Abstract
General anesthesia (GA) and local anesthesia (LA) evolved on separate tracks. Procedures that could not be performed under LA were typically conducted under GA. Decoding of afferent linkage of peripheral noxious stimuli has provided important understanding that may change the way we traditionally treat surgical pain. In the 1980s, animal studies suggested that preemptive peripheral blocking of painful (nociceptive) stimuli to the central nervous system with regional anesthesia or LA and nonsteroidal analgesics could be beneficial in attenuating postoperative pain. Clinical studies based on this knowledge suggest combining LA with GA, and perhaps non-steroidal analgesics with or without narcotics, to reduce the severity of postoperative pain. General anesthetics can be given in lower minimal alveolar concentration when combined with LA, and recovery characteristics are superior. Increasing evidence suggests that the combined use of GA and LA may reduce the afferent barrage of surgery, and that preemptive analgesia may reduce postoperative pain and should be used in patient care. This article reviews the evidence supporting the combined use of LA or analgesics with GA or sedation to provide improved pain management after surgery.
Keywords: General anesthesia; Local anesthesia; Nerve block; Analgesics, Postoperative pain; Pain
Acute pain is an essential warning signal that alerts the organism to either the presence or the potential of stimuli that cause tissue damage. The neuronal signal for acute pain is initiated by specialized sensory nerve fibers distributed throughout somatic and visceral structures. Unlike other sensory neurons, these nociceptive afferent neurons are specifically “tuned” to respond to high-intensity stimuli. The sensory input encoded by activated nociceptive afferent neurons is sent to the spinal or medullary dorsal horns, which send projection neurons to higher structures in the central nervous system (CNS). The thalamus sends neuronal projections in response to nociceptive signaling to regions of the cerebral cortex (eg, somatosensory cortex), resulting in the complex phenomenon called pain. Sensory and emotional components contribute to the complexity of pain.
Acute pain has had a profound impact on how some medical procedures are performed. Alternatives had to be developed to enable the performance of procedures that produced intolerable levels of pain. Several advances in anesthesiology enabled these procedures to be performed without significant interference from the patient's perception of pain. General anesthesia (GA) served as the main approach to surgical pain control in western medicine for more than 150 years. Despite the introduction and wide use of amide local anesthetics beginning in 1943, GA continued to be the sole agent for performing many painful procedures. The first recommendation for concomitant administration of both regional anesthesia and GA was made by Crile,1 who was in many ways the pioneer of the concept of preemptive analgesia (ie, administering analgesics before the onset of nociceptive stimulus as a means to prevent or reduce subsequent pain). This concept has expanded to include the use of systemically administered analgesic agents as well.
The preemptive use of systemic analgesics and the concomitant use of regional anesthesia during GA may offer potential advantages when compared with using GA alone. One of the possible important advantages of this “combined” technique may be an increase in the safety of the GA. Blocking nociceptive pathways, or reducing the amount of sensory information transmitted to the CNS, reduces the amount of pharmacological agents needed to produce a state of GA and favorably affects its safety. Reducing the amount of drug required to produce the state of GA is also likely to decrease the time needed to recover from the drug-induced CNS depression. Other benefits of this combined technique include a diminution in the subjective severity of postoperative pain, the resultant reduction in the amount or potency of analgesics used in the immediate recovery period, and the reduction of adverse effects these medications may produce. Some critics relate this to the lingering effect of local anesthesia (LA) given pre- or intra-operatively. Even so, this could still be seen as a clinical advantage in postoperative pain management. Last, the use of regional anesthesia may enable some procedures to be performed under sedation instead of GA.
A great deal of research has been conducted to evaluate the hypothesis that preemptive and combined analgesic administration prevents or reduces postoperative pain. The purpose of this article is to provide a scientific evidence base for the rationale of combining the above pain-control regimens as well as a comprehensive review of the studies conducted to test this hypothesis.
