLegend); CD93-APC (clone AA4.1), CD45.1-PECy5.5, IgM-PE/-APC/-PECy7 (clone 11/41), CD23-PECy7, CD1d-PE, CD36-PE, and CD11a-PE (eBioscience); and κ-PE, λ-biotin (clone R26-46), CD21-FITC/-PE, and CD43-FITC (BD Biosciences). These antibodies were used in combination with streptavidin-conjugated PE (Biomeda), APC (Biomeda), or Pacific Blue (BioLegend).
FACS analysis was performed on a digital LSRII (BD Biosciences) and data were analyzed using FlowJo software (Tree Star, Inc.). Cells were sorted on a FACSDiva or FACSAria (BD Biosciences). Sequence data from intact C57BL/6 and BAFF transgenic mice are derived from two to three independent cell sorts.
DNA and RNA isolation, cDNA preparation, PCR, cloning, and sequencing.
DNA and RNA were isolated from cell sorter–purified populations using AllPrep DNA/RNA kits (QIAGEN). PCR amplifications of DNA were performed for 40 cycles. Primers used for VH7183 V(DJ) rearrangements were AF303 (5′-CAGCTGGTGGAGTCTGGGGGA-3′) and AF591 (5′-CTTACCTGAGGAGACGGTGA-3′). 5–15 independent PCRs were performed on each sample to minimize PCR-generated duplicates, and at least two independent preparations of sorted cells were used. PCR products were gel purified and ligated into TOPO vectors (Invitrogen). Minipreps were made using QIAGEN kits and were sequenced with ABI-automated capillary sequencers (Thermo Fisher Scientific). The CDR3 was defined as including codon 95 through codon 102. Sequences that conformed to the triplet RF were identified as productive sequences. During sequence analysis, assigning an identity to prospective DH genes required a minimum of 5-bp homology with known DH genes. Nucleotides that could not be accounted for by VH, DH, or JH genes or their associated P nucleotides were categorized as N nucleotides. Duplicate sequences were only counted once, and no additional sequences were subsequently obtained from such PCRs.
H-CDR3 spectratype analyses.
cDNA was synthesized with QuantiTect Reverse Transcription kits (QIAGEN). A VHJ558 primer AF572 (5′-TCCAGCACAGCCTACATGCAGCTC-3′) in combination with the JH primer AF591 were used to amplify cDNA transcripts. PCR amplifications were performed for 40 cycles. A 5′ 6-FAM–labeled AF591 primer was subsequently used to fluorescently label PCR fragments, and samples were processed on an ABI 310 Genetic Analyzer (The Scripps Research Institute Center for Nucleic Acid Research). Spectratype data were analyzed using ABI Prism Genotyper software (Applied Biosystems).
Chimeric BM reconstitutions.
Lethally irradiated (1,100 rad) C57BL/6 TdT−/− mice were used as recipients for BM transfers. Donor cells were harvested from the BM of 6-wk-old C57BL/6 TdT+/+ (Ly5.1), and TdT−/− mice and were depleted of B cells using anti–B220-PE, and anti-PE–conjugated magnetic beads (Miltenyi Biotec). 5 × 106 cells were injected into the tail vein of each irradiated mouse, and chimeric mice were analyzed 5 wk after irradiation.
The data normalization process for our chimera data was a direct single step conversion to take into account variations in the actual reconstitution ratios of individual mice. In Fig. 2, we present data normalized with respect to the Fr. A compartment. After demonstrating that there was no difference between Fr. A, Fr. B+C, and Fr. D, we subsequently normalized the datasets with respect to the combined Fr. A–D compartments to facilitate the combined presentation of data from experiments using different BM surface-staining procedures that did not always allow the separate identification of the Fr. A compartment.
In mice reconstituted with equal proportions of TdT+/+ and TdT−/− BM precursors, if the reconstitution ratio in the combined Fr. A-D of a mouse was 0.7, and not precisely 1 as expected, then the Fr. A–D compartment is normalized to 1 and the reconstitution ratios in all other compartments of that mouse are multiplied by (1/0.7) to maintain their relativity. The normalized reconstitution ratios in each individual B cell compartment were averaged for all replicates, and the variability in reconstitution ratios among individual mice is directly reflected in the SE bars in the figures.
BrdU labeling.
The BrdU-labeling kinetics of spleen cells was assessed in TdT+/+/TdT−/− chimeric mice. These mice received i.p. injections of 100 μl (10 mg/ml) BrdU (Sigma-Aldrich) every 12 h for the duration of the indicated exposure periods. After tissue harvesting, cells were processed using BrdU flow kits (BD Biosciences) according to the manufacturer's protocol.
TNP-Fluorescein-Ficoll binding assay.
