Abstract
The inflamed liver in chronic hepatitis B virus (HBV) infection (CHB) is characterized by a large influx of non–virus-specific CD8 T cells. Little is known about the functional capacity of these lymphocytes, which could provide insights into mechanisms of failure of viral control and liver damage in this setting. We compared the effector function of total circulating and intrahepatic CD8 T cells in CHB patients and healthy donors. We demonstrated that CD8 T cells from CHB patients, regardless of their antigen specificity, were impaired in their ability to produce interleukin-2 and proliferate upon TCR-dependent stimulation. In contrast, these CD8 T cells had preserved production of the proinflammatory cytokines interferon-γ and tumor necrosis factor-α. This aberrant functional profile was partially attributable to down-regulation of the proximal T cell receptor signaling molecule CD3ζ, and could be corrected in vitro by transfection of CD3ζ or replenishment of the amino acid arginine required for its expression. We provide evidence for depletion of arginine in the inflamed hepatic microenvironment as a potential mechanism for these defects in global CD8 T cell signaling and function. These data imply that polarized CD8 T cells within the HBV-infected liver may impede proliferative antiviral effector function, while contributing to the proinflammatory cytokine environment.
Persistent infection with hepatitis B virus (HBV) remains a major global health threat, currently affecting over 350 million individuals worldwide and causing more than 1 million deaths annually. Immune clearance is thought to be mediated primarily through a strong virus-specific CD8 T cell response (1); marked quantitative and qualitative defects in this response have been described in patients with chronic HBV infection (CHB) (2–4). Patients with uncontrolled infection are distinguished from healthy HBV carriers by the presence of a large lymphocytic infiltrate in their livers, containing a high proportion of non–antigen-specific CD8 T cells (3, 5). Little is known about the characteristics of this generalized CD8 T cell population and its potential contribution to the failure of viral control and liver immunopathogenesis. In this study, we define the functional profile of non–HBV-specific CD8 T cells in patients with CHB, and investigate aberrant expression of the proximal TCR-associated signaling molecules CD3ζ and CD28 as putative mechanisms contributing to the defects seen.
In other diseases of chronic inflammation and high-load antigenic persistence analogous to the situation in CHB, selective defects in global CD8 function have been associated with down-regulation of CD3ζ ± CD28. These include autoimmune disorders (6, 7), malignancy (8), and chronic viral (9, 10) and bacterial (11) infections. The CD3ζ chain is a proximal TCR-associated signaling molecule comprised of a disulphide-linked homodimer (12). Upon TCR ligation, SRC family kinases phosphorylate the three ITAM residues on the intracytoplasmic domain of CD3ζ, and these then serve as docking sites for adaptor proteins such as ZAP-70, which potentiate further downstream signaling events. CD3ζ is structurally and evolutionarily unique from other CD3 components and plays a rate-limiting role in TCR–CD3 complex assembly and efficient signal amplification (13, 14). Along with CD3ζ, down-regulation of CD28, a proximal costimulatory molecule required for efficient signaling has also been described in viral infections (15) and associated with T cell anergy.
Selective defects in virus-specific CD8 T cell function have been described in several chronic viral infections and observed to be progressively lost in a predictable hierarchy according to the duration and strength of antigenic stimulation and availability of CD4 help (16, 17). Loss of CD8-derived IL-2 is an early defect that has recently been found to result in poor viral control and disease outcome in HIV infection (10, 18). Functional defects affecting nonantigen-specific CD8 would need to invoke factors other than excessive antigenic drive through MHC/peptide interactions. Bystander activation and effects of the large quantities of circulating HBV antigens are possible candidates. In addition, the liver microenvironment, where the nonantigen-specific CD8 T cells accumulate in HBV infection, has long been recognized to be immunotolerant. In active HBV infection, intrahepatic T cells would be exposed to a high level of production of HBV antigens and recurrent hepatic inflammation. Putative factors affecting non–antigen-specific T cells include high levels of proinflammatory cytokines, hepatocyte expression of tolerizing ligands (19), depletion of essential nutrients (20), or accumulation of toxic metabolites (21, 22).
In patients with CHB, we found a skewing of effector function in all CD8 T cells, regardless of their specificity. Compared with healthy donors, CHB patients had circulating and intrahepatic CD8 T cells with poor IL-2 production and proliferative potential. Our findings point to a dual pathogenic role for nonantigen-specific CD8 T cells within the liver, contributing to both liver inflammation and poor viral control. Investigation of the underlying signaling defects implicates a role for arginine deprivation triggering CD3ζ down-regulation and impairing T cell proliferation.
RESULTS
CD8 T cells from CHB patients display reduced IL-2 and proliferative capacity upon stimulation through the TCR
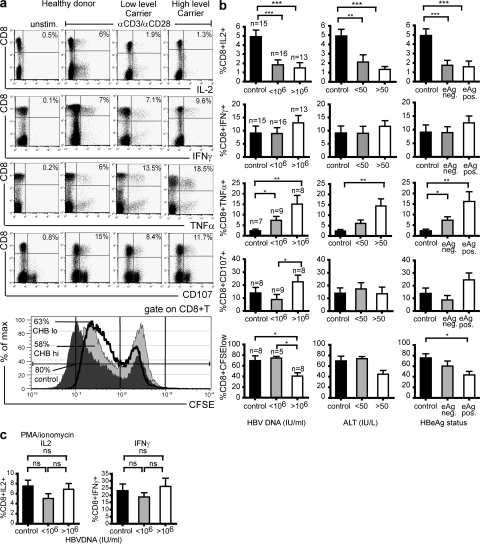
To investigate the functional properties of the global CD8 T cell population in CHB, we performed a cross-sectional study comparing patients with different HBV disease profiles and healthy controls (Table I). PBMCs were isolated and stimulated with either TCR-dependent mitogens (anti-CD3/anti-CD28) or TCR-bypassing mitogens (PMA/ionomycin); effector function was measured by intracellular cytokine or CFSE staining. Representative examples of CD8 effector functions in a control, low-level, and high-level CHB patient are shown in Fig. 1 a(top to bottom: IL-2, IFN-γ, TNF-α, and CD107). Cumulative data for 15 healthy controls, 16 CHB patients with viral load <106 IU/ml, and 13 CHB patients with viral load >1 × 106 IU/ml are shown in Fig. 1 b. Upon a TCR-dependent stimulus (anti-CD3 and -CD28), there was a significant impairment in CD8 T cell IL-2 production in CHB, with a mean fourfold decrease in patients with high viral load compared with the controls (P < 0.001, Mann-Whitney test). There was a trend to progressive impairment with high viral load (>106 IU/ml) and raised alanine transaminase (alanine aminotransferase [ALT] >50 IU/liter, surrogate marker for liver inflammation; Fig. 1 b).
Table I.
Clinical characteristics of patients and controls
| Overall CHB | Low viral load | High viral load | Healthy donor | |
|---|---|---|---|---|
| n | 58 | 35 | 23 | 28 |
| Median age (range) | 35 (23–69) | 35 (23–69) | 36 (23–63) | 28 (22–44) |
| Sex (M:F ratio) % male | (41:17) 70% | (25:10) 71% | (16:7) 70% | (14:14) 50% |
| Median serum HBVDNA (IU/ml; range) | 250,000 (BLQ–340 × 106) |
1,400 (BLQ–945700) |
78 × 106(1.1 × 106–340 × 106) | - |
| Median ALT (IU/liter; range) | 50 (21–498) | 36 (21–498) | 73 (24–363) | - |
| HBeAg status (% positive) | 11% | 10% | 87% | - |
BLQ, DNA positive but below limit of quantification, i.e., <50 IU/ml.
Figure 1.
Selective impairment in IL-2 production and proliferation, but elevated IFN-γ, TNF-α, and cytolysis, in CD8 T cells from patients with high viral load. PBMCs were stimulated with mitogens OKT3 (anti-CD3; 1 μg/ml) and anti-CD28 mAb (5 μg/ml), and cytokine production in CD8 T cells was determined by intracellular cytokine staining. (a) Representative dot plots show IL-2, IFN-γ, TNF-α, CD107, and CFSE staining by CD8 T cells in a healthy donor, patient with low viral load (<106 IU/ml HBVDNA), and a patient with high viral load (>106 IU/ml HBVDNA). (bottom) Percentage of CFSE low CD8 T cells in a control (dark gray histogram), low-level carrier (solid black line), and high-level carrier (light gray histogram). The y axis shows the percentage of maximum counts to standardize overlaid histogram heights. (b) Subjects were categorized into groups determined by their viral load/HBeAg status or level of liver inflammation (ALT as a surrogate marker of liver inflammation) and mean (± SEM) production of cytokines IL-2, IFN-γ, TNF-α, the cytolytic marker CD107, and divisions as determined by CFSE are shown for each group after anti-CD3/anti-CD28 stimulation. Background production of cytokines was determined in the unstimulated control, and subtracted from the final readings (*, P < 0.05; **, P < 0.01; ***, P < 0.001, all significance testing by the Mann-Whitney test). (c) Levels of intracellular IL-2 and IFN-γ in the different donors after TCR-independent stimulation with PMA/ionomycin.