Acute Pain, Hyperalgesia, and Allodynia
Acute pain is the result of activating nociceptive pathways in both the peripheral nervous system and the CNS. The origin of most acute pain from surgical stimulation is the mechanical trauma of the local tissue and the subsequent acute inflammatory response. This is in contrast to chronic pain, in which nociceptive signaling remains long after the initial tissue insult and healing of the damaged tissue appears complete. The peripheral terminals of nociceptive sensory neurons express a vast complement of receptors that become activated in the presence of specific ligands (eg, bradykinin, arachidonic acid metabolites) and physical stimuli (eg, high-intensity pressure, noxious heat). Once activated, these receptors facilitate the depolarization of the neuronal membrane via the direct gating of ions through channels that comprise the receptor or the activation of second messenger pathways and the subsequent alterations in the intracellular environment. The resulting action potential is conducted to the central terminals of these neurons located in the spinal and medullary dorsal horns. Excitatory amino acids (EAAs), such as glutamate, are among the neurotransmitters and neuromodulators that mediate the transmission of the signal from the central terminal of the peripheral neuron to neurons within the CNS (ie, projection neurons and local interneurons). These EAAs activate specific receptor classes such as the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, kainate, and N-methyl-D-aspartate (NMDA) ligandgated cation channels. Repeated peripheral nociceptive stimulation will generate changes in the sensitivity of the peripheral sensory neuron (termed activation-dependent plasticity in the nociceptor terminals) or neurons in the spinal and medullary dorsal horn (termed activation-dependent plasticity in the dorsal horn neurons or windup).
Descending Neuronal Pathways for Pain Modulation
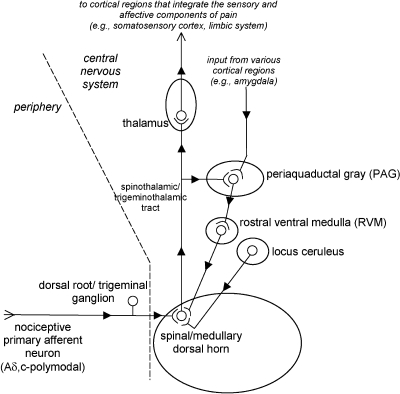
Human psychophysical studies have correlated stimulus intensity with the subject's report of pain intensity. Laboratory studies have demonstrated that responses to nociceptive primary afferent, spinal and medullary dorsal horn, thalamic, and certain cortical neurons all correlate well with the intensity of the noxious stimulus. These results are consistent with the hypothesis that the perception of pain is related to the intensity of the neuronal coding evoked by noxious stimuli. However, reports of pain severity outside the laboratory setting (ie, clinical) are often quite variable among persons with the same apparent injury. An example is the report of diminished pain severity among soldiers with battle wounds compared with elevated reports of pain by civilians with comparable injuries. These observations defy the simplicity of this hypothesis and help define the functional significance of pain modulatory mechanisms (Figure). The discovery of distinct neuronal circuits within the CNS that modulate the perception of pain has opened a new arena for developing analgesic strategies. The characterization of descending neuronal pathways and the mechanisms that modulate the perception of pain in response to seemingly arbitrary manipulations such as the administration of exogenous opioid analgesics or focal electric shock has led to a better understanding of endogenous pain-relieving systems. An understanding of these modulatory pathways and their activation is important, especially when attempting to recruit their beneficial characteristics in clinical situations. The efficacy of opioid analgesics and psychological manipulations such as hypnosis and distraction are among the many examples of how mimicking and recruiting descending neuronal pathways and mechanisms of analgesia can modulate the perception of pain.2
Pain transmission and modulation. Note: Ascending and descending pathways that influence the perception and modulation of pain. After the activation at the peripheral terminals of a select population of nociceptive primary afferent neurons (eg, Aδ and C-polymodal nociceptive afferent neurons), the neuronal signal is transferred to a population of projection neurons in the central nervous system at the dorsal and medullary dorsal horn. The signal is then propagated to the thalamus, which is responsible for routing the signal to cortical regions responsible for integrating the sensory and affective components of pain. Ascending nociceptive signals can also be directly routed to regions within the central nervous system that are responsible for modulating the signal (eg, periaquaductal gray). These centers responsible for signal modulation can also receive input from cortical regions of the brain in response to the perception of pain or stress. Collectively, the various descending modulatory pathways originating from the periaquaductal gray, rostral ventral medulla, and locus ceruleus reduce the amount of signaling that occurs at the spinal and medullary dorsal horn by reducing the amount of neurotransmitters released from the central terminals of nociceptive primary afferent neurons or by increasing the threshold of activation for projection neurons of the spinothalamic and trigeminothalamic tract.
The characterizations of neuronal pathways that are capable of regulating nociceptive transmission have advanced our understanding of how pain perception can be modulated. Producing profound analgesia by electrically stimulating discrete regions of the midbrain of rodents and humans was among the earliest observations to suggest the presence of descending analgesic pathways.3 Electrical stimulation of specific midbrain sites in humans selectively reduced the severe pain experienced by clinical patients. Electrical stimulation of distinct regions in the brainstem, midbrain, subcortex, and cortex has now defined the network of CNS pathways that modulate nociceptive neuronal signaling.
