Abstract
AIMS
The aim of this study was to investigate the incidence and reporting rate of drug-induced long-QT syndrome (LQTS) in France [defined by evidence of torsades de pointes (TdP), QT prolongation and exposure to a relevant drug] and to assess feasibility of case collection for drug-induced LQTS.
METHODS
A retrospective population-based study was carried out in Southwest France in five institutions: three main hospitals, one private clinic and one cardiac emergency unit, searched from 1 January 1999 to 1 January 2005 (population coverage of 614 000). The study population consisted of 861 cases with International Classification of Diseases-10 diagnostic codes for ventricular tachycardia (I147.2), ventricular fibrillation (I149.0) and sudden cardiac death (I146.1) from hospital discharge summaries, supplemented by cases reported to national or regional pharmacovigilance systems, and voluntary reporting by physicians, validated according to internationally defined criteria for drug-induced LQTS.
RESULTS
Of 861 patients coded with arrhythmias or sudden cardiac death, there were 40 confirmed surviving acquired cases of drug-induced LQTS. We estimated that the incidence of those who survive to reach hospital drug-induced LQTS is approximately 10.9 per million annually in France (95% confidence interval 7.8, 14.8).
CONCLUSIONS
Many cases of drug-induced LQTS may not survive before they reach hospital, as the reporting rate for drug-induced LQTS identified through the cardiology records and also reported to pharmacovigilance systems for the Midi-Pyrenees area is 3/40 (7.5%). Using the methods outlined it is possible to assemble cases to study genetic susceptibility to drug-induced LQTS and adapt these methods more widely.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Drug-induced long-QT syndrome (LQTS) is a potentially fatal condition that has led to a number of postmarketing withdrawals in recent years.
However, many cases may not survive long enough to reach hospital, and only a small proportion are reported to pharmacovigilance agencies.
The extent to which genetic determinants of susceptibility to LQTS are specific to particular drugs, or common to several classes of drug, remains to be determined.
WHAT THIS STUDY ADDS
We estimated population prevalence of drug-induced LQTS in the Midi-Pyrenees region, southwest France, using five different institutions and assessed feasibility of tracing potential cases (in addition to pharmacovigilance data), using hospital data and rigorous case definition.
These methods can be adapted to a wider region, used to augment pharmacovigilance reporting, and offer researchers the opportunity to study genetic susceptibility to drug-induced LQTS.
Keywords: arrhythmia, genetics, long-QT syndrome, pharmacoepidemiology, torsade de pointes
Introduction
Background
Relevance of studying the genetics of susceptibility to drug-induced long-QT syndrome
Drug-induced long-QT syndrome (LQTS) has led to several postmarketing drug withdrawals in the last decade [1]. These include drugs such as thioridazine, levoacetylmethadone and astemizole, after these drugs had been marketed for several years. In patients treated with antipsychotic agents such as thioridazine and droperidol, asymptomatic lengthening of the rate-corrected QT interval is common, and the risk of sudden cardiac death has been shown to be increased two-to-threefold [2, 3], but rarely appears on adverse drug reaction (ADR) reports. Failure to appreciate the magnitude of risk may delay withdrawal of a drug. For example, the eventual withdrawal of thioridazine in June 2005 was many years after the association with LQTS had been described, as the magnitude of the effect on sudden cardiac death became clear. Cases present with syncope and polymorphic ventricular tachycardia (VT) [torsades de pointes (TdP)] and prolongation of the QT interval. LQTS is likely to pass unrecognized as a cause of sudden cardiac death, as the diagnosis is unlikely to be made unless cases survive long enough to reach hospital.
Data from the World Health Organization Uppsala Monitoring Centre (UMC) have shown that most spontaneous reports (>50%) of LQTS recorded in the different pharmacovigilance systems in the world concern mainly class III antiarrhythmics (sotalol, amiodarone), followed by anti-infective drugs (macrolides, quinolones, imidazole antifungal drugs) and antihistaminic drugs. There is also evidence of a cumulative effect of ketoconazole or macrolide antibiotics on risk of drug-induced LQTS and of some antihistamines, especially diphenhydramine [4]. Although testing of new drugs for effect on the QT interval is now mandatory, such tests may fail to detect drugs that cause torsade in a small number of susceptible individuals. In addition to the low number of cases detected, techniques for detecting drug-induced LQTS are not always relevant; there is a lack of sensitivity of these methods, which include ECG measurement to Holter recording or more sophisticated techniques (post processing of ventricular repolarization). Using data from the UMC, DeBruin et al. found a statistically significant association between the risk of cardiac arrhythmia and sudden death and the level of antihuman ether-a-go-go-related gene (hERG) potassium channel activity of drugs [relative risk (RR) 1.93, 95% confidence interval (CI) 1.89, 1.98], although this did not allow for confounders such as co-prescription of drugs, hypokalaemia or comorbidity [5]. Currently, there is widespread testing of new drugs for ability to block hERG potassium channels, based on recommendations to assess the risk of QTc-interval prolongation and TdP for noncardiovascular drugs [6, 7]. Current use of noncardiac QTc-prolonging drugs in a Dutch population is associated with increased risk of cardiac death, especially with antipsychotics such as haloperidol, with highest reported risks in women and recent starters of medication [8].