In contrast, CD8 production of IFN-γ was maintained in patients with high HBV load, liver inflammation, and expression of eAg compared with healthy carriers or healthy donors (Fig. 1, a and b). Similarly, TNF-α production and cytolytic potential (measured by surface expression of CD107) of global CD8 T cells were maintained in CHB, and tended to be higher in patients with active disease (Fig. 1, a and b). Using PMA and ionomycin as a TCR bypassing signal, no differences in CD8 T cell effector function could be detected between CHB patients and controls (shown for IFN-γ and IL-2; Fig. 1 c). This indicated that the functional biases noted could not be attributed to the increase in CD27−CD45RA+ CD8 in CHB (unpublished data) because these “end-stage” effectors have impaired IL-2 production upon PMA/ionomycin stimulation (23). Instead, it pointed to the skewed CD8 effector function resulting from aberrations within the TCR-associated signaling machinery. This was supported by the fact that cytokine skewing was maintained upon substituting anti-CD3/CD28 stimulation with plate-bound anti-CD3 alone or anti-CD3 in combination with irradiated APC.
CD8 T cells with this altered IFN-γ/IL-2 ratio have recently been noted in other chronic viral infections, such as HIV and LCMV, and they were found to correlate with poor viral control and disease progression (17, 18). This impairment of IL-2 production would be expected to limit T cell clonal expansion potential, particularly within the liver, where CD4 help is restricted (24). To test this, we measured the proliferative capacity of CD8 T cells upon mitogenic stimulation by CFSE dilution. Consistent with their impaired IL-2 production and/or a direct block of cell cycle progression, fewer CD8 T cells from patients with CHB divided, and those that did divide underwent fewer divisions than CD8 T cells from controls (Fig. 1, a and b, bottom). The proportion of CD8 T cells able to undergo at least one division was significantly reduced in CHB patients with high viral load and expression of eAg, and showed a negative correlation with liver inflammation within the patient group (r2 = 0.6; P < 0.04; unpublished data). Impaired CD8 division was seen upon stimulation of whole PBMCs, indicating that the available CD4 help could not compensate for the CD8 defect in these patients. Compatible with this, reduced IL-2 production by CD4 T cells was also noted in patients with HBV infection compared with healthy controls (unpublished data).
Skewed IFN-γ/IL-2 ratio in CD8 T cells from CHB patients regardless of specificity
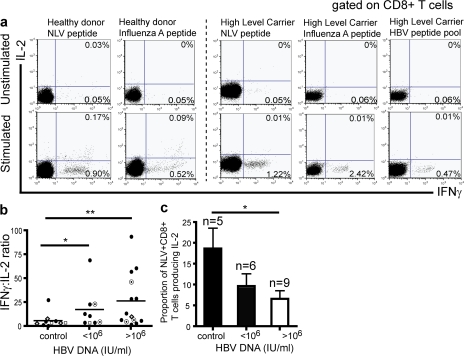
HBV-specific CD8 T cells are markedly depleted in patients with CHB (3), such that circulating responses are barely detectable, even when overlapping peptides covering the whole genome are applied (25, 26). Based on this finding, it is logical to assume that the majority of the CD8 T cells we are studying in the periphery of these patients are non-HBV specific. To confirm that the qualitative dysfunction we observed was not restricted to the HBV-specific CD8 T cells but also extended to other virus specificities, PBMCs from HLA-A2–positive donors were stimulated directly ex vivo with peptides representing immunodominant HLA-A2–restricted viral epitopes from CMV, EBV, and influenza A, in addition to HBV (Fig. 2 a). Virus-specific CD8 from healthy donors produced predominantly IFN-γ, but had a clear population of dual IFN-γ/IL-2–responding cells, whereas those from CHB patients only produced IFN-γ (Fig. 2 a). The percentage of IFN-γ–positive to dual IFN-γ/IL-2–positive peptide-specific CD8 T cells was plotted as a ratio after subtraction of background readings from controls without peptide. As shown in Fig. 2 b, regardless of the virus specificity, there was a significantly higher IFN-γ/IL-2 ratio in patients with active HBV infection compared with healthy donors (P < 0.01). Because CMV infection has also been reported to skew the repertoire of T cell responses (27), and was therefore a potential confounding factor, we also compared exclusively CMV-specific responses in a subset of CHB patients and controls who were known to be CMV seropositive. The proportion of CD8 able to produce IL-2 in response to the immunodominant HLA-A2–restricted NLV epitope from pp65 showed a stepwise reduction from healthy donors to low- and high-level CHB patients (Fig. 2 c). These data further confirm that functional impairment is present in the generalized T cell population in patients with CHB, regardless of antigen specificity.
Figure 2.
Determination of IL-2 and IFN-γ production by CD8 T cells after stimulation with HLA-A2–restricted viral peptides. (a) Representative flow cytometric dot plots to show distribution of IFN-γ and IL-2–positive responder cells after ex vivo stimulation with peptides (for IFN-A, CMV, HBV envelope pool) in a healthy donor and in a high-level carrier. All ex vivo responses were in the range of 0.1–4% after subtraction of background readings. A response was determined as any reading > mean + 2SD of background. (b) Cumulative data to show mean IFN-γ/IL-2 ratio after stimulation with peptides (NLV, •; EBV, ⋄; FLU, □; HBV c18-27/ env pool, ⊙) for controls, and patients with low (<106 IU/ml) and high (>106 IU/ml) viral load. (c) Histogram to show proportion of double-positive (IFN-γ+IL-2+/IFN-γ+) CD8 T cells in controls and patients after stimulation with the NLV peptide of CMV ex vivo. Mean and SEM are shown.
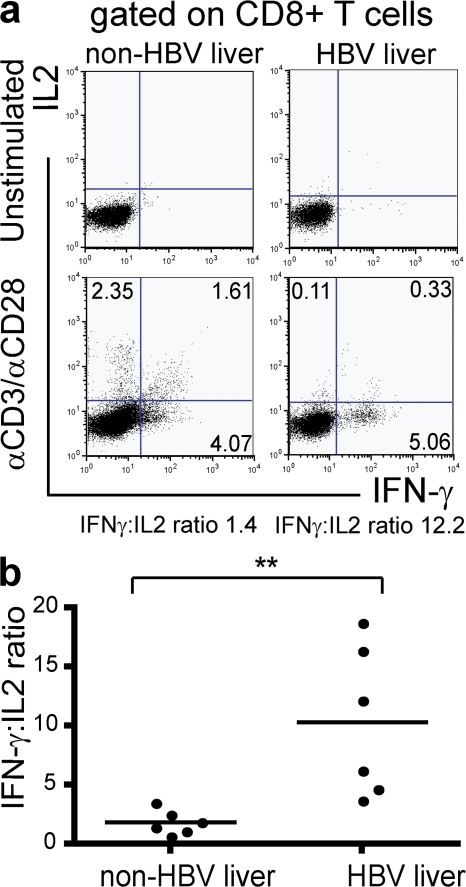
Intrahepatic CD8 T cells produce less IL-2 in patients with CHB than in controls
CD8 T cells are enriched in the liver compared with the periphery (24), with CD8 T cells constituting 60–90% of intrahepatic T cells. To better understand what role this population may play in HBV-associated inflammation and/or viral control, intrahepatic CD8 T cells were isolated and studied. Liver tissue was obtained from routine diagnostic biopsies or resected tissue from six patients with HBV-related liver disease and six controls without HBV infection. Control liver samples included liver tissue with normal architecture and uninflamed histology taken distal to a metastasis and tissue from patients with other inflammatory liver diseases (Table II). After isolation, intrahepatic lymphocytes were stimulated directly ex vivo with TCR-dependent mitogens, and IFN-γ and IL-2 production was determined in gated CD8 T cells (Fig. 3 a). In non–HBV-infected patients, the following three populations of responder cells could be seen: IFN-γ single-positive, IFN-γ/IL-2 double-positive, and IL-2 single-positive. In each of the six CHB-infected donors, however, intrahepatic CD8 T cells almost exclusively produced IFN-γ, resulting in a significantly raised mean IFN-γ/IL-2 ratio compared with the non–HBV-infected group (P < 0.01; Fig. 3 b). These data revealed a dysfunction in global intrahepatic CD8 T cell IL-2 production in CHB.
Table II.
Clinical characteristics of patients and controls for liver samples
| Subject | Pathology | ALT (IU/liter) |
HBeAg | Sex | Age |
|---|---|---|---|---|---|
| H1a | CHB mild fibrosis | 45 | NEG | Male | 31 |
| H2a | CHB mild fibrosis | 100 | POS | Male | 23 |
| H3a | CHB moderate fibrosis | 246 | POS | Male | 42 |
| H4ab | CHB cirrhosis | 498 | NEG | Male | 34 |
| H5a | CHB no fibrosis | 48 | NEG | Male | 43 |
| H6a | CHB mild fibrosis | 63 | NEG | Male | 43 |
| H7b | CHBd | 40 | - | Male | 60 |
| H8b | CHBd | 56 | - | Male | 66 |
| H9b | CHBd | 110 | - | Male | 56 |
| H10b | CHBd | 79 | - | Male | 44 |
| H11b | CHBd | 45 | - | Female | 48 |
| H12c | CHB cirrhosis | 31 | - | Male | 54 |
| H13c | CHB fibrosis | 67 | NEG | Male | 49 |
| H14c | CHB cirrhosis | 54 | NEG | Male | 53 |
| H15c | CHB cirrhosis | 47 | NEG | Male | 50 |
| H16c | CHB + hepatocellular carcinoma | 19 | NEG | Male | 61 |
| C1ab | Nonprimary liver carcinomae |
140 | N/A | Female | 34 |
| C2a | Nonprimary liver carcinomae |
26 | N/A | Female | 56 |
| C3ab | Cholangiocarcinoma | 41 | N/A | Female | 34 |
| C4a | Nonprimary liver carcinomae |
34 | N/A | Male | 60 |
| C5ab | Nonprimary liver carcinomae |
17 | N/A | Female | 67 |
| C6a | Nonprimary liver carcinomae |
209 | N/A | Male | 66 |
| C7b | Hepatitis C virus infection | 54 | N/A | Female | 41 |
| C8b | Nonprimary liver carcinomae |
30 | N/A | Female | 60 |
| C9b | Nonprimary liver carcinomae |
30 | N/A | Male | 54 |
| C10b | Nonalcoholic steatohepatitis | 108 | N/A | Female | 32 |
| C11c | Healthy transplant donor | - | N/A | Male | 56 |
| C12c | Alcoholic cirrhosis | 19 | N/A | Male | 63 |
| C13c | Primary sclerosing cholangitis | 138 | N/A | Female | 29 |
| C14c | Primary sclerosing cholangitis | 69 | N/A | Female | 60 |
| C15c | Primary sclerosing cholangitis | - | - | Female | - |
Used for functional ICS.