Studying the mechanisms of opioidmediated analgesia within the CNS has also been an important tool in defining the descending neuronal pathways that modulate pain.4 One of the ways that opioids produce analgesia is by decreasing the transmission of nociceptive information from the spinal and medullary dorsal horn, which occurs by distinct mechanisms that depend on the activation of μ, κ, and δ opioid receptors. One of the mechanisms of opioidmediated analgesia at the dorsal horn is to reduce the amount of excitatory neurotransmitters released from the central terminals of nociceptive afferent neurons. Opioids can also reduce nociceptive transmission at the dorsal horn by reducing the responsiveness of postsynaptic nociceptive neurons to excitatory neurotransmitters. The activation of opioid receptors expressed on the membranes of postsynaptic neurons produces a state of hyperpolarization, making these neurons less responsive.
Similar to the experiments in which analgesia results from the electrical stimulation of specific regions in the brain stem and midbrain, the microinjection of opioids into the rostral ventral medulla, the periaquaductal gray, and the amygdala also produce analgesia.4 Interestingly, the analgesic effects of opioids injected into the rostral ventral medulla and periaquaductal gray can be attenuated by the local administration of receptor antagonists selective for serotonin and noradrenergic receptors at the dorsal horn. These findings are consistent with the hypothesis that one of the mechanisms for producing opioidmediated analgesia is indirect, relying on the release of nonopioid neurotransmitters and the activation of nonopioid receptors at the spinal and medullary dorsal horn.
The existence and expression of opioid receptors is related to the function of endogenous opioid peptides (ie, enkephalins, endomorphins, dynorphins, and endorphins), which are capable of modulating pain. Similar to the inhibitory actions of exogenously administered opioids in the CNS, the endogenous opioid peptides also decrease the amount of excitatory neurotransmitter release from the central terminals of nociceptive afferent neurons and reduce the responsiveness of postsynaptic nociceptive neurons to excitatory neurotransmitters at the dorsal horn via activation. These endogenous actions are mediated by the same complement of opioid receptors that are expressed at various spinal and supraspinal locations. Besides recognizing the existence of endogenous neural networks that can reduce pain, it is also important to determine the physiological, psychological, and environmental conditions that regulate their activity. The observation that aversive experimental stimuli (eg, electrical shock, restraint, forced swimming, moderately intense white noise) produce analgesia in laboratory animals was an important step in determining how these modulatory systems are activated.5 Stressful stimuli of biological relevance (eg, exposure to predators) also produce analgesia in laboratory animals.6,7 These examples suggest that fear is a powerful stimulant capable of activating endogenous analgesic pathways in acute pain. Experimental manipulations have further demonstrated that analgesic responses can be decreased in animals by administering drugs that decrease fear (eg, anxiolytic drugs) and can be increased by drugs that increase fear (eg, anxiogenic drugs).4 Certainly, the ability to flee from threatening and potentially harmful situations would be compromised if the guarding and recuperative behaviors induced by pain were allowed to dominate.
It is important to recall the considerable evidence for nonopioid systems in descending pathways that modulate pain.8 Although the analgesia observed with physiological and psychological stress, acupuncture, hypnosis, and the placebo effect have been attributed to descending mechanisms that use opioid receptors, it is often not possible to block this analgesia completely with opioid receptor antagonists, which suggests that nonopioid mechanisms are also involved. Current understanding and characterization of these nonopioid pathways, neurotransmitters, and mechanisms is limited. The inhibitory neurotransmitter gamma-aminobutyric acid (GABA) is strongly implicated in nonopioid mechanisms of pain modulation. GABA can be found in various CNS sites, including those in which nociceptive neural transmission takes place. Presynaptic binding of GABA-to-GABA binding sites reduces the release of neurotransmitters from central terminals of nociceptive primary afferent neurons, and binding at postsynaptic sites reduces the excitability of dorsal horn neurons. Similar to the effect of opioids, the pre- and postsynaptic binding of GABA also attenuates nociceptive neuronal signaling.