Feasibility studies – preliminary work
To collect large numbers of cases of LQTS a European collaboration is required, as no single country can generate enough cases. We have therefore established a network (EUDRAGENE) to establish a DNA case–control collection for studying the genetic basis of susceptibility to various classes of ADR, based on case ascertainment through the pharmacovigilance system. For LQTS/TdP, however, it is not feasible to ascertain cases through the pharmacovigilance system as the reporting rate is too low. In France, for example, which has one of the most well-organized pharmacovigilance systems in Europe, the reporting rate is only about 0.8 per million per year, and a pilot study has shown that only one-third of these meet our strict case definition for LQTS/TdP.
The only feasible method to ascertain large numbers of cases is by a systematic search of discharge records from many hospitals. This is laborious, because standard diagnostic coding of hospital discharges [International Classification of Diseases (ICD)-9 or ICD-10] does not specifically classify LQTS, and it is necessary to search all diagnostic codes for ventricular arrhythmia and associated outcomes such as ventricular fibrillation (VF) and sudden cardiac death, combined with medical records in hospital as described below.
Methods
Setting
A retrospective population-based study was carried out in the administrative area of Midi-Pyrenees (Southwest France) and specifically in three cities with a population coverage of 614 000 (Toulouse, Montauban and Albi). The study was done in four hospitals: the regional university tertiary hospital, two main hospitals and one private clinic, all with cardiology department and cardiac emergency units. We searched from hospital medical information system all cases hospitalized from 1 January 1999 to 1 January 2005, with ICD-10 diagnostic codes of VT (I147.2), VF (I149.0) and sudden cardiac death (I146.1) in the discharge summaries. Additional information from ECGs and medical records was sought with the appropriate consents, and all putative cases were validated according to internationally defined criteria (Appendix 1).
This study was carried out with the approval of the French Drug Agency (AFSSAPS) and with the ethical approval of the regional committee for biomedical research according to French law. The project also followed guidelines from the ethical review board of the European Commission as part of the EUDRAGENE project (http://www.eudragene.org), including informed patient consent where relevant.
Source population: Toulouse, Albi and Montauban
The population served by the Toulouse University Hospital is about 540 000 (60% of the metropolitan area of Toulouse). The public and the private hospital in Albi cover about 50 000 inhabitants and the public hospital in Montauban approximately 24 000. From the hospital's information system, we obtained a list of 861 hospitalizations during the period 1 January 1999 to 1 January 2005 with the ICD-10 codes of interest, for whom we examined medical files. We also used data recorded in the French Pharmacovigilance database, in order to identify cases spontaneously reported to the Midi-Pyrenees pharmacovigilance centre (covering an area of 2.4 millions inhabitants). Letters of invitation were sent to the general practitioners (GPs) of all patients eligible for study and alive at the time of last entry in the case notes. All cases identified via hospital databases were subsequently reported to the regional pharmacovigilance centre, according to the French guidelines for pharmacovigilance reporting.
Case definition
Electronic discharge summaries were selected with ICD-10 diagnostic codes of VT and examined by the research team to classify these records for confirmation of diagnoses in patients aged ≥18 years. For these records, case notes were retrieved and examined by the research assistant. Copies were taken of any ECGs recorded during the hospital episode and any additional ECGs recorded on a previous occasion (before starting the drug) or subsequently (after recovery). Mean QTc and RR intervals were measured on these ECGs by a trained cardiologist for independent verification of prolonged QT interval according to predefined criteria (Appendix 1). A list of drugs known to cause LQTS was adapted from the University of Arizona Centre for Education and Research on Therapeutics (http://www.torsades.org) and from the French system of pharmacovigilance, specifically for drugs available in Europe or for new drugs.
We supplemented the case collection that met the strict definition with additional cases of torsade without QT prolongation and cases not exposed to drugs, to allow researchers to test at a later date whether associations detected with drug-induced LQTS are also seen with torsades without QT prolongation, or with cases not exposed to drugs.