Used for CD3ζ staining.
Used for arginase activity.
CHB, histology unavailable.
Resection specimens adjacent to nonprimary liver carcinoma; all tissue was of normal architecture.
Figure 3.
Intrahepatic CD8 T cells from patients with CHB are biased toward IFN-γ over IL-2 production. (a) Intrahepatic CD8 T cells were isolated from liver biopsies, and cytokine production was determined by intracellular staining after anti-CD3/anti-CD28 stimulation. Representative dot plots show IL-2 and IFN-γ production by CD8 T cells for a typical patient with chronic HBV and a patient with nonviral liver pathology. (b) After mitogenic stimulation, the ratio of IFN-γ/IL-2 production by intrahepatic CD8 T cells was determined for six patients with CHB and six patients without CHB. Ratios are plotted in the histogram shown.
CD8 T cells from patients with CHB have selective down-regulation of the CD3ζ chain, which is more marked in the intrahepatic compartment
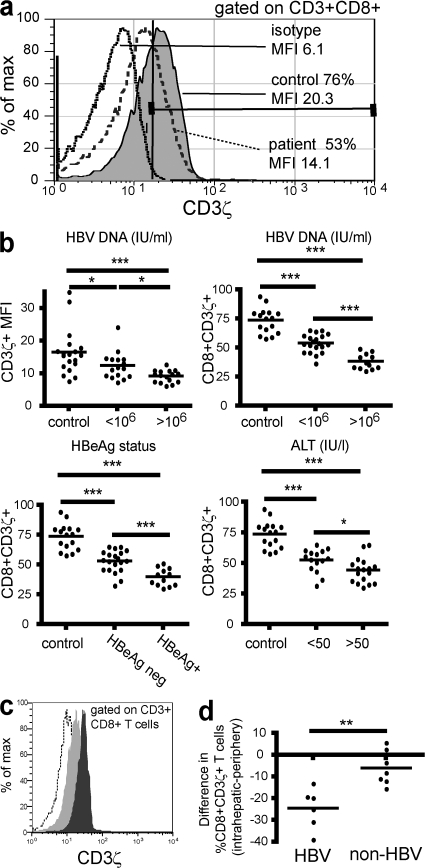
The identification of a selective defect in IL-2 and proliferation after a TCR-dependent stimulus, but not upon a TCR-bypassing stimulus, pointed to dysfunction within the TCR signaling machinery. In other chronic infectious diseases such as HIV (10), impairment in IL-2 production has been partially attributed to down-regulation of the CD3ζ chain, a proximal TCR-associated signaling molecule (12). This molecule can be down-regulated independently of other CD3 signaling molecules, which maintain their expression on the cell surface. T cells with low (10, 15, 28) or absent (29) CD3ζ maintain IFN-γ, but not IL-2 production, and become refractory to TCR-induced proliferation. To determine whether CD3ζ down-regulation could be a factor contributing to the functional defects seen, we compared CD3ζ expression between controls (n = 16) and patients with low (n = 20) and high HBV load (n = 12). CD3ζ expression was determined by intracellular staining after gating on CD3ε, so that detected differences in the expression of CD3ζ would not be caused by down-regulation of the entire CD3 complex, but to isolated down-regulation of the CD3ζ homodimer (anti-CD3ε binds an extracellular domain on the CD3ε chain and does not interfere with binding of anti-CD3ζ to its intracellular determinant). Representative CD3ζ expression after gating on CD3ε+CD8 cells is shown for a CHB patient and healthy donor in Fig. 4 a.
Figure 4.
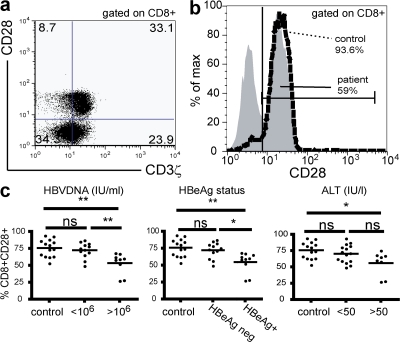
CD3ζ is down-regulated in both peripheral and intrahepatic CD8 from patients with chronic HBV. PBMCs or intrahepatic lymphocytes isolated from biopsy tissue were surface stained for CD3ε and CD8, fixed, permeabilized, and stained for intracellular CD3ζ or its isotype. A tight lymphocyte gate and secondary gate over CD3ε+CD8 cells has been applied. A marker was applied at the edge of the isotype control (solid black line) and the proportion of CD8 to the right of this line represented the CD3ζ+ fraction. (a) Representative flow cytometric histogram illustrating CD3ζ expression (as a percentage and as mean fluorescence intensity [MFI]) for a chronic HBV-infected patient with high viral load (>106 IU/ml; dashed line) and a healthy control (gray). (b) CD3ζ levels, shown as both MFI and percentage of CD3ζ+ in the total CD8 are shown against viral load (top), and percentage values are further broken down by HBeAg status and ALT (bottom). (c) Histogram to illustrate differential expression of CD3ζ by peripheral (dark gray) and intrahepatic (light gray) CD8 from a CHB donor (dotted line, isotype). (d) The difference in CD3ζ expression between intrahepatic and peripheral CD8 is plotted for each patient with HBV/non-HBV–related liver disease.
To determine whether CD3ζ down-regulation correlated with disease status, patients were categorized by their viral load (<106 or >106 IU/ml), HBeAg status, or ALT level (raised >50 IU/liter). Patients were classed as ALT high if their ALT was raised at the time of sampling or had been raised in the previous 6 mo to take account of patients with cyclical flares of liver inflammation. CD3ζ expression in CD8 T cells correlated inversely with each of the three parameters (P < 0.0001; Fig. 4 b). Patients with the most active HBV infection had the lowest CD3ζ expression in their CD8 T cells, with a mean reduction of 40% compared with controls. Expression of CD3ζ was also reduced in CD4 T cells from these patients, but to a lesser degree than the CD8 T cells (unpublished data), as noted previously in HIV infection (9).
CD3ζ down-regulation has been attributed to exposure to sustained inflammatory cytokines and antigenic stimulation; therefore, we hypothesized that it would be more pronounced in the intrahepatic compartment, which is the site of HBV replication and associated pathology. Intrahepatic lymphocytes were therefore isolated from six CHB patients and seven non–HBV-infected patients undergoing diagnostic liver biopsies. For each patient, CD3ζ expression in intrahepatic and peripheral T cells was compared (Fig. 4 c). CD3ζ expression was lower in intrahepatic than peripheral CD8 T cells for all CHB patients, with a mean of 24% fewer CD3ζ+ cells in the liver compared with the periphery (Fig. 4 d). However, in the seven non–HBV-infected patients, we found significantly less difference in CD3ζ expression between peripheral and intrahepatic T cells (Fig. 4 d). These data suggest that CD3ζ down-regulation may be potentiated by the combination of chronic viral-driven inflammation and other factors that are more pronounced in the CHB liver microenvironment.
CD28 is also down-regulated in CHB patients
Like CD3ζ, down-regulation of CD28 has been described in chronic viral infections such as HIV and CMV and has been associated with T cell anergy (15). CD8 T cells that are CD28− in CHB are more likely to have down-regulated CD3ζ than their CD28+ counterparts, but these two proximal signaling molecules are not always down-regulated together (Fig. 5 a). To investigate whether CD28 down-regulation could be playing a role in chronic HBV, we compared surface CD28 staining of CD8 T cells from patients and controls. The percentage of CD8 T cells expressing CD28 was reduced in CHB compared with healthy controls (Fig. 5 b). CD28 was significantly down-regulated in CD8 T cells from patients with high viral load, eAg expression, and liver inflammation (Fig. 5 c). The expression of CD28 is known to be affected by CMV status and age (27); data were therefore reanalyzed, including only age-matched CMV-seropositive individuals in the control and CHB groups; CD28 was still reduced in CHB patients with high viral load compared with low-level carriers or controls (unpublished data).
Figure 5.
CD28 is down-regulated in CD8 T cells from patients with high viral load. (a) Representative dot plot to show the coexpression of CD28 and CD3ζ in CD8 T cells in a patient with high viral load. (b) Flow cytometric histogram to show the proportion of CD8 T cells that are CD28+ in a patient with high viral load (gray) and a healthy donor (dashed line). (c) Cumulative data for CD28 expression is shown against viral load, HBeAg status, and ALT level.