Sensitization
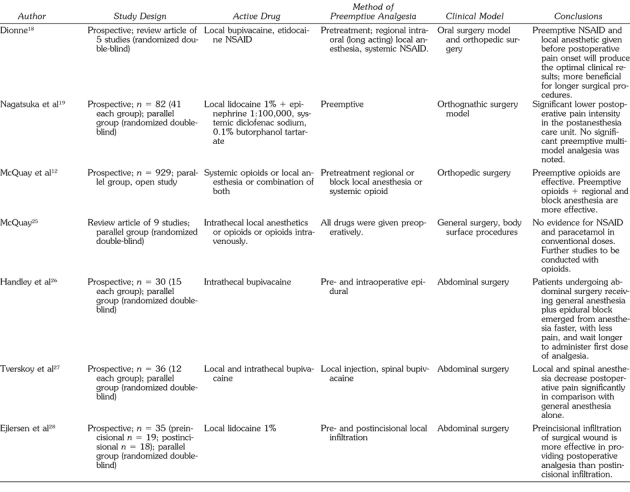
Sensitization is the reversible heightening of peripheral and central neuron excitability. This process is activated by intracellular signal transduction cascades, leading to an increase in synaptic response and reduced inhibition, which yield firing at normally ineffective input.9,10 Sensitization in central pain pathways is triggered by peripheral nociceptor input and results in enhanced responsiveness of pain-transmission neurons. These neurons either require low-intensity peripheral stimuli for activation or continue to fire long after an initiating input.9
In contrast to inflammation, peripheral nerve injury will decrease substance P calcitonin gene-related peptide, VR1 (capsaicin sensitive), SNS/PN39 (responsive to anticonvulsive drugs), and μ opiate receptors9 and will increase brain III sodium channels. Brain III sodium channels may mediate spontaneous activity in injured sensory neurons, whereas decreased μ opiate receptors may play a role in diminished opiate sensitivity. Increase in brain-derived neurotrophic factor is common to both inflammation and peripheral nerve injury.9
It has been suggested that blocking painful input from the site of injury to the CNS with LA prevents changes at the spinal cord level.9 Without this peripheral block, spinal cord changes may sustain or amplify pain long after the original stimulus and, as a result, would involve larger areas of the CNS (dynamic receptive field plasticity). It was also shown that delivering a brief conditioning stimulus to C-fibers (deep muscle conditioning) initiated a change at the spinal cord level, and this processing of sensory signals persists up to 1 hour after the administration of original stimulus.9
Receptive field expansion was shown to occur when dorsal horn neurons respond to cutaneous test stimuli beyond the original receptive field after peripheral injury.11 Increase in receptive field size is a result of central sensitization (windup), modulation of second-order neurons such as wide dynamic range and nociceptive specific, and repeated C-fiber stimulus.10 These phenomena result in afferent input from dermatomes that did not previously activate the neurons, which now evoke a prominent response. Moreover, low-threshold tactile stimulation also becomes increasingly effective.9 As acute pain progresses toward chronicity,10 plasticity of input-output relationship occurs, manifesting modulation in the dorsal spinal cord and causing either hyperalgesia or allodynia. The basic mechanism of the above phenomena may relate to EAAs such as glutamate and aspartate that increase the excitability of the NMDA receptor site and are responsible for the dynamic receptive field plasticity.9 Other EAA receptors subtypes such as non-NMDA (α-amino-3-hydroxy-5-methyl-4-isoxazo-lepropionic acid or kainate and metabotropic receptors) found postsynaptically on the spinal dorsal horn cells are also involved in the process.10
In other studies of receptive field expansion, substance P and calcitonin gene-related peptide are also found to be involved in these phenomena.10 An analgesic given intrathecally before exposure to a noxious peripheral stimulus is more effective than the same dose given after the same stimulus. Preventing the hyperexcitability aroused in the spinal cord by the nociceptive event may reduce the subsequent analgesic requirement.
These studies provide evidence related to the mechanisms by which the use of LA and regional anesthesia minimizes a chain of events in the CNS after painful peripheral stimuli.
Clinical Studies Demonstrating the Efficacy of Preemptive Analgesia
Systemic Preemptive Analgesics
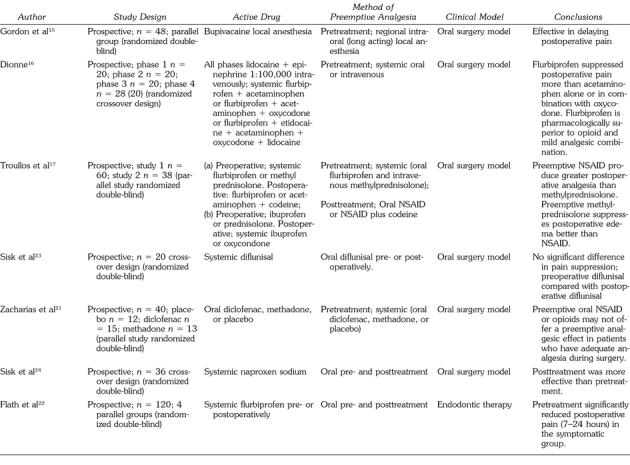
McQuay et al12 used preemptive opioid analgesics, LA, and GA in orthopedic patients and found a delayed request for postoperative analgesics. When preoperative opioids were used, the first request for postoperative analgesia was delayed by 3 hours; when a combination of preemptive LA and morphine was used, an even greater delay (6 hours) was experienced, suggesting an additive effect.