Mice were injected (tail i.v.) with 500 μl of 1 mg/ml TNP-fluorescein-ficoll (Biosearch Technologies, Inc.) in PBS. 1 h later, mice were killed and spleens were harvested. Single cell suspensions were then surface stained for B cell subset markers. FACS analysis was performed on a digital LSRII, and TNP-ficoll binding by B cells was revealed by fluorescein fluorescence.
Statistical analysis.
Analyses were performed using Prism 4 (GraphPad Software, Inc.). Where appropriate, differences between populations were assessed by ξ2, Fisher's exact test (two-tailed), one-way analysis of variance (ANOVA) with Bonferroni's Multiple Comparison test, Student's T test, and two-tailed or two-way ANOVA. Data are represented as means ± SEM (where applicable).
Online supplemental material.
Fig. S1 shows that TdT deficiency restricts H-CDR3 length. Fig. S2 demonstrates that BAFF overexpression does not alter the levels of N− H-CDR3 in FO and MZ B cell populations. Fig. S3 shows VH7183 and DH gene usage in MZ and FO B compartments. Fig. S4 shows the amino acid usage and hydrophobicity of MZ and FO H-CDR3. Fig. S5 displays the somatic hypermutation levels in MZ and FO H-CDR3. Figs. S6–10 show the flow cytometry gates used to identify individual B cell subsets. Fig. S11 demonstrates that C57BL/6 TdT−/− mice have smaller FO compartments than wild-type mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20080559/DC1.
Acknowledgments
We thank Sara Salerno, Siddarth Menon, and Timothy Wong for their excellent technical assistance, Dr. Jared Purton and David Kim for help with the BM chimeras, and Drs. Michael McHeyzer-Williams and David Nemazee for critically reading this manuscript and for helpful discussions.
This work was funded by National Institutes of Health grants RO1 AI 29672 and AI 61167 to A.J. Feeney. J.B. Carey was supported by National Institutes of Health training grant T32-AI007244.
The authors have no conflicting financial interests.
Abbreviations used: ANOVA, analysis of variance; BCR, B cell receptor; CDR, complementarity-determining region; FO, follicular; Fr., fraction; MZ, marginal zone; MZP, MZ precursor; RF1, reading frame 1; TdT, terminal deoxynucleotidyl transferase.
References
- 1.Hardy, R.R., P.W. Kincade, and K. Dorshkind. 2007. The protean nature of cells in the B lymphocyte lineage. Immunity. 26:703–714. [DOI] [PubMed] [Google Scholar]
- 2.Allman, D., and S. Pillai. 2008. Peripheral B cell subsets. Curr. Opin. Immunol. 20:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopes-Carvalho, T., and J.F. Kearney. 2004. Development and selection of marginal zone B cells. Immunol. Rev. 197:192–205. [DOI] [PubMed] [Google Scholar]
- 4.Tung, J.W., D.R. Parks, W.A. Moore, L.A. Herzenberg, and L.A. Herzenberg. 2004. Identification of B-cell subsets: an exposition of 11-color (Hi-D) FACS methods. Methods Mol. Biol. 271:37–58. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava, B., R.C. Lindsley, N. Nikbakht, and D. Allman. 2005. Models for peripheral B cell development and homeostasis. Semin. Immunol. 17:175–182. [DOI] [PubMed] [Google Scholar]
- 6.Martin, F., and J.F. Kearney. 2002. Marginal-zone B cells. Nat. Rev. Immunol. 2:323–335. [DOI] [PubMed] [Google Scholar]
- 7.Pillai, S., A. Cariappa, and S.T. Moran. 2005. Marginal zone B cells. Annu. Rev. Immunol. 23:161–196. [DOI] [PubMed] [Google Scholar]
- 8.Hayakawa, K., and R.R. Hardy. 2000. Development and function of B-1 cells. Curr. Opin. Immunol. 12:346–353. [DOI] [PubMed] [Google Scholar]
- 9.Montecino-Rodriguez, E., H. Leathers, and K. Dorshkind. 2006. Identification of a B-1 B cell-specified progenitor. Nat. Immunol. 7:293–301. [DOI] [PubMed] [Google Scholar]
- 10.Tanigaki, K., H. Han, N. Yamamoto, K. Tashiro, M. Ikegawa, K. Kuroda, A. Suzuki, T. Nakano, and T. Honjo. 2002. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat. Immunol. 3:443–450. [DOI] [PubMed] [Google Scholar]
- 11.Saito, T., S. Chiba, M. Ichikawa, A. Kunisato, T. Asai, K. Shimizu, T. Yamaguchi, G. Yamamoto, S. Seo, K. Kumano, et al. 2003. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity. 18:675–685. [DOI] [PubMed] [Google Scholar]