Transfection of CD3ζ/CD28 corrects the IL-2 defect in CD8 T cells from CHB patients
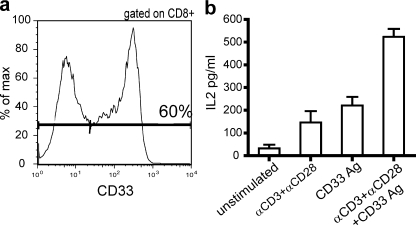
Our data suggested an association between down-regulated CD3ζ, and impaired IL-2 production, with a possible further contribution from CD28 down-modulation in patients with the most active disease. To confirm this functional association, we sought to specifically reconstitute levels of these two signaling molecules in CD8 T cells from CHB patients and assess the impact on their functional skewing. Freshly isolated, unstimulated CD3ζCD28lo CD8 T cells from patients with CHB were transfected by electroporation with plasmid DNA encoding a chimeric receptor with intracellular CD3ζ and CD28 domains, as previously described (30, 31). These chimeric receptors had the human antibody P67 specificity for CD33 antigen; detection using fluorescent, soluble CD33Ag indicated a transfection efficiency of at least 60% (Fig. 6 a). After an overnight rest, transfected PBMCs were then stimulated with anti-CD3 and -CD28, which activated the cells through the TCR, with or without the addition of CD33Ag, which bound to the extracellular P67 domain of the chimeric receptor. The same experiments were repeated on untransfected cells that had also been passed through the AMAXA transfection protocol with PBS in place of DNA. When PBMCs or negatively selected CD8 T cells were stimulated through the TCR or through CD33 alone, low levels of IL-2 production were detectable by ELISA. Addition of both stimuli together substantially increased IL-2 production (Fig. 6b). Production of IL-2 by the dual stimulus exceeded the combined additive effect of the TCR-dependent or transfection-specific stimuli separately. This suggests that the newly introduced CD3ζ and CD28 may not only be activated after the CD33Ag stimulus, but may also be recruited to TCR signaling after CD3/CD28 stimuli. Untransfected cells showed no increase in IL-2 production upon addition of CD33Ag stimulation. We were unable to exclude the possibility that some of the functional enhancement derived from increased signaling of “normal” cells rather than recovery of the specific population of cells deficient in CD3ζ/CD28. However, these data at least directly confirm the link between overall levels of CD3ζ/CD28 and capacity for IL-2 production in CD8 T cells from CHB patients.
Figure 6.
Reconstitution of CD3ζ and CD28 levels with a chimeric receptor reverses the IL-2 defect. (a) Histogram shows transfection efficiency in a representative patient after transfection of a chimeric receptor with an extracellular CD33-binding domain and intracellular CD3ζ and CD28 domains. (b) After antigen-specific stimulation through the chimeric receptor, TCR (anti-CD3/anti-CD28), or both together, IL-2 production was determined by ELISA. Mean and SEM of IL-2 production is shown for three individuals after the varying stimuli.
Functional reconstitution of CD3ζ in CD8 T cells from CHB patients is partially dependent on l-arginine
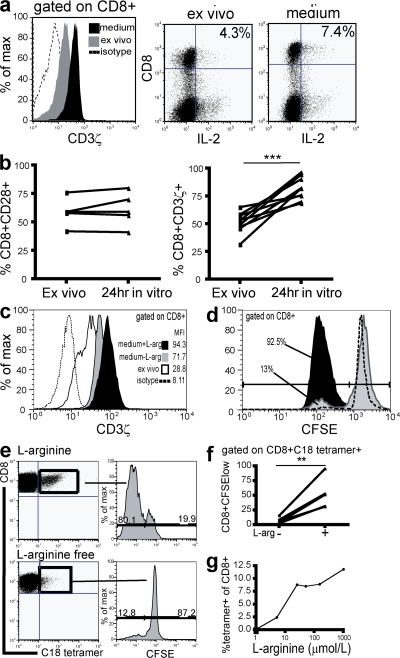
We observed that after overnight culture of CD8 T cells from CHB patients in complete medium, levels of intracellular CD3ζ were reconstituted to levels found in healthy controls (Fig. 7 a, histogram). This CD3ζ reconstitution was accompanied by a recovery in IL-2 production from the reduced levels produced by CD8 directly ex vivo to normal levels (Fig. 7 a, FACS plots). Recovery of CD3ζ expression was seen consistently after overnight culture for all samples from CHB patients, whereas CD28 levels in these patients did not show a concurrent increase over the same period (Fig. 7 b). The fact that substantial functional reconstitution accompanied CD3ζ up-regulation in cells that had not altered their CD28 levels indicated that CD3ζ down-regulation may be the more relevant defect in this situation.
Figure 7.
Replenishment of arginine restores CD8 expression of CD3ζ, accompanied by a recovery in IL-2 production and proliferation. (a) Histogram illustrates effect of overnight culture in medium on CD3ζ levels (black histogram) compared with when measured directly ex vivo (gray histogram). Isotype control is shown as the dotted line. IL-2 production was determined in parallel before and after culture and representative plots are shown. (b) Cumulative data to show change in CD28 (P = nonsignificant) and CD3ζ expression (P < 0.0001) after in vitro culture, compared with ex vivo culture, in five and nine patients, respectively. (c) CD3ζ expression in CD8 T cells was determined directly ex vivo, and then again after overnight culture in medium with or without l-arginine (0.2 g/liter). (d) To investigate whether the presence of l-arginine affects CD8 T cell proliferation, PBMCs were preincubated with medium with or without l-arginine for 24 h, stained with CFSE, and resuspended in their respective mediums in the presence of a CD3ζ-dependent stimulus for 6 d. A gate has been applied on CD8 T cells. (e) To determine the effect of l-arginine on proliferation of HBV-specific CD8+ T cells, PBMCs from HLA-A2+ patients who had resolved HBV infection were stimulated with core 18–27 peptide for 7 d and detected with an HLA-A2/c18-27–specific tetramer. Representative dot plots depict the HBV-specific CD8+ population expanded at 1 wk, along with the number of divisions these populations have undergone, determined by CFSE dilution, in the presence (top) or absence (bottom) of l-arginine. (f) Cumulative data illustrate the effect of depleting l-arginine on the percentage of tetramer-positive populations dividing by 1 wk in 5 patients (P < 0.01). (g) HLA-A2/core18-27 tetramer-positive CD8 were compared after 7-d peptide stimulation of PBMCs incubated in a range of l-arginine concentrations (0 μM, 5 μM, 25 μM, 50 μM, 150 μM, and 1 mM).
These findings suggested that removal or replenishment of a factor in vitro enabled CD3ζ to be reexpressed at physiological levels in CD8 T cells from patients with CHB. One candidate for such an effect was l-arginine because depletion of this conditionally essential amino acid has been associated with CD3ζ down-regulation in human T cells (32). To investigate whether l-arginine levels modulate CD3ζ expression in T cells of patients with CHB, PBMCs were cultured overnight in medium with or without l-arginine and CD3ζ expression was subsequently determined. When medium depleted of l-arginine was used, up-regulation of CD3ζ was attenuated compared with the reconstitution that could be achieved upon the addition of l-arginine at the concentration found in the usual complete medium (Fig. 7 c). To investigate whether functional differences could be attributed to these altered levels of CD3ζ reconstitution, proliferative capacity of CD8 T cells was determined after 6 d of culture with a TCR-dependent stimulus in medium with or without l-arginine. PBMCs were rested in their respective mediums for 24 h before stimulation, to allow sufficient time for CD3ζ levels to be influenced by the extracellular l-arginine microenvironment at the point of triggering of proliferation. We observed that the proliferation of CD8 T cells taken from CHB patients and cultured in l-arginine–free medium was lower than that for CD8 T cells cultured in medium with l-arginine (Fig. 7 d). In the absence of arginine supplementation, CD8 T cells from CHB patients had a profound block in proliferation, and a partial block to proliferation persisted in serum supplemented with arginine in the physiological concentration range (unpublished data). These data suggest that the CD3ζ down-modulation and IL-2/proliferative deficiency observed directly ex vivo in CD8 T cells from patients with CHB may be partially attributable to deprivation of l-arginine. In addition to loss of proliferative function caused by CD3ζ down-regulation, l-arginine deprivation has also been directly linked to growth arrest at the G0-G1 phase of the cell cycle (33), which is compatible with the striking loss of CD8 proliferation we observed. Once these cells were removed from factors present in the HBV-infected patient, they were susceptible to functional reconstitution.
We then investigated the potential impact of this arginine-dependent functional defect on the HBV-specific CD8 T cell response, which is essential to control viraemia (1). HBV-specific responses were efficiently expanded from the blood of patients who had controlled HBV infection (as reflected by the percentage of CD8 staining with a HLA-A2/core 18–27-specific tetramer), and by 1 wk in culture the majority had divided as assessed by CFSE staining (Fig. 7 e, top). When these responses were cultured in an arginine-depleted environment, they resembled CD8 from patients with high HBV load (Fig. 1) (3), showing a marked reduction in clonal expansion (Fig. 7 e, bottom). Only a minority of HBV-specific CD8 had divided by 1 wk in arginine-depleted medium, and in every case there was inhibition of proliferation compared with responses in the presence of arginine (Fig. 7 f; P < 0.01). This proliferative impairment resulted in reduced in vitro expansion of HBV-specific CD8 (Fig. 7 e). When arginine-free medium was supplemented with increasing concentrations of arginine, a dose-dependent impairment in expansion of HBV-specific CD8 was observed (Fig. 7 g).