The wisdom tooth extraction model, in which patients undergo surgical removal of impacted wisdom teeth, provides a paradigm of surgical pain, frequently with bilateral similarity, in otherwise healthy individuals. This model was used in a number of studies investigating systemic preemptive analgesics. Ibuprofen and flubiprofen, when administered preoperatively, suppressed postoperative pain better than did placebo, acetaminophen, or acetaminophen plus oxycodone administered preoperatively.13,14
Plasma levels of β-endorphin (a marker for the activation of nociceptive pathways) have been used to assess the effect of preemptive techniques in the oral surgery model. The β-endorphin plasma levels were similar among groups at baseline and at induction of GA and intubation, they increased nearly threefold during surgery in the placebo group not given long-acting nerve blocks, and they remained elevated for 1 hour postoperatively.15 In contrast, negligible changes in plasma β-endorphin levels were observed in the bupivacaine group. This study also demonstrated that postoperative scores for pain and unpleasantness at 48 hours post-treatment were significantly higher in the placebo group when compared with the group who received block anesthesia with bupivacaine. The placebo group self-administered more codeine for postoperative pain 24–48 hours after tooth extraction.
In another study using the wisdom tooth extraction model, pre- and postoperative administration of flurbiprofen led to superior pain relief when compared with acetaminophen alone or in combination with oxycodone.16 Preemptive and postoperative ibuprofen and flurbiprofen also provided greater initial analgesia than did steroids when tested in a similar study.17 A literature review supports the hypothesis that preoperative use of analgesic drugs attenuates the development of pain and may be more beneficial for longer surgical procedures.18 In an orthognathic surgical model, Nagatsuka et al19 found that patients preemptively receiving κ receptor antagonists, butorphanol and diclofenac sodium, reported significant lower postoperative pain intensity than did the control group. However, multimodal analgesia was not observed.
After testing long-acting local anesthetics injected regionally in the oral surgery model, Gordon et al15 stated that preemptive bupivacaine is effective in delaying postoperative pain. In a study largely designed to detect preemptive bupivacaine cardiovascular toxicity,20 the control group scored higher Visual Analog Scale (VAS) median scores up to 16 hours postoperatively when compared with tested groups. However, no significant difference in VAS scores was found at any time between the groups. The authors also concluded that VAS median scores of their control group were consistently higher than in the tested groups, and no significant difference was found in the VAS scores at any time. These conflicting results can be attributed to dexamethsone, which might have contaminated the experimental model, and the bupivacaine regional nerve block anesthesia given to the control group at the end of the procedure.
Another recent study concluded that preoperative nonsteroidal anti-inflammatory drugs (NSAIDs) and opiates may not offer a preemptive analgesic effect in patients who have had adequate analgesia during surgery.21 Three other studies also did not reveal any significant preemptive effects on pain intensity.22–24 However, McQuay25 noted that these last 3 studies lacked the sensitivity and adequate size needed to differentiate between pre- and postoperative dosing.
LA and Regional Anesthesia Given in Combination With GA
The effect of the combined use of GA with LA and regional anesthesia on postoperative pain in various surgical procedures has been examined in a number of studies. When the recovery characteristics associated with combined GA and epidural analgesia were compared with GA alone in patients after abdominal surgery, it was found that patients receiving GA and epidural emerged from the anesthesia faster and with less pain.26 These patients had better psychomotor function at 2 hours and were less drowsy at 4 hours postoperatively.
Another study compared postoperative pain characteristics in 3 different groups: (a) patients receiving GA alone; (b) patients receiving GA and LA infiltration; and (c) patients receiving GA combined with spinal anesthesia.27 A significant reduction in postoperative hyperalgesia in the second and third groups was reported, and regional anesthesia combined with GA was found to reduce postoperative pain. Ejlersen et al28 published similar results showing significant delay in analgesic remedication in a group receiving preincision local infiltration for hemorrhoidectomy.
Safety in Combined Anesthesia
The potential hazard from using LA containing epinephrine with GA or bupivacaine20 must be counterbalanced by the potential benefits, as demonstrated in a recent study by Mamiya et al.29 The use of block LA can minimize the required depth of GA while still keeping the patient unconscious without motor interference, resulting in safer management of patients with cardiovascular disease or elderly patients requiring cardiovascular stability during surgery. In the Mamiya et al study, patients who did not receive the LA block demonstrated a significant elevation in systolic blood pressure, plasma norepinephrine, and heart rate under GA. The authors concluded that the combination of GA with block analgesia promotes safer anesthesia for patients.