- 12.Wen, L., J. Brill-Dashoff, S.A. Shinton, M. Asano, R.R. Hardy, and K. Hayakawa. 2005. Evidence of marginal-zone B cell-positive selection in spleen. Immunity. 23:297–308. [DOI] [PubMed] [Google Scholar]
- 13.Martin, F., and J.F. Kearney. 2000. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity. 12:39–49. [DOI] [PubMed] [Google Scholar]
- 14.Feeney, A.J. 1992. Comparison of junctional diversity in the neonatal and adult immunoglobulin repertoires. Int. Rev. Immunol. 8:113–122. [DOI] [PubMed] [Google Scholar]
- 15.Herzenberg, L.A., and L.A. Herzenberg. 1989. Toward a layered immune system. Cell. 59:953–954. [DOI] [PubMed] [Google Scholar]
- 16.Feeney, A.J. 1990. Lack of N regions in fetal and neonatal mouse immunoglobulin V-D-J junctional sequences. J. Exp. Med. 172:1377–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu, H., I. Forster, and K. Rajewsky. 1990. Sequence homologies, N sequence insertion and JH gene utilization in VHDJH joining: implications for the joining mechanism and the ontogenetic timing of Ly1 B cell and B-CLL progenitor generation. EMBO J. 9:2133–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yancopoulos, G.D., B.A. Malynn, and F.W. Alt. 1988. Developmentally regulated and strain-specific expression of murine VH gene families. J. Exp. Med. 168:417–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perlmutter, R.M., J.F. Kearney, S.P. Chang, and L.E. Hood. 1985. Developmentally controlled expression of immunoglobulin VH genes. Science. 227:1597–1601. [DOI] [PubMed] [Google Scholar]
- 20.Feeney, A.J. 1992. Predominance of VH-D-JH junctions occurring at sites of short sequence homology results in limited junctional diversity in neonatal antibodies. J. Immunol. 149:222–229. [PubMed] [Google Scholar]
- 21.Carvalho, T.L., T. Mota-Santos, A. Cumano, J. Demengeot, and P. Vieira. 2001. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7−/− mice. J. Exp. Med. 194:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hao, Z., and K. Rajewsky. 2001. Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J. Exp. Med. 194:1151–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dammers, P.M., A. Visser, E.R. Popa, P. Nieuwenhuis, and F.G. Kroese. 2000. Most marginal zone B cells in rat express germline encoded Ig VH genes and are ligand selected. J. Immunol. 165:6156–6169. [DOI] [PubMed] [Google Scholar]
- 24.Schelonka, R.L., J. Tanner, Y. Zhuang, G.L. Gartland, M. Zemlin, and H.W. Schroeder Jr. 2007. Categorical selection of the antibody repertoire in splenic B cells. Eur. J. Immunol. 37:1010–1021. [DOI] [PubMed] [Google Scholar]
- 25.Hayakawa, K., R.R. Hardy, L.A. Herzenberg, and L.A. Herzenberg. 1985. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J. Exp. Med. 161:1554–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindsley, R.C., M. Thomas, B. Srivastava, and D. Allman. 2007. Generation of peripheral B cells occurs via two spatially and temporally distinct pathways. Blood. 109:2521–2528. [DOI] [PubMed] [Google Scholar]
- 27.Cariappa, A., C. Boboila, S.T. Moran, H. Liu, H.N. Shi, and S. Pillai. 2007. The recirculating B cell pool contains two functionally distinct, long-lived, posttransitional, follicular B cell populations. J. Immunol. 179:2270–2281. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava, B., W.J. Quinn III, K. Hazard, J. Erikson, and D. Allman. 2005. Characterization of marginal zone B cell precursors. J. Exp. Med. 202:1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allman, D., R.C. Lindsley, W. DeMuth, K. Rudd, S.A. Shinton, and R.R. Hardy. 2001. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J. Immunol. 167:6834–6840. [DOI] [PubMed] [Google Scholar]
- 30.Guinamard, R., M. Okigaki, J. Schlessinger, and J.V. Ravetch. 2000. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat. Immunol. 1:31–36. [DOI] [PubMed] [Google Scholar]