In vivo levels of arginine and arginase correlate with HBV disease activity
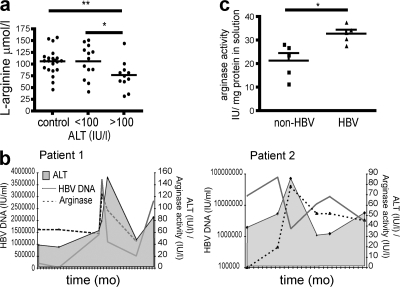
To investigate whether T cells would be exposed to an environment depleted of arginine in patients with CHB, we initially measured l-arginine levels in their serum. We found significantly reduced levels of arginine in the circulation of CHB patients with marked liver inflammation (ALT > 100 IU/liter; Fig. 8 a). These reductions in arginine concentration, although subtle, were in the dose range that we had shown to impair expansion of the HBV-specific response in vitro (Fig. 7 g). We would expect a more striking local depletion of arginine within the liver, but this is difficult to quantitate ex vivo because of activation of arginase during the homogenization of liver tissue required for arginine measurement. Despite this caveat, we did find significantly lower levels of arginine in the liver homogenates from CHB patients than those from patients with other types of liver disease (P < 0.05; unpublished data).
Figure 8.
Depletion of serum l-arginine is associated with elevated serum and liver arginase activity during periods of HBV disease activity in patients with CHB. (a) Serum l-arginine levels were compared in healthy donors (n = 21), patients with low-to-moderate liver inflammation (ALT < 100 IU/liter; n = 13), and patients with high liver inflammation (ALT > 100 IU/liter; n = 11; control vs. high ALT P < 0.01; low ALT vs. high ALT P < 0.05). (b) Graphs depict longitudinal fluctuations in serum arginase activity (dashed line, IU/liter) in association with viral load (solid gray line, HBV DNA, IU/ml) and ALT (shaded histogram, IU/liter) for two patients with flares of CHB. (c) The arginase activity of liver homogenates is shown for five individuals with CHB and five individuals with nonviral liver pathology.
The conditionally essential amino acid l-arginine is predominantly depleted by arginase I, which breaks it down into its constituents, urea and ornithine (20). Hepatocytes have the potential to be a potent source of arginase I (34), as do myeloid suppressor cells (20) and macrophages exposed to Th2 cytokines such as IL-10 (35). We investigated whether HBV-related liver inflammation resulted in an up-regulation of arginase activity to account for our findings of arginine depletion-dependent T cell defects in these patients.
Arginase activity was measured by its capacity to produce urea from an excess of arginine substrate (and standardized against the quantity of total protein extracted in the case of liver samples). Ex vivo serum arginase levels showed a nonsignificant trend to be higher in CHB patients with active liver inflammation, but showed considerable variability within patient groups and controls (unpublished data). To remove the problem of interpatient variability, we therefore compared changes in arginase activity within two individuals with eAg-negative CHB who we were able to sample repeatedly over periods of rapid disease fluctuation (spontaneous hepatic flares). Arginase activity in their serum showed large fluctuations upon longitudinal assessment, with peaks correlating with flares of disease activity as measured by HBV DNA and ALT levels (Fig. 8 b). Elevated arginase levels in the serum have been shown to reflect increased release from the relevant tissue source: the placenta in pregnancy (36) and the liver in orthoptic transplantation (34). We therefore compared levels of arginase activity in extracts prepared from liver samples from patients with or without CHB (Table II). Arginase I activity was significantly increased in liver extracts taken directly ex vivo from patients with CHB compared with those from patients with other types of liver pathology (Fig. 8 c).
DISCUSSION
CHB is characterized by an intrahepatic influx of nonantigen-specific lymphocytes that fail to suppress viral replication and may instead give rise to liver damage (3, 5, 37). In this study, we demonstrated a functional bias of CD8 T cells in CHB, which could contribute to both the failure of viral control and the pathogenesis of the resultant liver inflammation. We found that the global CD8 population (including CD8 specific for HBV and for other chronic viruses), both in the circulation and the liver, were skewed toward IFN-γ/TNFα production and impaired in their ability to produce IL-2 and proliferate. By scrutinizing the global CD8 T cells in CHB, we elucidated a mechanism that may contribute to the failure of virus-specific CD8 to survive and proliferate sufficiently to control HBV replication.
The study of HBV-specific CD8 has been restricted by the difficulty in detecting these cells in patients failing to control HBV infection. We have previously noted that the scanty populations detectable in patients with CHB have an impaired capacity for clonal expansion (3, 26, 38) and an enhanced propensity to Bim-mediated apoptosis (39). In this study, we identify a mechanism that could contribute to both of these defects, resulting in an impairment of HBV-specific CD8 proliferation and a generalized intrahepatic T cell deficiency in IL-2 production in CHB. CD8 T cells have been shown to depend on autologous IL-2 production to drive their replication in settings of impaired CD4 help (18), such as the CD4-depleted liver in CHB (24, 40). A local depletion in IL-2 could also contribute to the propensity of HBV-specific CD8 to undergo apoptosis through the IL-2-dependent Bcl-2 pathway (39). The impairment in IL-2 production manifested by nonantigen-specific CD8 T cells could therefore impact upon the ability of the HBV-specific populations to both expand and survive. A generalized defect in the ability to mount efficient T cell responses in patients with CHB is supported by the long-standing clinical observation that these patients are significantly less likely to reject a liver transplant than patients with other chronic liver diseases (41, 42). It might also be expected to result in an impaired ability of CHB patients to control concurrent intrahepatic infections, particularly with other persistent noncytopathic viruses that are highly dependent on long-term T cell expansion and survival for their control. We suggest that the HBV-specific response, already depleted by other mechanisms such as PD-1–induced anergy (26) and Bim-mediated apoptosis (39), becomes more vulnerable to this additional global mechanism of intrahepatic T cell impairment than other more robust antiviral responses.
We observed that global CD8 T cells capable of IFN-γ and TNF-α release are maintained in CHB patients with high viral load and liver inflammation. Although these two cytokines have the potential for noncytopathic inhibition of HBV replication (43), they may also potentiate hepatocyte damage (5). IFN-γ is a proinflammatory cytokine that induces chronic hepatitis when constitutively expressed in the liver of transgenic mice (44) and may also increase susceptibility to TNF-mediated liver damage (45). TNF-α has been demonstrated to mediate potent hepatocyte destruction, particularly when expressed in a cell-bound form (46). This effect could be heightened in the HBV-infected liver, through the capacity of the HBx protein of HBV to sensitize hepatocytes to TNF-mediated apoptosis (47). IFN-γ has been shown to stimulate the release of chemokines such as CXCL9 and CXCL10 from hepatocytes, driving chemotaxis of the nonantigen-specific hepatic infiltrate both in the transgenic mouse model of HBV (48) and in human HCV infection (49). Similarly, lung pathology resulting from the failure of CD8 control of influenza and the subsequent infiltration of non–antigen-specific lymphocytes can be prevented by blocking IFN-γ (50). A recent study has shown that TNF-α can also switch the liver from a site of immunoprivilege to one susceptible to immune destruction by the T cells it indirectly chemoattracts (51). Thus, a propensity of bystander CD8 T cells in CHB to produce IFN-γ and TNF-α could further drive the nonantigen-specific lymphocytic infiltrate and propagate liver inflammation.
Studies have shown that HBV-specific CD8 T cells are present at extremely low frequencies in the circulation and liver of patients with chronic infection (3, 38), even when overlapping peptides are used to screen for responses to the whole viral genome (25, 26). The dysfunction we have observed, affecting all CD8 T cells, must therefore involve a ‘bystander’ effect, and we have specifically shown that these defects extend to CD8 of unrelated specificities. Such a bystander defect, affecting all CD8 T cells regardless of specificity, has been attributed to down-regulation of the ζ-chain component of the CD3 signaling complex in several malignant, autoimmune, and infectious diseases (for review see [12]). These conditions are all characterized by chronic antigenic stimulation in the setting of persistent or recurrent inflammation, as is CHB. A mouse model of chronic bacterial infection dissected the mechanism whereby this combination led to global T cell ζ down-regulation and dysfunction, and confirmed the requirement for IFN-γ together with sustained antigenic exposure (11, 52), both of which are found in the HBV-infected liver. Because most of our CHB patients with high viral load also had liver inflammation, we could not distinguish whether HBV infection and/or the inflammatory milieu associated with chronic liver damage drove the signaling and functional defects we found.
High local levels of IFN-γ are postulated to recruit myeloid-derived suppressor cells (MDSC), which mediate global down-regulation of CD3ζ in surrounding T cells, an effect that typically then extends from the infiltrating cells to affect circulating populations (11, 12, 53). Whether there is an intrahepatic accumulation of MDSC in CHB needs to be investigated, but would be in keeping with the infiltration of the HBV transgenic mouse liver with many cell types, including granulocytes in response to IFN-γ–induced chemokines (54, 55). One of the mechanisms through which MDSCs may exert their effect is through the consumption of l-arginine (56, 57); reduced levels of this conditionally essential amino acid have been associated with CD3ζ down-regulation and impaired proliferation (20, 32, 56). Hepatocytes are also known to be a potent potential source of arginase I, which catabolizes arginine, and reduced l-arginine levels have been found after orthoptic liver transplantation (34). The capacity of macrophages to take up and catabolize arginine has been shown to be regulated by Th2 cytokines such as IL-10 and enhanced by an LPS-rich environment such as the liver (35). We have recently found an increase in IL-10 in patients with flares of HBV infection (58), and there is a close temporal correlation between induction of IL-10 and the increases in arginase activity we noted with HBV disease activity (unpublished data). Whether enhanced arginase activity in these patients is dependent on the degree or type of liver inflammation and/or on HBV itself, and whether it is mediated by hepatocytes and/or infiltrating MDSCs or macrophages, remains to be determined.