A large-scale epidemiological study (n = 85,412) demonstrated only rare, minor complications combining GA with regional anesthesia.30 An overall complication rate of 0.9/1000 and a rate of 1.5/1000 in central blocks established the safety of combined anesthesia in children. These results provide strong support for the safe use of peripheral and even central blocks together with GA.
The safety and acceptability of surgically removing bilateral impacted wisdom teeth by using a bilateral LA was examined.31 Two of 45 patients in the 1-stage group where all 4 wisdom teeth were extracted at 1 visit desaturated in the recovery process (SaO2 < 90), which indicates that 4-quadrant LA persists after GA has ceased and could interfere with airway patency in postoperative recovery.
Hempenstall et al32 compared the effect of intravenous conscious sedation versus GA on a number of stress variables in the oral surgery model. They reported that in the intravenous sedation group, only the levels of growth hormones and prolactin rose significantly. On the other hand, the GA group showed a significant rise in heart rate; systolic blood pressure; mean arterial pressure; and levels of plasma adrenaline, adrenocorticotropic hormone, cortisol, prolactin, and plasma glucose. This study demonstrates that intravenous conscious sedation, when compared with GA, is associated with fewer stress responses in patients undergoing oral surgery.
Discussion
The concept of preemptive analgesia is derived from basic research in neuroscience. The clinical efforts to apply this hypothesis used basically 3 pharmacologic methods of preemptive analgesia or a combination of them: (a) LA peripherally or centrally injected (local infiltration regional and intrathecal nerve blocks), (b) preemptive systemic NSAIDs, acetaminophen, or opioids, and (c) intrathecal opioids. The testing clinical models in the reviewed studies can be categorized into 3 groups: (a) orofacial model (including oral surgery and endodontics), (b) orthopedic surgery (including orthognatic surgery), and (c) general and abdominal surgery. The Table summarizes the clinical articles dealing with preemptive analgesia.
Concise Review of Articles (According to Clinical Model)
Concise Review of Articles (According to Clinical Model) (Continued)
Controlled clinical evidence has yet to decisively prove the theory of preemptive analgesia and LA as a supplement to GA. In the McQuay25 extensive review of preemptive local anesthetics (infiltration and epidural caudal) in the abdominal model, only 1 local anesthetic study showed a preemptive effect, whereas 5 other studies (spinal and nerve block) did not. However, McQuay notes that these 5 local anesthetic studies may have been contaminated by the administration of opioids. He also gives other possible explanations for the lack of preemptive analgesia in other studies.
According to Kissin,33 there are 3 causes for failure of obvious preemptive analgesia in clinical studies: (a) incomplete effect in the preemptive group, insufficient duration of antinociceptive protection during surgery and the initial postoperative period (inflammatory phase), and insufficient degree of preventive blockade; (b) partial preemptive effect in the control group and the use of opioids during induction of anesthesia and during surgery; and (c) surgery with low-intensity noxious stimuli.
Most of the supportive evidence for preemptive efficacy comes from oral surgery model studies.13–18 This can be attributed to the certain specificity of the trigeminal nerve (pathofunctional differences) between trigeminal and other segmental innervation fields34 as well as the difference in embryonic origin. Whereas most of the trigeminal afferents derive from ectodermal placoda, other segmental innervations fields derive from segmental neuronal crest.
Conclusions
Clinical studies may support the hypothesis derived from basic science that preemptive analgesia will attenuate postoperative pain in the oral surgery model. To establish the preemptive effect more decisively, different strategies of experimental design are needed. For example, trials with fewer variables involved (local anesthetics without narcotics or steroidal anti-inflammatories) should be used. There is a need for separation between preemptive doses and surgical procedures because of the possible lingering effect of local anesthetics.
More refined studies are needed to establish whether the timing of administration and the mode of application (local anesthetics, NSAIDs, opioids, or a combination) will be more effective, keeping in mind the possible rare adverse effects. Difficulties in achieving a reliable model must be taken into consideration when reviewing data taken from other fields such as orthopedic or abdominal surgery.