As LQTS is one of several predisposing factors for TdP, all other potential causes were excluded by careful examination of medical records by a cardiologist at the time of their hospital appointment. Examples of exclusion were: structural cardiac diseases, metabolic abnormalities, conduction defects, systemic diseases and hereditary disturbances of the rhythm associated with sudden cardiac death such as Brugada syndrome.
Comorbidities
Medical records were searched to examine comorbidities for drug-induced LQTS. These included patients in whom arrhythmia occurred only during acute coronary ischaemia (supported by ECG changes or elevated cardiac enzymes) or within 3 months of an acute coronary event, and diagnosed cases of Mendelian forms of LQTS, who were excluded from the study. We also collected electrolyte data and excluded cases with abnormal potassium levels (≤3.5 mmol l−1 or ≥5.5 mmol l−1). Data were also collected on use of co-medication at the time of the event.
Statistical analyses
Analyses were conducted using stata version 9 (STATA Corp., College Station, TX, USA). The incidence of nonfatal drug-induced LQTS was calculated by the ratio of events per year divided by 1 000 000 population, based on the coverage area of the hospitals and centres involved, with 95% CI assuming a Poisson distribution. The denominator over the time of the study was approximately 3.68 million patient-years. We did not calculate specific incidences for separate drug classes due to the small numbers involved, although approximately 50% of cases were taking antiarrhythmic medication.
Results
Among the total of 861 cases diagnosed as ‘ventricular tachycardia’, 628 (73%) were male; of those diagnosed presumed alive with possible torsades, without conduction defect, atrial fibrillation or myocardial infarction, from data available, 25/49 (51%) cases were female and 24/49 (49%) were male (P < 0.001). For four men and two women the diagnosis of torsades could not be confirmed as medical notes were incomplete. (One man was not known to be taking any medication.) The flowchart (Figure 1) gives further breakdown of the excluded cases. From 99 dead cases diagnosed as VT, cause of death was listed in 29/99 (29%) cases as: cardiorespiratory arrest (n = 8), cardiogenic shock or insufficiency (n = 6), TdP and VT, other arrhythmias (n = 7) (there was no mention of LQTS in any of the causes of death) and death from natural causes (n = 5), sudden death (n = 1), cerebrovascular haemorrhage (n = 1) and septicaemia (n = 1). All cases were of European ethnicity. In surviving patients the mean age for men was 72.7 (SD 18.2, range 22–106 years) and for women 78.3 (SD 24.1, range 15–106 years). From Toulouse, Montauban, Albi and the pharmacovigilance database, for 56 of the cases alive classified as ‘possible acquired LQTS or torsades’, 36 case notes or ADR reports were successfully retrieved to date. Of 56 presumed live cases documented as either torsades or LQTS, replies or medical files were unavailable from doctor for 11 patients, nine patients refused or were medically unfit to participate (of these, four were confirmed TdP/LQTS) and nine patients or their doctors were untraceable (of these, data were incomplete for six; three were confirmed TdP/LQTS). For the untraceable/unavailable cases for which further information was obtained, seven names of the reporting doctors were not available (anonymized by pharmacovigilance centre), one report did not contain the doctor's name, one patient had left for a convalescent home and one patient was no longer in contact with their GP. Of the remaining 40 confirmed traceable cases, 28 (70%) had prolonged QTc measured on ECG (together with VT or VF), 30 (75%) had syncope with documented premature ventricular contractions, and 24 (60%) LQTS and torsades measured on ECG (mean QTc 547 ms, SD 105). Twenty-four patients agreed to take part and donated blood samples for which genetic analyses are planned at a later date. For those on whom drug information was available (surviving and dead cases), 21/55 (38%) were taking amiodarone (including amiodarone combinations), and 19/55 (35%) suspected cases were taking a combination of two or more drugs known to cause LQTS. All cases ascertained through pharmacovigilance systems were also identified from hospital records.
Figure 1.