We found a dependence on l-arginine for complete recovery of CD3ζ in T cells from HBV patients, consistent with it being a contributory factor. CD8 T cell proliferative capacity was more strikingly dependent on arginine replenishment, compatible with the recent observation that arginine depletion also causes G0-G1 phase growth arrest independently of CD3ζ (33). CD8 T cells from CHB patients showed some reconstitution of CD3ζ once they were removed from the source of antigenic stimulation and inflammation, even in the absence of arginine. Other factors are therefore likely to play a role in the inflamed liver environment, such as depletion of other essential nutrients, inflammatory or immunosuppressive cytokines, and accumulation of reactive oxygen intermediates and other toxic metabolites. In particular, depletion of tryptophan and accumulation of tryptophan catabolites has also been shown to inhibit T cell proliferation via CD3ζ down-regulation (21, 22). CD3ζ down-regulation and arginine deprivation have been noted to be most marked at the site of pathology (in the case of tumors for example), but to also extend to affect T cells in the periphery. In keeping with this, we found CD3ζ down-regulation and arginase induction to be most accentuated in the liver but also detectable in the periphery. It is possible that other persistent infections capable of triggering an analogous inflammatory milieu in the liver would similarly bias T cell signaling and function. Along these lines it is worth noting that HCV has been found to have a pervasive influence on non–antigen-specific CD8 T cells (59).
In summary, we demonstrate for the first time that CD8 T cells from patients with CHB have impaired IL-2 and proliferative capacity, regardless of their specificity. Such functional skewing could impair their survival and anti-viral potential, particularly for persistent noncytopathic viruses in the CD4-depleted liver environment. Their capacity for IFN-γ/TNF-α production could contribute to the proinflammatory milieu, thereby exacerbating mechanisms underlying their dysfunction. The functional bias is corrected upon removal from the pathogenic environment and reversal of underlying signaling defects. Our data suggest that chronic exposure to the microenvironment of an inflamed liver could impair T cell effector functions critical to the control of this virus. A major limitation to the success of therapeutic vaccination in the setting of persistent virus infection such as HBV is thought to be the impaired T cell proliferative capacity (60). Approaches to remedy these T cell defects in patients with CHB could therefore form an important adjunct to future immunotherapeutic strategies.
MATERIALS AND METHODS
Subjects.
58 patients with CHB, 3 patients who had resolved acute HBV infection, and 28 healthy donors participated in the study. Informed consent was obtained, and the study was approved by the respective local ethical committees for the three participating clinics (Camden Primary Care Ethics Review Board, Royal Free Hospital and Medical School Research Ethics Committee, and Cambridge Research Ethics Committee). There was no significant difference in demographic variables (age/gender) between patient and control groups (Table I). 24 of the CHB patients were positive for HBeAg, and 19 of these also had raised ALT (>50 IU/liter) and high HBV DNA (>106 IU/ml) as quantified by an in-house PCR-based assay. All other patients were HBeAg negative, and positive for serum anti-HBe antibody. Two of the patients in this study who had spontaneous flares of eAg-negative CHB were followed up longitudinally and sampled repeatedly over several time points. Patients on antiviral therapy were excluded from the study. All patients were negative for antibodies to hepatitis C virus, delta virus, HIV-1, and HIV-2.
In addition, liver samples were obtained from 16 CHB-infected patients and from 15 patients with nonviral liver disease undergoing diagnostic liver biopsies or from resected tissue. Liver tissue was suspended in RPMI 1640 (Applied Biosciences) and macerated in a Petri dish. The cell suspension was then passed through a 70-μm filter, and intrahepatic lymphocytes were isolated.
Separation of PBMCs and detection of CD28 and intracellular CD3ζ.
PBMCs were isolated from fresh heparinized blood by Ficoll-Hypaque density gradient centrifugation and resuspended in RPMI 1640 and 10% heat-inactivated FBS (Invitrogen). PBMCs were then plated (2 × 105 cells/well) into a 96-well round bottomed plate and incubated at 4°C for 20 min with saturating concentrations of anti-CD3 PerCP-Cy5.5 mAb (BD Biosciences), anti-CD8–APC mAb (BD Biosciences), and anti-CD28-FITC mAb. After an additional wash in PBS, cells were fixed and permeabilized according to the manufacturer's protocol with Cytoperm/Cytofix for 30 min at 4°C, after which intracellular staining was performed using anti-TCRζ-PE mAb (Coulter Immunotech) or its corresponding isotype control, IgG1-PE mAb in PBS containing 0.1% saponin to permeabilize the cells. Washed cells, resuspended in a solution of PBS, 0.1% formaldehyde, and 10% FBS, were acquired on a FACSCalibur (BD Biosciences) using Cell Quest Software, and data files were analyzed using FlowJo software (Tree Star, Inc.).
Detection of IFN-γ/IL-2/TNF-α production.
PBMCs were incubated at 37°C for 16 h in the presence of either anti-CD3mAb and -CD28mAb (BD Biosciences; reagents that act via the TCR) or phorbol myristic acetate (PMA at 3 ng/ml) and ionomycin (100 ng/ml; TCR-bypassing reagents) or medium alone as a negative control. 10 μg/ml Brefeldin A (Sigma-Aldrich) was added 1 h into the incubation time. PBMCs were then washed in PBS, stained with anti-CD8–Cychrome (PE-Cy5) mAb, and permeabilized and fixed with Cytoperm/Cytofix as detailed above. After a further wash in PBS, cells were stained with anti-IFN-γ-PE mAb (R&D Systems) and anti-IL-2-FITC mAb (R&D Systems) together or anti-TNF-α PE mAb alone in PBS containing 0.1% saponin at 4°C for 30 min. After two additional washes, cells were fixed and acquired immediately on a FACSCalibur flow cytometer using Cell Quest Software.
CD107 degranulation assay.
PBMCs were stimulated for 16 h at 37°C in the presence of anti-CD3 and -CD28 in addition to anti-CD107a FITC. Monensin was added 1 h into the incubation time. Cells were then surface stained with anti-CD8-Cychrome (PE-Cy5; BD biosciences) and acquired on the FACSCalibur Cytometer.
Stimulation with synthetic viral peptides.
The following peptides representing HLA-A2–restricted viral epitopes were used: CMV pp65 (NLVPMVATV), EBV BMLF1 (GLCTLVAML), influenza matrix 1 (GILGFVFTL), HBV C18-27 (FLPSDFFPSV), and HBV envelope pool (FLLTRILTI, WLSLLVPFV, LLVPFVQWFV, and GLSPTVWLSV). PBMCs were stimulated with 10 μM peptide for 12 h and incubated at 37°C in the presence of Brefeldin A (which was added 1 h into the incubation).
Detection of CD3ζ after culture in l-arginine free medium.
PBMCs were plated in a 96-well round bottomed plate and incubated at 37°C for 6 d in either l-arginine–depleted medium (GRH Biosciences) or l-arginine–free medium supplemented with l-arginine (at 0.2 g/liter; Sigma-Aldrich). CD3ζ expression in CD8 T cells was then measured using on day 6.
CFSE proliferation assay.
PBMCs were washed 3 times in PBS, resuspended at 5 × 106 ml in PBS and incubated for 10 min at 37°C with 0.5 μM CFSE (Invitrogen). An equal volume of FBS was added to quench the reaction, and cells were subsequently washed twice in medium and 10% FBS, and then cultured with anti-CD3 mAb and anti-CD28 mAb. Cells were harvested at day 6 and stained with anti-CD8–Cychrome (PE-Cy5). Cells were then washed and resuspended in PBS with 0.1% formaldehyde and 1% FBS and acquired on a FACSCalibur. To determine proliferation of HBV-specific CD8 T cells, PBMCs were stained for CFSE, and then stimulated with HBV c18-27 peptide, and on day 7, HBV-specific CD8 were detected by costaining with anti-CD8-Cychrome (PE-Cy5) and an HLA-A2/c18-27-specific tetramer (PE; provided by A. Turner and P. Klenerman, Oxford University, Oxford, England, UK). These latter experiments were done in l-arginine–free medium, with or without the addition of l-arginine at the range of concentrations indicated.
Transfection of lymphocytes with chimeric receptor.
PBMCs were transfected with a human CD33-specific chimeric receptor with intracellular CD28 in series with CD3ζ using the AMAXA electroporation protocol as previously described (30). In brief, 1–3 μg of plasmid DNA was added to 5 × 106 cells, and resuspended in 100 μl of nucleofactor solution for T cells. Cells were then immediately electroporated in the AMAXA device with program U-13 (Amaxa Biosystems), and then transferred into RPMI complete medium supplemented with 10% FBS at 37°C.
Detection of IL-2 production by ELISA.
Transfected cells were rested overnight at 37°C, after which 2 × 105 cells were seeded into appropriate wells in a 96-well flat bottomed plate precoated with either 5 μg/ml CD33 antigen, 1 μg/ml anti-CD3, both anti-CD3 and CD33 antigen, or neither as a negative control. Soluble anti-CD28 was added to the appropriate wells (5 μg/ml). Cells were then left in the incubator at 37°C for 48 h, after which supernatants were harvested. Enzyme-linked immunosorbent assays were then conducted in duplicate using the supernatants collected, according to the manufacturer's instructions (R&D Systems).