- 31.Cariappa, A., C. Chase, H. Liu, P. Russell, and S. Pillai. 2007. Naive recirculating B cells mature simultaneously in the spleen and bone marrow. Blood. 109:2339–2345. [DOI] [PubMed] [Google Scholar]
- 32.Cariappa, A., I.B. Mazo, C. Chase, H.N. Shi, H. Liu, Q. Li, H. Rose, H. Leung, B.J. Cherayil, P. Russell, et al. 2005. Perisinusoidal B cells in the bone marrow participate in T-independent responses to blood-borne microbes. Immunity. 23:397–407. [DOI] [PubMed] [Google Scholar]
- 33.Wang, J.H., N. Avitahl, A. Cariappa, C. Friedrich, T. Ikeda, A. Renold, K. Andrikopoulos, L. Liang, S. Pillai, B.A. Morgan, and K. Georgopoulos. 1998. Aiolos regulates B cell activation and maturation to effector state. Immunity. 9:543–553. [DOI] [PubMed] [Google Scholar]
- 34.Merrell, K.T., R.J. Benschop, S.B. Gauld, K. Aviszus, D. Decote-Ricardo, L.J. Wysocki, and J.C. Cambier. 2006. Identification of anergic B cells within a wild-type repertoire. Immunity. 25:953–962. [DOI] [PubMed] [Google Scholar]
- 35.Teague, B.N., Y. Pan, P.A. Mudd, B. Nakken, Q. Zhang, P. Szodoray, X. Kim-Howard, P.C. Wilson, and A.D. Farris. 2007. Cutting edge: Transitional T3 B cells do not give rise to mature B cells, have undergone selection, and are reduced in murine lupus. J. Immunol. 178:7511–7515. [DOI] [PubMed] [Google Scholar]
- 36.Robey, I.F., M. Peterson, M.S. Horwitz, D.H. Kono, T. Stratmann, A.N. Theofilopoulos, N. Sarvetnick, L. Teyton, and A.J. Feeney. 2004. Terminal deoxynucleotidyltransferase deficiency decreases autoimmune disease in diabetes-prone nonobese diabetic mice and lupus-prone MRL-Fas(lpr) mice. J. Immunol. 172:4624–4629. [DOI] [PubMed] [Google Scholar]
- 37.Feeney, A.J., B.R. Lawson, D.H. Kono, and A.N. Theofilopoulos. 2001. Terminal deoxynucleotidyl transferase deficiency decreases autoimmune disease in MRL-Fas(lpr) mice. J. Immunol. 167:3486–3493. [DOI] [PubMed] [Google Scholar]
- 38.Conde, C., S. Weller, S. Gilfillan, L. Marcellin, T. Martin, and J.L. Pasquali. 1998. Terminal deoxynucleotidyl transferase deficiency reduces the incidence of autoimmune nephritis in (New Zealand Black x New Zealand White)F1 mice. J. Immunol. 161:7023–7030. [PubMed] [Google Scholar]
- 39.Kearney, J.F., J. Bartels, A.M. Hamilton, A. Lehuen, N. Solvason, and M. Vakil. 1992. Development and function of the early B cell repertoire. Int. Rev. Immunol. 8:247–257. [DOI] [PubMed] [Google Scholar]
- 40.Wu, T.T., G. Johnson, and E.A. Kabat. 1993. Length distribution of CDRH3 in antibodies. Proteins. 16:1–7. [DOI] [PubMed] [Google Scholar]
- 41.Schroeder, H.W. Jr., and P.M. Kirkham. 2000. Marriage, divorce, and promiscuity in human B cells. Nat. Immunol. 1:187–188. [DOI] [PubMed] [Google Scholar]
- 42.Wasserman, R., Y.S. Li, S.A. Shinton, C.E. Carmack, T. Manser, D.L. Wiest, K. Hayakawa, and R.R. Hardy. 1998. A novel mechanism for B cell repertoire maturation based on response by B cell precursors to pre–B receptor assembly. J. Exp. Med. 187:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keyna, U., S.E. Applequist, J. Jongstra, G.B. Beck-Engeser, and H.M. Jack. 1995. Ig mu heavy chains with VH81X variable regions do not associate with lambda 5. Ann. N. Y. Acad. Sci. 764:39–42. [DOI] [PubMed] [Google Scholar]
- 44.Hayakawa, K., R.R. Hardy, and L.A. Herzenberg. 1986. Peritoneal Ly-1 B cells: genetic control, autoantibody production, increased lambda light chain expression. Eur. J. Immunol. 16:450–456. [DOI] [PubMed] [Google Scholar]
- 45.Kantor, A.B., C.E. Merrill, L.A. Herzenberg, and J.L. Hillson. 1997. An unbiased analysis of V(H)-D-J(H) sequences from B-1a, B-1b, and conventional B cells. J. Immunol. 158:1175–1186. [PubMed] [Google Scholar]
- 46.Cariappa, A., M. Tang, C. Parng, E. Nebelitskiy, M. Carroll, K. Georgopoulos, and S. Pillai. 2001. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity. 14:603–615. [DOI] [PubMed] [Google Scholar]
- 47.Gavin, A.L., B. Duong, P. Skog, D. Ait-Azzouzene, D.R. Greaves, M.L. Scott, and D. Nemazee. 2005. deltaBAFF, a splice isoform of BAFF, opposes full-length BAFF activity in vivo in transgenic mouse models. J. Immunol. 175:319–328. [DOI] [PubMed] [Google Scholar]