The concomitant administration of LA and GA is safer than GA alone as evidenced by greater physiological stability in patients.29 Consideration should be given to combining preemptive analgesics or the concomitant use of LA with lighter levels of GA or sedation. This strategy is directed at achieving the highest degree of efficacy while simultaneously limiting the amount and magnitude of unwanted adverse effects that are associated with using any technique alone. The evidence is convincing that preoperative preemptive LA or analgesic use with GA provides improved patient care and should be routinely considered.
References
- Crile GW. The kinetic theory for shock and its prevention through anociassociation (shockless operation) Lancet. 1913;ii:7–16. [Google Scholar]
- Fields HL, Basbaum AI. Central nervous system mechanisms of pain modulation. In: Wall PD, Melzack R, editors. Textbook of Pain. 4th ed. New York, NY: Churchill Livingstone; 1999. pp. 309–329. [Google Scholar]
- Fields HL. Pain. New York, NY: McGraw-Hill; 1987. pp. 99–132. [Google Scholar]
- McNally GP. Pain facilitatory circuits in the mammalian central nervous system: their behavioral significance and role in morphine analgesic tolerance. Neurosci Biobehav Rev. 1999;23:1059–1078. doi: 10.1016/s0149-7634(99)00040-8. [DOI] [PubMed] [Google Scholar]
- Amit Z, Galinia ZH. Stress-induced analgesia: adaptive pain suppression. Phys Rev. 1986;66:1091–1120. doi: 10.1152/physrev.1986.66.4.1091. [DOI] [PubMed] [Google Scholar]
- Lester LS, Fanselow MS. Exposure to a cat produces opioid analgesia in rats. Behav Neurosci. 1985;99:756–759. doi: 10.1037//0735-7044.99.4.756. [DOI] [PubMed] [Google Scholar]
- Kavaliers M. Brief exposure to a natural predator, the short tail weasel, induces benzodiazepine sensitive analgesia in white-footed mice. Phys Behav. 1988;42:29–32. doi: 10.1016/0031-9384(88)90236-3. [DOI] [PubMed] [Google Scholar]
- Yaksh T. Central pharmacology of nociceptive transmission. In: Wall PD, Melzack R, editors. Textbook of Pain. 4th ed. New York, NY: Churchill Livingstone; 1999. pp. 253–308. [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1789. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Sorkin LS. Pain: nociceptive and neuropathic mechanisms. Anesthesiol Clin North America. 1997;15:235–249. [Google Scholar]
- Cook AJ, Woolf CJ, Wall PD, MacMahon SB. Dynamic receptive field plasticity in rat spinal cord dorsal horn following C-primary afferent input. Nature. 1987;325:151–153. doi: 10.1038/325151a0. [DOI] [PubMed] [Google Scholar]
- McQuay HJ, Dawn C, Moore RA. Postoperative orthopaedic pain—the effect of opiate premedication and local anaesthetic blocks. Pain. 1988;33:289–291. doi: 10.1016/0304-3959(88)90287-4. [DOI] [PubMed] [Google Scholar]
- Dionne RA, Campbell RA, Cooper SA, Hall DL, Buckingham B. Suppression of postoperative pain by preoperative administration of ibuprofen in comparison to placebo, acetaminophen, and acetaminophen plus codeine. J Clin Pharmacol. 1983;23:37–43. doi: 10.1002/j.1552-4604.1983.tb02702.x. [DOI] [PubMed] [Google Scholar]
- Dionne RA, Sisk AL, Fox PC, Wirkzek PR, Gracely RH, Dubner R. Suppression of postoperative pain by preoperative administration of flurbiprofen in comparison to acetaminophen and oxycodone plus acetaminophen. Curr Ther Res. 1983;34:15–29. [Google Scholar]
- Gordon SM, Dionne RA, Brahim J, Jabir F, Dubner R. Blockade of peripheral neuronal barrage reduces postoperative pain. Pain. 1997;70:209–215. doi: 10.1016/s0304-3959(96)03315-5. [DOI] [PubMed] [Google Scholar]
- Dionne RA. Suppression of dental pain by the preoperative administration of flurbiprofen. Am J Med. 1986;80:41–49. doi: 10.1016/0002-9343(86)90110-5. [DOI] [PubMed] [Google Scholar]
- Troullos ES, Hargreaves KM, Butler DP, Dionne RA. Comparison of nonsteroidal anti-inflammatory drugs, ibuprofen and flurbiprofen, with methylprednisolone and placebo for acute pain, swelling and trismus. J Oral Maxillofac Surg. 1990;48:945–952. doi: 10.1016/0278-2391(90)90007-o. [DOI] [PubMed] [Google Scholar]