Measurement of arginase activity and arginine levels in serum and liver tissue homogenates.
Liver tissue was suspended in a TRIS/HCL buffer containing a protease inhibitor cocktail (Sigma-Aldrich) and PMSF, and manually macerated with a glass mortar and pestle. The suspension was then centrifuged, and the clear homogenate was collected leaving the precipitate behind. 20 μl liver homogenate or serum was then combined with 20 μl MnCl2 (10 mM), 150 μl Tris Buffer (pH 7.4, 50 mM), and 100 μl of l-arginine (50 mM; Sigma-Aldrich) for 30 min at 37°C. Arginine hydrolysis was stopped with 0.5 ml Tungstic acid solution (150 mM) containing concentrated hydrochloric and sulphuric acid (100 mM each). Unwanted particulate matter was pelleted by centrifuging at 5,000 RPM for 10 min at 4°C. 200 μl of clear solution was used for detection of urea on a COBAS INTEGRA 400 using a UREAL kit assay (Roche). To subtract background serum urea levels, each experiment was duplicated substituting the l-arginine substrate with 100 μl water. 1 U of arginase activity is defined as the amount of enzyme required to catalyze formation of 1 μM urea per minute. To avoid any bias in arginase activity attributable to baseline differences in protein content of the liver sections, protein content for each sample was determined by the Biurret test. This allowed arginase activity to be standardized against protein content, expressed as IU/liter serum or IU/mg protein.
l-arginine levels were determined by electrospray mass spectrometry. Samples (50 μl) were deproteinized with acetonitrile, chromatographed (acetonitrile/water, 1:1, with 0.025% formic acid) on a Teicoplanin guard column 10 mm × 2.1 mm ID (Chirobiotic T; ASTEC Ltd.), and analyzed using a SCIEX API4000 (Applied Biosystems) in positive ion multiple reaction monitoring mode.
Statistical analysis.
Statistical significance was calculated in all cases by the nonparametric Mann-Whitney U test. A P value of <0.05 was deemed significant.
Acknowledgments
We thank the staff and patients at the Mortimer Market Centre, University College London, Royal Free Hospital Centre for Hepatology, and Addenbrooke's Hospital, Cambridge for samples. We are grateful to R. Neil Dalton and Charles Turner (WellChild Laboratory, Evelina Children's Hospital at St Thomas' Hospital) for arginine quantitation and to Andrew Copas (Mortimer Market Centre and Medical Research Council Clinical Trials Unit) for statistical advice.
This work was funded by the Medical Research Council (PhD studentship to A. Das and Clinician Scientist Award to M.K. Maini).
The authors have no conflicting financial interests.
Abbreviations used: ALT, alanine aminotransferase; CHB, chronic HBV infection; HBV, hepatitis B virus; MDSC, myeloid-derived suppressor cell.
References
- 1.Thimme, R., S. Wieland, C. Steiger, J. Ghrayeb, K.A. Reimann, R.H. Purcell, and F.V. Chisari. 2003. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 77:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chisari, F.V. 1997. Cytotoxic T cells and viral hepatitis. J. Clin. Invest. 99:1472–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maini, M.K., C. Boni, C.K. Lee, J.R. Larrubia, S. Reignat, G.S. Ogg, A.S. King, J. Herberg, R. Gilson, A. Alisa, et al. 2000. The role of virus-specific CD8+ cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 191:1269–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reignat, S., G.J. Webster, D. Brown, G.S. Ogg, A. King, S.L. Seneviratne, G. Dusheiko, R. Williams, M.K. Maini, and A. Bertoletti. 2002. Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J. Exp. Med. 195:1089–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertoletti, A., and M.K. Maini. 2000. Protection or damage: a dual role for the virus-specific cytotoxic T lymphocyte response in hepatitis B and C infection? Curr. Opin. Immunol. 12:403–408. [DOI] [PubMed] [Google Scholar]
- 6.Tsuzaka, K., I. Fukuhara, Y. Setoyama, K. Yoshimoto, K. Suzuki, T. Abe, and T. Takeuchi. 2003. TCR zeta mRNA with an alternatively spliced 3′-untranslated region detected in systemic lupus erythematosus patients leads to the down-regulation of TCR zeta and TCR/CD3 complex. J. Immunol. 171:2496–2503. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan, S., J.G. Kiang, C.U. Fisher, M.P. Nambiar, H.T. Nguyen, V.C. Kyttaris, B. Chowdhury, V. Rus, and G.C. Tsokos. 2005. Increased caspase-3 expression and activity contribute to reduced CD3zeta expression in systemic lupus erythematosus T cells. J. Immunol. 175:3417–3423. [DOI] [PubMed] [Google Scholar]
- 8.Nakagomi, H., M. Petersson, I. Magnusson, C. Juhlin, M. Matsuda, H. Mellstedt, J.L. Taupin, E. Vivier, P. Anderson, and R. Kiessling. 1993. Decreased expression of the signal-transducing zeta chains in tumor-infiltrating T-cells and NK cells of patients with colorectal carcinoma. Cancer Res. 53:5610–5612. [PubMed] [Google Scholar]
- 9.Trimble, L.A., and J. Lieberman. 1998. Circulating CD8 T lymphocytes in human immunodeficiency virus-infected individuals have impaired function and downmodulate CD3 zeta, the signaling chain of the T-cell receptor complex. Blood. 91:585–594. [PubMed] [Google Scholar]
- 10.Trimble, L.A., P. Shankar, M. Patterson, J.P. Daily, and J. Lieberman. 2000. Human immunodeficiency virus-specific circulating CD8 T lymphocytes have down-modulated CD3zeta and CD28, key signaling molecules for T-cell activation. J. Virol. 74:7320–7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bronstein-Sitton, N., L. Cohen-Daniel, I. Vaknin, A.V. Ezernitchi, B. Leshem, A. Halabi, Y. Houri-Hadad, E. Greenbaum, Z. Zakay-Rones, L. Shapira, and M. Baniyash. 2003. Sustained exposure to bacterial antigen induces interferon-gamma-dependent T cell receptor zeta down-regulation and impaired T cell function. Nat. Immunol. 4:957–964. [DOI] [PubMed] [Google Scholar]
- 12.Baniyash, M. 2004. TCR zeta-chain downregulation: curtailing an excessive inflammatory immune response. Nat. Rev. Immunol. 4:675–687. [DOI] [PubMed] [Google Scholar]
- 13.Sussman, J.J., J.S. Bonifacino, J. Lippincott-Schwartz, A.M. Weissman, T. Saito, R.D. Klausner, and J.D. Ashwell. 1988. Failure to synthesize the T cell CD3-zeta chain: structure and function of a partial T cell receptor complex. Cell. 52:85–95. [DOI] [PubMed] [Google Scholar]
- 14.Pitcher, L.A., and N.S. van Oers. 2003. T-cell receptor signal transmission: who gives an ITAM? Trends Immunol. 24:554–560. [DOI] [PubMed] [Google Scholar]
- 15.Trimble, L.A., L.W. Kam, R.S. Friedman, Z. Xu, and J. Lieberman. 2000. CD3zeta and CD28 down-modulation on CD8 T cells during viral infection. Blood. 96:1021–1029. [PubMed] [Google Scholar]
- 16.Kaech, S.M., E.J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2:251–262. [DOI] [PubMed] [Google Scholar]
- 17.Wherry, E.J., J.N. Blattman, K. Murali-Krishna, R. Van Der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmerli, S.C., A. Harari, C. Cellerai, F. Vallelian, P.A. Bart, and G. Pantaleo. 2005. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. USA. 102:7239–7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crispe, I.N. 2003. Hepatic T cells and liver tolerance. Nat. Rev. Immunol. 3:51–62. [DOI] [PubMed] [Google Scholar]
- 20.Bronte, V., and P. Zanovello. 2005. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 5:641–654. [DOI] [PubMed] [Google Scholar]
- 21.Frumento, G., R. Rotondo, M. Tonetti, G. Damonte, U. Benatti, and G.B. Ferrara. 2002. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 196:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fallarino, F., U. Grohmann, S. You, B.C. McGrath, D.R. Cavener, C. Vacca, C. Orabona, R. Bianchi, M.L. Belladonna, C. Volpi, et al. 2006. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J. Immunol. 176:6752–6761. [DOI] [PubMed] [Google Scholar]
- 23.Hamann, D., P.A. Baars, M.H. Rep, B. Hooibrink, S.R. Kerkhof-Garde, M.R. Klein, and R.A. van Lier. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186:1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doherty, D.G., S. Norris, L. Madrigal-Estebas, G. McEntee, O. Traynor, J.E. Hegarty, and C. O'Farrelly. 1999. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J. Immunol. 163:2314–2321. [PubMed] [Google Scholar]
