Abstract
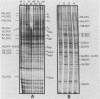
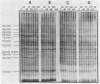
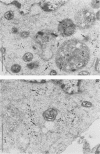
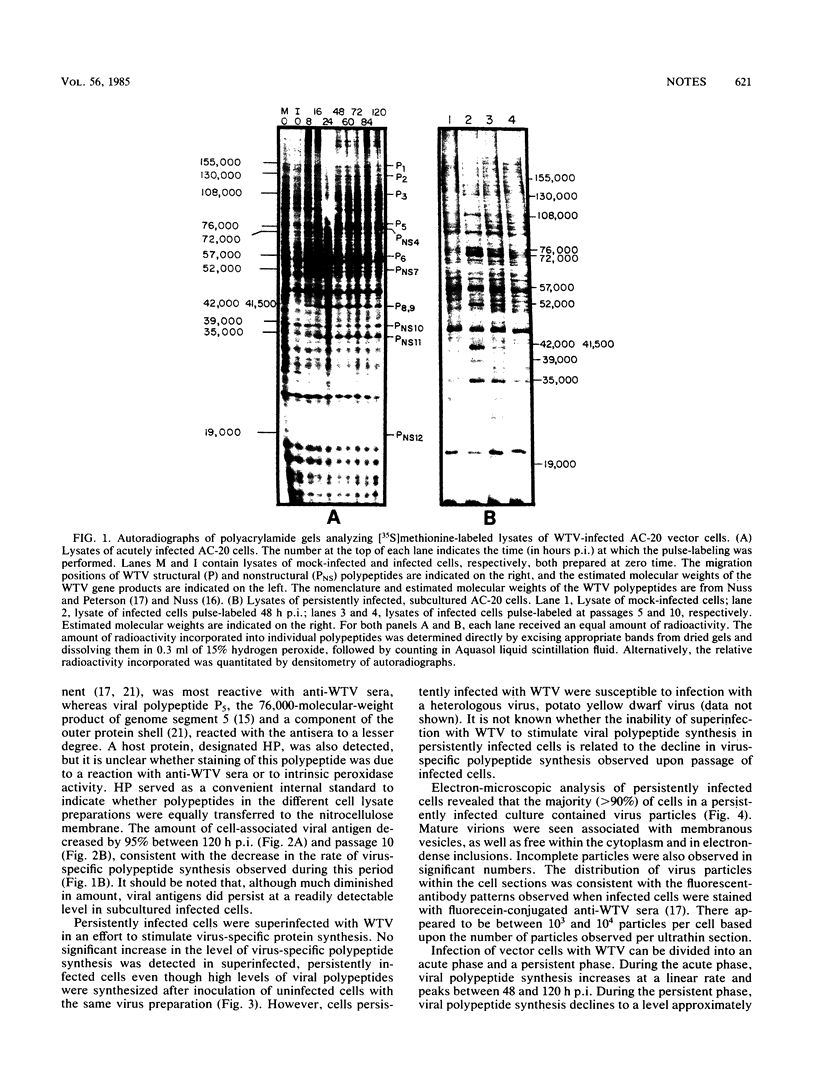
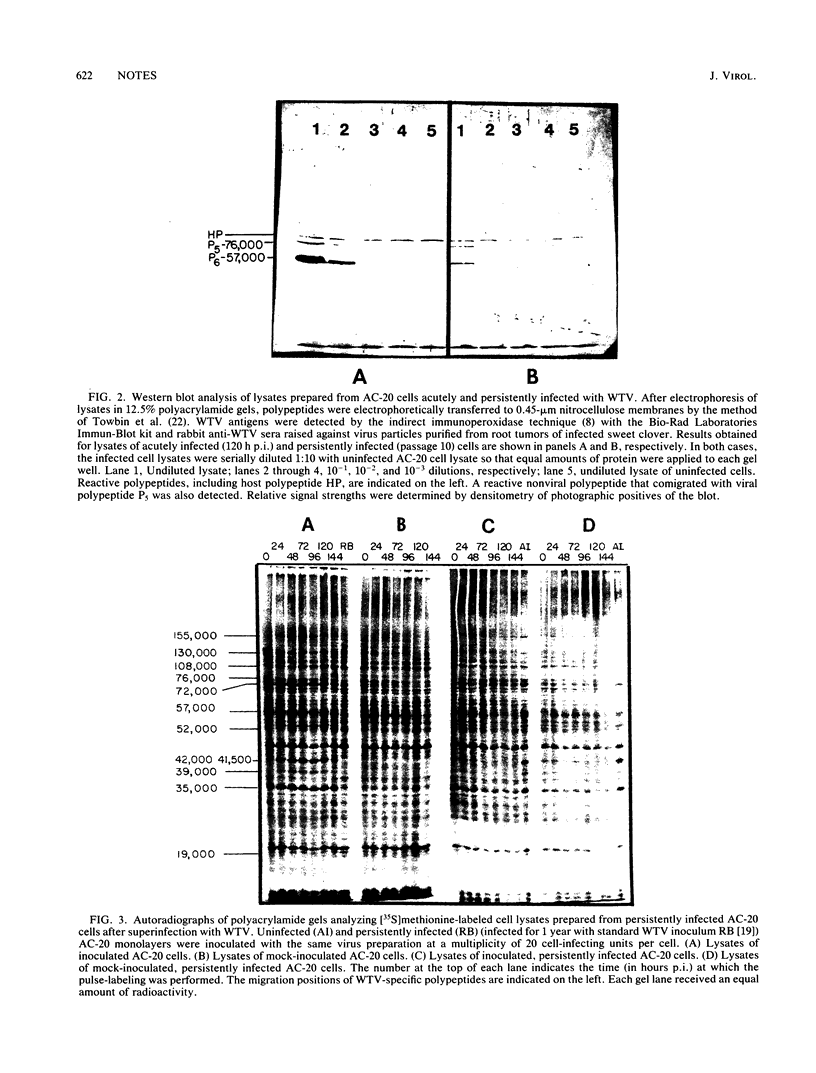
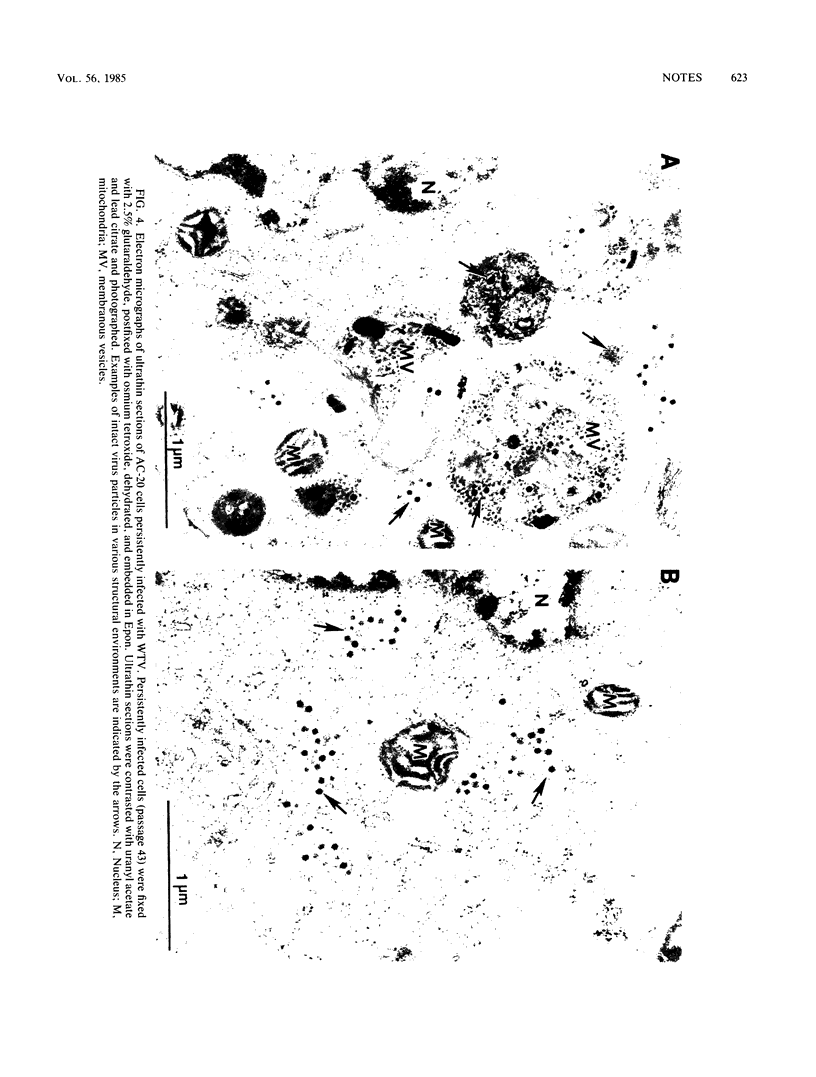
Inoculation of the leafhopper cell line AC-20 with wound tumor virus resulted in a productive noncytopathic infection with no detectable alteration of cellular protein synthesis. Virus-specific polypeptide synthesis, detectable by 8 h postinoculation, increased in a linear fashion, reaching a peak (approximately 10 to 15% of total protein synthesis) by 48 h postinoculation. The rate of viral protein synthesis continued at this level for several days but declined, relative to cellular protein synthesis, as infected cells were passaged. By passage 10, the synthesis of viral polypeptides was reduced to a level approximately 5% of that observed at 48 h postinoculation. Viral protein synthesis was not stimulated by superinfection. Viral antigens and infectious virus persisted in the majority (greater than 90%) of cells in an infected culture even after more than 100 passages. The synthesis of wound tumor virus polypeptides in infected insect vector cells appears to be regulated in a coordinated and selective manner.