- Dionne RA. Preemptive vs preventive analgesia: which approach improves clinical outcome. Compend Contin Educ Dent. 2000;21:50–56. [PubMed] [Google Scholar]
- Nagatsuka C, Ichnohe T, Kaneko Y. Preemptive effects of a combination of preoperative diclofenac, butorphanol, and lidocaine on postoperative pain management following orthognathic surgery. Anesth Prog. 2000;47:119–124. [PMC free article] [PubMed] [Google Scholar]
- Younessi OJ, Punnia-Moorthy A. Cardiovascular effects of bupivacaine and the role of this agent in preemptive dental analgesia. Anesth Prog. 1999;46:56–62. [PMC free article] [PubMed] [Google Scholar]
- Zacharias M, Hunter KM, Baker AB. Effectiveness of preoperative analgesics on postoperative dental pain: a study. Anesth Prog. 1996;43:92–96. [PMC free article] [PubMed] [Google Scholar]
- Flath RK, Hicks ML, Dionne RA, Pelleu GB. Pain suppression after pulpotomy with preoperative flurbiprofen. J Endodotics. 1987;13:339–347. doi: 10.1016/S0099-2399(87)80116-4. [DOI] [PubMed] [Google Scholar]
- Sisk AL, Mosly RO, Martin RP. Comparison of pre-operative and postoperative diflunisal for suppression of postoperative pain. J Oral Maxillofac Surg. 1989;47:464–468. doi: 10.1016/0278-2391(89)90278-4. [DOI] [PubMed] [Google Scholar]
- Sisk AL, Grover BJ. A comparison of preoperative and postoperative naproxen sodium for suppression of postoperative pain. J Oral Maxillofac Surg. 1990;48:674–678. doi: 10.1016/0278-2391(90)90048-7. [DOI] [PubMed] [Google Scholar]
- McQuay HJ. Do preemptive treatments provide better pain control. In: Gebhardt GF, Hammond DL, Jensen TS, editors. Proceedings of the 7th World Congress on Pain. Seattle, Wash: IASP Press; 1994. pp. 709–723. [Google Scholar]
- Handley GH, Silbert BS, Mooney PH, Schweitzer SA, Allen NB. Combined general and epidural anesthesia versus general anesthesia for major abdominal surgery: postanesthesia recovery characteristics. Reg Anesth. 1997;22:435–441. doi: 10.1016/s1098-7339(97)80030-2. [DOI] [PubMed] [Google Scholar]
- Tverskoy M, Cozacov C, Ayache M, Bradley EL, Kissin I. Postoperative pain after inguinal herniorrhaphy with different types of anesthesia. Anesth Analg. 1990;70:29–35. doi: 10.1213/00000539-199001000-00006. [DOI] [PubMed] [Google Scholar]
- Ejlersen E, Andersen HB, Eliasen K, Mogensen T. A comparison between preincisional and postincisional lidocaine infiltration and postoperative pain. Anesth Analg. 1992;74:495–498. doi: 10.1213/00000539-199204000-00004. [DOI] [PubMed] [Google Scholar]
- Mamiya H, Ichinohe T, Kaneko Y. Effects of block analgesia on attenuating intraoperative stress responses during oral surgery. Anesth Prog. 1997;44:101–105. [PMC free article] [PubMed] [Google Scholar]
- Giaufré E, Dalens B, Gombert A. Epidemiology and morbidity of regional anesthesia in children. Anesth Analg. 1996;83:904–912. doi: 10.1097/00000539-199611000-00003. [DOI] [PubMed] [Google Scholar]
- Holland IS, Stassen LFA. Bilateral block: is it safe and more efficient during removal of third molars. Br J Oral Maxillofac Surg. 1996;34:243–247. doi: 10.1016/s0266-4356(96)90278-8. [DOI] [PubMed] [Google Scholar]
- Hempenstall PD, Campball JPS, Bajurnow AT, Reade PC, McGrath B, Harrison LC. Cardiovascular, biochemical, and hormonal responses to intravenous sedation with local analgesia versus general anesthesia in patients undergoing oral surgery. J Oral Maxillofac Surg. 1986;44:441–446. doi: 10.1016/s0278-2391(86)80008-8. [DOI] [PubMed] [Google Scholar]
- Kissin I. Preemptive analgesia. Anesthesiology. 1996;84:1015–1019. doi: 10.1097/00000542-199605000-00001. [DOI] [PubMed] [Google Scholar]
- Tal M, Devor M. Ectopic discharge in injured nerves: comparison of trigeminal and somatic afferents. Brain Res. 1992;972:520–526. doi: 10.1016/0006-8993(92)90753-v. [DOI] [PubMed] [Google Scholar]