- 25.Chang, J.J., F. Wightman, A. Bartholomeusz, A. Ayres, S.J. Kent, J. Sasadeusz, and S.R. Lewin. 2005. Reduced hepatitis B virus (HBV)-specific CD4+ T-cell responses in human immunodeficiency virus type 1-HBV-coinfected individuals receiving HBV-active antiretroviral therapy. J. Virol. 79:3038–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boni, C., P. Fisicaro, C. Valdatta, B. Amadei, P. Di Vincenzo, T. Giuberti, D. Laccabue, A. Zerbini, A. Cavalli, G. Missale, et al. 2007. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 81:4215–4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almanzar, G., S. Schwaiger, B. Jenewein, M. Keller, D. Herndler-Brandstetter, R. Wurzner, D. Schonitzer, and B. Grubeck-Loebenstein. 2005. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J. Virol. 79:3675–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang, Z., C.L. Gorman, A.C. Vermi, C. Monaco, A. Foey, S. Owen, P. Amjadi, A. Vallance, C. McClinton, F. Marelli-Berg, et al. 2007. TCRzetadim lymphocytes define populations of circulating effector cells that migrate to inflamed tissues. Blood. 109:4328–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krymskaya, L., W.H. Lee, L. Zhong, and C.P. Liu. 2005. Polarized development of memory cell-like IFN-gamma-producing cells in the absence of TCR zeta-chain. J. Immunol. 174:1188–1195. [DOI] [PubMed] [Google Scholar]
- 30.Finney, H.M., A.N. Akbar, and A.D. Lawson. 2004. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCRzeta chain. J. Immunol. 172:104–113. [DOI] [PubMed] [Google Scholar]
- 31.Plunkett, F.J., O. Franzese, H.M. Finney, J.M. Fletcher, L.L. Belaramani, M. Salmon, I. Dokal, D. Webster, A.D. Lawson, and A.N. Akbar. 2007. The loss of telomerase activity in highly differentiated CD8+CD28-CD27- T cells is associated with decreased Akt (Ser473) phosphorylation. J. Immunol. 178:7710–7719. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez, P.C., A.H. Zea, K.S. Culotta, J. Zabaleta, J.B. Ochoa, and A.C. Ochoa. 2002. Regulation of T cell receptor CD3zeta chain expression by L-arginine. J. Biol. Chem. 277:21123–21129. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez, P.C., D.G. Quiceno, and A.C. Ochoa. 2007. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 109:1568–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roth, E., R. Steininger, S. Winkler, F. Langle, T. Grunberger, R. Fugger, and F. Muhlbacher. 1994. L-Arginine deficiency after liver transplantation as an effect of arginase efflux from the graft. Influence on nitric oxide metabolism. Transplantation. 57:665–669. [DOI] [PubMed] [Google Scholar]
- 35.Modolell, M., I.M. Corraliza, F. Link, G. Soler, and K. Eichmann. 1995. Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow-derived macrophages by TH1 and TH2 cytokines. Eur. J. Immunol. 25:1101–1104. [DOI] [PubMed] [Google Scholar]
- 36.Kropf, P., D. Baud, S.E. Marshall, M. Munder, A. Mosley, J.M. Fuentes, C.R. Bangham, G.P. Taylor, S. Herath, B.S. Choi, et al. 2007. Arginase activity mediates reversible T cell hyporesponsiveness in human pregnancy. Eur. J. Immunol. 37:935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ando, K., T. Moriyama, L.G. Guidotti, S. Wirth, R.D. Schreiber, H.J. Schlicht, S.N. Huang, and F.V. Chisari. 1993. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J. Exp. Med. 178:1541–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webster, G.J., S. Reignat, D. Brown, G.S. Ogg, L. Jones, S.L. Seneviratne, R. Williams, G. Dusheiko, and A. Bertoletti. 2004. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J. Virol. 78:5707–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopes, A.R., P. Kellam, A. Das, C. Dunn, A. Kwan, J. Turner, D. Peppa, R.J. Gilson, A. Gehring, A. Bertoletti, and M.K. Maini. 2008. Bim-mediated deletion of antigen-specific CD8 T cells in patients unable to control HBV infection. J. Clin. Invest. 118:1835–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karrer, U., S. Sierro, M. Wagner, A. Oxenius, H. Hengel, U.H. Koszinowski, R.E. Phillips, and P. Klenerman. 2003. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J. Immunol. 170:2022–2029. [DOI] [PubMed] [Google Scholar]
- 41.Adams, D.H., S.G. Hubscher, J.M. Neuberger, P. McMaster, E. Elias, and J.A. Buckels. 1991. Reduced incidence of rejection in patients undergoing liver transplantation for chronic hepatitis B. Transplant. Proc. 23:1436–1437. [PubMed] [Google Scholar]
- 42.Farges, O., F. Saliba, H. Farhamant, D. Samuel, A. Bismuth, M. Reynes, and H. Bismuth. 1996. Incidence of rejection and infection after liver transplantation as a function of the primary disease: possible influence of alcohol and polyclonal immunoglobulins. Hepatology. 23:240–248. [DOI] [PubMed] [Google Scholar]
- 43.Guidotti, L.G., T. Ishikawa, M.V. Hobbs, B. Matzke, R. Schreiber, and F.V. Chisari. 1996. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 4:25–36. [DOI] [PubMed] [Google Scholar]
- 44.Toyonaga, T., O. Hino, S. Sugai, S. Wakasugi, K. Abe, M. Shichiri, and K. Yamamura. 1994. Chronic active hepatitis in transgenic mice expressing interferon-gamma in the liver. Proc. Natl. Acad. Sci. USA. 91:614–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morita, M., Y. Watanabe, and T. Akaike. 1995. Protective effect of hepatocyte growth factor on interferon-gamma-induced cytotoxicity in mouse hepatocytes. Hepatology. 21:1585–1593. [PubMed] [Google Scholar]
- 46.Maeda, S., L. Chang, Z.W. Li, J.L. Luo, H. Leffert, and M. Karin. 2003. IKKbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFalpha. Immunity. 19:725–737. [DOI] [PubMed] [Google Scholar]
- 47.Su, F., and R.J. Schneider. 1997. Hepatitis B virus HBx protein sensitizes cells to apoptotic killing by tumor necrosis factor alpha. Proc. Natl. Acad. Sci. USA. 94:8744–8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kakimi, K., T.E. Lane, S. Wieland, V.C. Asensio, I.L. Campbell, F.V. Chisari, and L.G. Guidotti. 2001. Blocking chemokine responsive to γ-2/interferon (IFN)-γ inducible protein and monokine induced by IFN-γ a activity in vivo reduces the pathogenetic but not the antiviral potential of hepatitis B virus–specific cytotoxic T lymphocytes. J. Exp. Med. 194:1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shields, P.L., C.M. Morland, M. Salmon, S. Qin, S.G. Hubscher, and D.H. Adams. 1999. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J. Immunol. 163:6236–6243. [PubMed] [Google Scholar]
- 50.Moskophidis, D., and D. Kioussis. 1998. Contribution of virus-specific CD8+ cytotoxic T cells to virus clearance or pathologic manifestations of influenza virus infection in a T cell receptor transgenic mouse model. J. Exp. Med. 188:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lang, K.S., P. Georgiev, M. Recher, A.A. Navarini, A. Bergthaler, M. Heikenwalder, N.L. Harris, T. Junt, B. Odermatt, P.A. Clavien, et al. 2006. Immunoprivileged status of the liver is controlled by Toll-like receptor 3 signaling. J. Clin. Invest. 116:2456–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poli, G., and C. Bordignon. 2003. Unplugging the T cell receptor. Nat. Immunol. 4:943–944. [DOI] [PubMed] [Google Scholar]
- 53.Ezernitchi, A.V., I. Vaknin, L. Cohen-Daniel, O. Levy, E. Manaster, A. Halabi, E. Pikarsky, L. Shapira, and M. Baniyash. 2006. TCR zeta down-regulation under chronic inflammation is mediated by myeloid suppressor cells differentially distributed between various lymphatic organs. J. Immunol. 177:4763–4772. [DOI] [PubMed] [Google Scholar]
- 54.Sitia, G., M. Isogawa, K. Kakimi, S.F. Wieland, F.V. Chisari, and L.G. Guidotti. 2002. Depletion of neutrophils blocks the recruitment of antigen-nonspecific cells into the liver without affecting the antiviral activity of hepatitis B virus-specific cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA. 99:13717–13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sitia, G., M. Isogawa, M. Iannacone, I.L. Campbell, F.V. Chisari, and L.G. Guidotti. 2004. MMPs are required for recruitment of antigen-nonspecific mononuclear cells into the liver by CTLs. J. Clin. Invest. 113:1158–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodriguez, P.C., A.H. Zea, J. DeSalvo, K.S. Culotta, J. Zabaleta, D.G. Quiceno, J.B. Ochoa, and A.C. Ochoa. 2003. L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J. Immunol. 171:1232–1239. [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez, P.C., C.P. Hernandez, D. Quiceno, S.M. Dubinett, J. Zabaleta, J.B. Ochoa, J. Gilbert, and A.C. Ochoa. 2005. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J. Exp. Med. 202:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dunn, C., M. Brunetto, G. Reynolds, T. Christophides, P.T. Kennedy, P. Lampertico, A. Das, A.R. Lopes, P. Borrow, K. Williams, et al. 2007. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell–mediated liver damage. J. Exp. Med. 204:667–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lucas, M., A.L. Vargas-Cuero, G.M. Lauer, E. Barnes, C.B. Willberg, N. Semmo, B.D. Walker, R. Phillips, and P. Klenerman. 2004. Pervasive influence of hepatitis C virus on the phenotype of antiviral CD8+ T cells. J. Immunol. 172:1744–1753. [DOI] [PubMed] [Google Scholar]
- 60.Wherry, E.J., J.N. Blattman, and R. Ahmed. 2005. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J. Virol. 79:8960–8968. [DOI] [PMC free article] [PubMed] [Google Scholar]