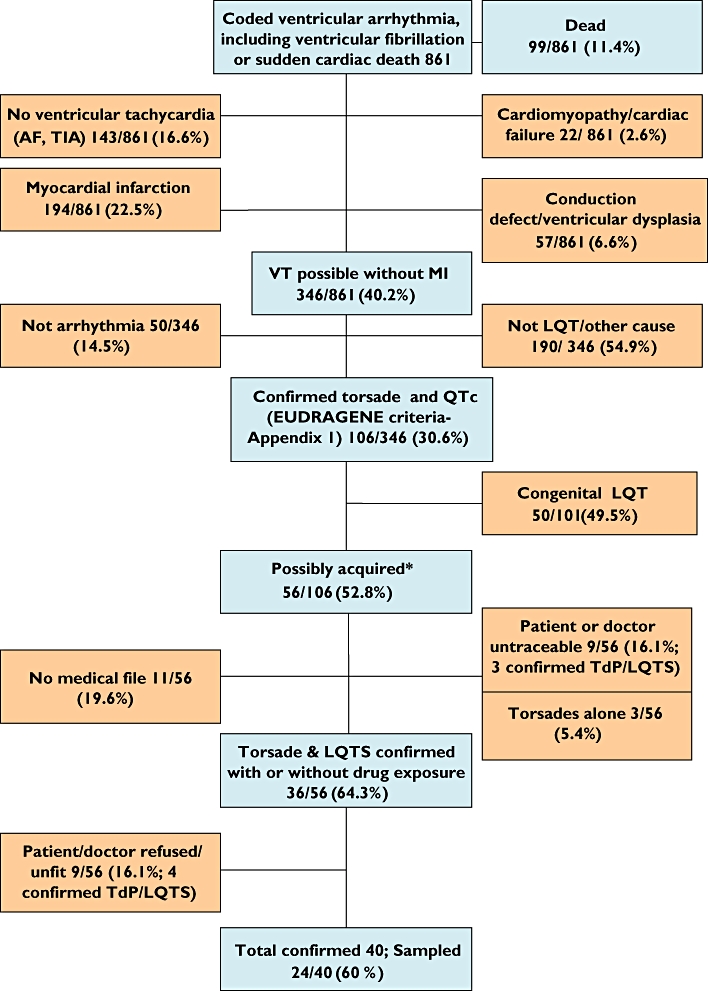
Flowchart of case ascertainment in Toulouse, Albi, Montauban and pharmacovigilance database, Midi-Pyrenees area
From the centres selected in the Midi-Pyrenees area, 40 confirmed cases of nonfatal torsades with QT prolongation, excluding those with other causes of VT, such as underlying heart disease or Mendelian syndromes, were included in the study. In a population of 614 000 over 6 years, we estimate the incidence of diagnosed nonfatal torsades as around 10.9 per million per year (95% CI 7.8, 14.8). We estimate that, with the strict definition of LQTS that we are using, 98% of cases without underlying heart disease have been exposed to a relevant drug. Results for Toulouse, Albi, Montauban and the pharmacovigilance database, Midi-Pyrenees area are presented in the flowchart (Figure 1).
Discussion
Ascertainment of cases
This study has a response rate of 60% of those confirmed eligible. Other studies that have attempted to contact cases of drug-induced LQTS have had much lower response rates (<10%) [9] and illustrate difficulties of contacting physicians and patients. It is not possible to contact patients directly without the consent of their physician or GP, although almost all of the physicians contacted agreed to participate. This study did not examine fatal cases of drug-induced LQTS, although this would be possible provided the diagnoses could be confirmed from the medical records.
Incidence of TdP/LQTS
From data from hospital and clinic discharge summaries in France we estimate the true incidence of torsades/LQTS to be around 5–7% of cases diagnosed as ‘ventricular tachycardia’, ‘ventricular fibrillation’ or ‘sudden cardiac death’, excluding acute coronary syndromes or congenital LQTS. Although the methodology for case ascertainment used ICD-9 or -10 coding, all potential cases were subjected to a rigorous medical record and ECG review by a trained cardiologist, based on internationally defined criteria for drug-induced LQTS and TdP (Appendix 1).
Our study showed women have a greater susceptibility to TdP in drug-induced LQTS than men (51% vs. 49%), which is consistent with other studies and thought possibly to be due to regulation of ion channel by sex hormones [10]. Extrapolating from the Toulouse, Albi and Montauban coverage areas, we estimated the incidence of torsades (both sexes) using definite medical record/ECG confirmation to be approximately 10.9 per million per year (95% CI 7.8, 14.8). This is comparable to the estimate of 40 per million per year, excluding acute ischaemic-related cases (derived 95% CI 21.9, 67.1) obtained in a Swedish study (Medical Products Agency), in which cases of torsades were notified prospectively by cardiologists [11]. However, the Swedish study included fatal and nondrug-induced cases. It should be noted, however, that this estimate was based on 14 cases of TdP and data extrapolated from a 28-day study period, which may result in some uncertainty around the accuracy of these estimates. If drug-induced cases represent 10/14 torsades cases [11], this would give an incidence in Sweden of approximately 30 per million year−1. Table 1 compares European reporting rates for TdP/LQTS using pharmacovigilance data.
Table 1.