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asamizu T., Summers D., Motika M. B., Anzola J. V., Nuss D. L. Molecular cloning and characterization of the genome of wound tumor virus: a tumor-inducing plant reovirus. Virology. 1985 Jul 30;144(2):398–409. doi: 10.1016/0042-6822(85)90281-8. [DOI] [PubMed] [Google Scholar]
- Black L. M. Vector cell monolayers and plant viruses. Adv Virus Res. 1979;25:191–271. doi: 10.1016/s0065-3527(08)60571-0. [DOI] [PubMed] [Google Scholar]
- Chiu R. J., Black L. M. Monolayer cultures of insect cell lines and their inoculation with a plant virus. Nature. 1967 Sep 2;215(5105):1076–1078. doi: 10.1038/2151076a0. [DOI] [PubMed] [Google Scholar]
- Chiu R., Reddy D. V., Black L. M. Inoculation and infection of leafhopper tissue cultures with a plant virus. Virology. 1966 Nov;30(3):562–566. doi: 10.1016/0042-6822(66)90131-0. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hirumi H., Granados R. R., Maramorosch K. Electron microscopy of a plant-pathogenic virus in the nervous system of its insect vector. J Virol. 1967 Apr;1(2):430–444. doi: 10.1128/jvi.1.2.430-444.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura I., Black L. M. Some factors affecting infectivity assays of wound-tumor virus on cell monolayers from an insect vector. Virology. 