European annual reporting rates for torsades de pointes/long-QT syndrome (TdP/LQTS) per 10 million total population from pharmacovigilance data
| Country | TdP | LQTS |
|---|---|---|
| France | 4.2 | 3.9 |
| Belgium | 2 | 2.8 |
| Germany | 1.3 | 1.3 |
| Sweden | 12 | |
| Spain | 0.75 | 0.95 |
| Italy (three regions) | 0.11 | 0.66 |
In France it is clear that this is likely to be an underestimate of true LQTS cases, as many cases will not survive before they reach hospital. These differences may also reflect different prescribing practices in France and Sweden. A recent amiodarone drug utilization study in our area found no difference in the use of this drug according to gender [12].
From this study, searches from the three main general hospitals and clinics serving about 614 000 people in Toulouse, Albi and Montauban yielded 861 discharge records coded as ventricular arrhythmia in the last 6 years. It is possible that the potential source population may actually be slightly higher (due to possible referrals to city hospitals from rural areas), but we do not expect this would affect our result greatly. Of these, about 40 were identified as possible cases of drug-induced LQTS on the basis of information in the cardiology discharge summary satisfying inclusion criteria (ECGs and cardiologist discharge letters searched electronically to confirm diagnosis or exclude unlikely cases). Of these, about 30 were alive and eligible for study, and 24 of these responded to invitation. Collection of 600 cases will thus require searching 100 public hospitals across France.
Table 2 shows categorization of possible torsades cases. Of people alive included in the study with drug-induced torsades and available ECGs or data from ECG recordings, mean QTc was 547 ms, SD 105. Differences in the cut-off criteria for prolonged QT interval or correction used made very little difference to the overall result. Only two cases had documented LQT without torsades.
Table 2.
Categorization of 40 cases confirmed as ‘Possible alive torsades cases without conduction defect, atrial fibrillation or myocardial infarction’ with records available (hospitals in Toulouse, Montauban, Albi and pharmacovigilance database, Midi-Pyrenees area)
| Categories (documented by discharge summaries, medical records or ECG) | No. (%) | Prolonged QTc documented in records | QTc measured on ECG |
|---|---|---|---|
| Possible TdP/LQTS*– all | 40 | 28 (70%) | 24 (60%) |
| Polymorphic ventricular tachycardia or fibrillation (PVT)† | 19 | 11 (58%) | 9 (47%) |
Possible torsades is syncope plus either premature ventricular contraction or PVT plus LQT.
Documented in medical records or on ECG recording.
Most LQTS cases are taking class 3 antiarrhythmic drugs or β-blockers, with very few on antipsychotic (one case with possible torsades) or antibiotic drug classes (Table 3). It is unclear whether low rates of torsades associated with antipsychotic drugs are due to lower prescribing rates of drugs such as thioridazine (discontinued from June 2005) following changes in prescribing as seen in the UK [13], or high case fatalities associated with these drugs. Prescribing data from France suggest that rates of antipsychotic reimbursement have decreased over recent years (Figure 2), which could reflect lower prescribing rates [14]. However, French national sales data for diuretics, β-blockers and antiarrhythmic drugs are relatively stable (http://afssaps.sante.fr/pdf/5/rapmed05.pdf).
Table 3.
Data on drugs taken in all patients diagnosed with ‘Ventricular arrhythmia or sudden cardiac death’ and ‘all possible torsades without conduction defect/atrial fibrillation or myocardial infarction’
| Drug class | Possible torsades (PVT or syncope and PVC) + LQT | Coded ‘ventricular arrhythmia or sudden cardiac death’ |
|---|---|---|
| Class III antiarrhythmics | ||
| Amiodarone | 10 | 58 |
| Amiodarone combinations | 11 | 11 |
| β-Blocker (sotalol; acebutolol, atenolol, bisoprolol) | 8 | 22 |
| β-Blocker combinations | 3 | 3 |
| Other cardiovascular drugs: | ||
| Digoxin | 2 | 6 |
| Digoxin combinations digoxin + flecainide or disopyramide (Class 1 antiarrhythmic) | 2 | 2 |
| ACE/ACE inhibitor + diuretic (benazepril + hydrochlorothiazide) | 2 | 2 |
| Class 1 antiarrhythmic (flecainide quinidine/hydroxyquinidine) | 3 | 11 |
| Class 1 antiarrhythmic combinations | 1 | 1 |
| Calcium channel antagonist (bepridil) | 2 | 2 |
| Other antihypertensives: vincamine (alkaloid), urapidil, alphagan (α-blocker) | 1 | 3 |
| Diuretic-(furosemide) | 0 | 1 |
| Other drugs: | ||
| H1 antihistamine (alimemazine) | 0 | 1 |
| Anti-infectious drugs (clofamizine, lomefloxacine, itraconazole) | 2 | 4 |
| COX-2 inhibitor (celecoxib) | 1 | 1 |
| Neuroleptics (amisulpride, thiorazidine, periciazine) | 1 | 8 |
| Antiepileptic (sodium valproate) | 1 | 1 |
| SSRI antidepressant (fluvoxamine) | 0 | 1 |
| Benzodiazepine | 1 | 1 |
| No drug | 1 | 1 |
| Data unavailable or not requested as other exclusion criteria | 3 | 721 |
| Total | 55 | 861 |
PVC, premature ventricular contraction; PVT, polymorphic ventricular tachycardia; ACE angiotensin converting enzyme; COX, cyclooxygenase; SSRI, selective serotonin reuptake inhibitor.
Figure 2.
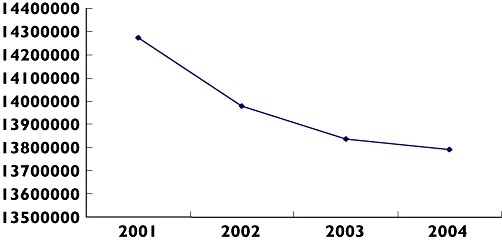
Graph showing number of antipsychotic units re-imbursed in France from 2001–4. Based on French Insurance System database (MEDICAM) concerning drugs reimbursed in ambulatory care in France (http://www.ameli.fr//)
The pharmacovigilance system has a much lower yield of drug-induced LQTS/TdP; pharmacovigilance reports estimated a total of 245 cases over 5 years, i.e. around 0.8 per million per year. In comparison with other ADRs, the reporting rate for drug-induced LQTS/TdP is low, as many cases will result in a fatal outcome that may not be attributable to the drug as they will not reach hospital.
Drug classes associated with LQTS
The website lists all drugs currently acknowledged to be associated with acquired LQTS (http://www.eudragene.org). Most cases are attributed to sotalol and amiodarone-like compounds, although in recent years amiodarone has tended to replace quinidine. β-Blockers may also identify patients with underlying cardiovascular disorder such as hypertension and angina, which may interact to promote drug-induced long QT due to co-prescriptions. For the cases collected, data were recorded on co-prescribed medication where available.
A few drugs implicated in drug-induced LQTS were prescribed as combinations [e.g. angiotensin converting enzyme inhibitor and diuretic (benazepril & hydrochlorothiazide)], where it is possible that LQTS was secondary to diuretic-induced hypokalaemia (data were unavailable from the records). One individual not previously known with congenital LQTS had non drug-associated LQTS that was unexplained by other factors.
Ways to improve case ascertainment
One of the main problems in detecting cases of drug-induced LQTS is that there is no specific ICD-10 code and that cases could be coded otherwise in relationship to associated disease or complication. A revision of ICD-10 codes to include a separate category for drug-induced LQTS/TdP would enhance sensitivity of searches, prior to validation by internationally defined criteria (Appendix 1).
The average population coverage of such hospitals varies between 50–300 000. In France and other European countries, hospital discharge records are coded for multiple diagnoses according to ICD-9 or -10 (Belgium, Sweden, France, UK, the Netherlands, Spain, Italy and Ireland). In principle, this approach can be extended to each hospital, searching the database of hospital discharges during the last 5 years to retrieve all records with any of the following diagnostic codes, excluding records with additional codes for acute coronary episodes such as myocardial infarction.
National pharmacovigilance data and cardiology networks; other data sources
Where possible, pharmacovigilance data from other centres were used to identify further possible cases of drug-induced LQTS/TdP, some of whom have already agreed to participate in the study. Although numbers are small, the sensitivity rates of cases identified in this manner is high.
Cardiology networks and other centres specializing in arrhythmias in France may aid further recruitment. Revision of hospital databases to allow drugs on admission to be linked to text of discharge summary would help identify further cases. Computer programs able to search free text of discharge letters would also help in improving search strategies. In several European countries, there are databases covering many general practices that link clinical diagnoses to drug prescription records. In some of these databases, such as NIN-GP (the Netherlands), diagnostic information is stored as free text (rather than only as diagnostic codes). However, as the total coverage of these databases is only a few million people, they can contribute only a small proportion of cases.
Summary
Many cases of drug-induced LQTS may not survive until they reach hospital, therefore it is likely that there is under-reporting of LQTS by cardiologists and other medical staff to pharmacovigilance agencies. Ascertaining cases from hospitals can be used to supplement pharmacovigilance reporting for LQTS. Our estimate of drug-induced LQTS in France (10.9 per million per year, 95% CI 7.8, 14.8), ascertained from hospital discharge summaries and confirmed by medical records, will be an underestimate of the true incidence as we did not include fatal cases or cases for whom we were unable to trace records. This study has shown a feasible method of ascertaining potential cases of LQTS (in addition to pharmacovigilance data), using hospital data and rigorous case definition that can be adapted to a wider region, and offers researchers the opportunity to study genetic susceptibility to drug-induced LQTS.