1971 Nov;46(2):266–276. doi: 10.1016/0042-6822(71)90029-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MARAMOROSCH K. Arthropod transmission of plant viruses. Annu Rev Entomol. 1963;8:369–414. doi: 10.1146/annurev.en.08.010163.002101. [DOI] [PubMed] [Google Scholar]
- Nuss D. L. Molecular biology of wound tumor virus. Adv Virus Res. 1984;29:57–93. doi: 10.1016/s0065-3527(08)60405-4. [DOI] [PubMed] [Google Scholar]
- Nuss D. L., Peterson A. J. Expression of wound tumor virus gene products in vivo and in vitro. J Virol. 1980 May;34(2):532–541. doi: 10.1128/jvi.34.2.532-541.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy D. V., Black L. M. Deletion mutations of the genome segments of wound tumor virus. Virology. 1974 Oct;61(2):458–473. doi: 10.1016/0042-6822(74)90282-7. [DOI] [PubMed] [Google Scholar]
- Reddy D. V., Black L. M. Increase of wound tumor virus in leafhoppers as assayed on vector cell monolayers. Virology. 1972 Nov;50(2):412–421. doi: 10.1016/0042-6822(72)90393-5. [DOI] [PubMed] [Google Scholar]
- Reddy D. V., MacLeod R. Polypeptide components of wound tumor virus. Virology. 1976 Apr;70(2):274–282. doi: 10.1016/0042-6822(76)90270-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER D. L. THE VIRAL CARRIER STATE IN ANIMAL CELL CULTURES. Prog Med Virol. 1964;6:111–148. [PubMed] [Google Scholar]