Competing interests
None to declare.
Webpage citations
OMIM Online Mendelian Inheritance in Man (http://www.ncbi.nlm.nih.gov)
EUDRAGENE (http://www.eudragene.org)
University of Arizona Centre for Education and Research on Therapeutics (http://www.torsades.org)
French Insurance System database (MEDICAM) (http://www.ameli.fr)
AFSSAPS: Drug sales from ambulatory and hospital care in France, 1994–2004 (http://afssaps.sante.fr/pdf/5/rapmed05.pdf)
Appendix 1: Case definition
For each suspected case, each of the criteria listed below will be scored
| V | Polymorphic ventricular tachycardia (≥3 beats at rate >100 min−1) or ventricular fibrillation, documented by an ECG recording or statement in the case record |
| S | Syncope recorded in case notes, and polymorphic ventricular premature contractions documented by an ECG recording in the case record |
| Q | ECG recording during admission showing Fridericia rate-corrected QT interval (QT/RR1/3) >440 ms in men or >450 ms in women. |
| D | Exposure (within an interval short enough for the drug to be present) to a drug known to cause LQTS (based on the drugs listed as causes of torsades, supplemented with other drugs available in Europe, http://www.torsades.org) |
| C | Acute coronary ischaemia (supported by ECG changes or elevated cardiac enzymes) in last 3 months or other underlying heart disease |
| M | Diagnosed as congenital LQTS |
Probable drug-induced LQTS is defined as
[(V or S) and*Q] and D and [not (C or M)]
This follows the definition for ‘high confidence in drug-induced LQTS diagnosis’ given by Barbey, Lazzara and Zipes [15] (http://www.arizonacert.org/medical-pros/class-cases.htm). Although the reliability of measurement of the rate-corrected QT interval based on a single ECG recording is limited [16], the criterion chosen is sufficiently stringent to be specific.
The diagnosis of drug-induced LQTS will be excluded where an ECG taken before exposure to the suspected drug shows a normal QT interval, or an ECG taken after an interval long enough for the drug to be cleared shows that the QT interval is still prolonged.
Torsades without structural heart disease is defined as
(Polymorphic ventricular tachycardia documented by ECG) and [not (C or M)]
Appendix 2: Genetics of LQTS
Genetics of susceptibility to drug-induced LQTS
Research on genetic susceptibility to drug-induced LQTS has focused mainly on genes that cause Mendelian forms of the syndrome, and on genes that influence drug metabolism, notably CYP2D6 and CYP3A4, which is inhibited by drugs including macrolide antibiotics and imidazole antifungals. Six genes in which mutations cause Mendelian forms of LQTS have been identified by family linkage studies (Table 4): five encoding potassium channels (KCNQ1 on chromosome 11p, KCNH2 on chromosome 7q, KCNE1 and KCNE2 on chromosome 21q, KCNJ2 on chromosome 17q) and one encoding a sodium channel (SCN5A on chromosome 3p). Another locus (LQT4) causing a sub-phenotype of LQTS has been mapped to chromosome 4q and recently sequenced by Mohler et al., who identified the underlying defect as a glu1425-to-gly (E1425G) missense mutation in the ankyrin-B gene [17], although this may represent a clinical entity distinct from classic LQTS; prolonged rate-corrected QT interval is not a consistent feature and the identified protein is not an ion channel or channel subunit. It has been suggested that the syndrome previously ‘LQT4’ be renamed as ‘sick sinus syndrome associated with bradycardia’[18].
Table 4.
Known genes associated with long-QT syndrome [Online Mendelian Inheritance in Man (http://www.ncbi.nlm.nih.gov)]
| Linkage | Gene | Chromosome |
|---|---|---|
| LQT1 | KCNQ1 (KvLQT1) | 11 |
| LQT2 | KCNH2 (hERG) | 7 |
| LQT3 | SCN5A | 3 |
| LQT4 | ANK2, GLU1425GLY | 4 |
| LQT5 | KCNE1 | 21 |
| LQT6 | KCNE2 | 21 |
| LQT7 | KCNJ2 | 17 |
KCNQ1 is the commonest variant on congenital LQT syndrome, of which there are two forms: Romano Ward is the autosomal dominant form; and Jervell Lange-Nielsen (KCNQ1/KCNE1) is an autosomal recessive/compound heterozygote that is associated with deafness, and is particularly common in Norway. KCNJ2, known as the Anderson syndrome, may represent a subtype, as often these cases show different ventricular arrhythmias from types 1–6 [19].
Total prevalence at birth of these Mendelian syndromes is estimated as 1–2 per 10 000. Not all individuals who bear disease-causing mutations are affected, suggesting that the effects of these genes depend upon other ‘modifier’ loci. Although patients with Mendelian forms of LQTS are susceptible to drug-induced arrhythmias, missense mutations in genes that cause these Mendelian syndromes are uncommon in patients with drug-induced long QT [20]. A study looking at three congenital mutations in LQT1-3 showed DNA variants in coding regions of these genes can only be identified in 10–15% of acquired (drug-induced) LQTS [21]. A recent study identifying cases of drug-induced QTc prolongation and cardiac arrest in the Netherlands screened 4/45 eligible cases for genetic mutations and identified variants in KCNH2, SCN5A and KCNE1 [9].
Studying the genetic basis of susceptibility to drug-induced LQTS may allow development of tests to identify susceptible individuals, and may also identify target proteins against which molecules can be screened to exclude those that are likely to cause LQTS or even to develop novel antiarrhythmic agents. The extent to which genetic determinants of susceptibility to LQTS are specific to particular drugs, or common to several classes of drug, remains to be determined.
The authors are grateful to Olivia Boeuf and Emmanuelle Guitton for their help with the data collection. We thank Dorothea Nitsch for her advice regarding the statistical models used. We also thank all the physicians who reported cases to regional pharmacovigilance centres, and the French pharmacovigilance network. This study was supported by a concerted action grant from the European Union 5th Framework QLRI-CT-2002-02757. Funding for this study has been received from Pfizer Pharmaceuticals.
REFERENCES
- 1.Shah RR. Pharmacogenetic aspects of drug-induced torsade de pointes: potential tool for improving clinical drug development and prescribing. Drug Saf. 2004;27:145–72. doi: 10.2165/00002018-200427030-00001. [DOI] [PubMed] [Google Scholar]
- 2.Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SHQ. Tc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet. 2000;355:1048–52. doi: 10.1016/s0140-6736(00)02035-3. [DOI] [PubMed] [Google Scholar]
- 3.Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SH. Thioridazine and sudden unexplained death in psychiatric in-patients. Br J Psychiatry. 2002;180:515–22. doi: 10.1192/bjp.180.6.515. [DOI] [PubMed] [Google Scholar]
- 4.Hanrahan JP, Choo PW, Carlson W, Greineder D, Faich GA, Platt R. Terfenadine-associated ventricular arrhythmias and QTc interval prolongation. A retrospective cohort comparison with other antihistamines among members of a health maintenance organization. Ann Epidemiol. 1995;5:201–9. doi: 10.1016/1047-2797(94)00039-v. [DOI] [PubMed] [Google Scholar]
- 5.De Bruin ML, Pettersson M, Meyboom RH, Hoes AW, Leufkens HG. Anti-HERG activity and the risk of drug-induced arrhythmias and sudden death. Eur Heart J. 2005;26:590–7. doi: 10.1093/eurheartj/ehi092. [DOI] [PubMed] [Google Scholar]
- 6.Committee for Proprietary Medicinal Products (CPMP) Points to Consider: the Assessment for the Potential for QT Interval Prolongation by Non-Cardiovascular Medicinal Products. London: European Agency for the Evaluation of Medicinal Products; 1997. [Google Scholar]
- 7.Committee for Proprietary Medicinal Products (CPMP) Notes for Guidance on Safety Pharmacologic Studies for Assessing the Potential for Delayed Ventricular Repolarization (QT Interval Prolongation) by Human Pharmaceuticals. London: European Agency for the Evaluation of Medicinal Products; 2002. [Google Scholar]
- 8.Straus SM, Sturkenboom MC, Bleumink GS, Dieleman JP, van der LJ, de Graeff PA, Kingma JH, Stricker BH. Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur Heart J. 2005;26:2007–12. doi: 10.1093/eurheartj/ehi312. [DOI] [PubMed] [Google Scholar]
- 9.De Bruin ML, van Puijenbroek EP, Bracke M, Hoes AW, Leufkens HG. Pharmacogenetics of drug-induced arrhythmias: a feasibility study using spontaneous adverse drug reactions reporting data. Pharmacoepidemiol Drug Saf. 2006;15:99–105. doi: 10.1002/pds.1194. [DOI] [PubMed] [Google Scholar]
- 10.James AF, Choisy SC, Hancox JC. Recent advances in understanding sex differences in cardiac repolarization. Prog Biophys Mol Biol. 2007;94:265–319. doi: 10.1016/j.pbiomolbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Darpo B. Spectrum of drugs prolonging QT interval and the incidence of torsade de pointes. Eur Heart J. 2001;3(Suppl K):70–80. [Google Scholar]
- 12.Bongard V, Marc D, Philippe V, Jean-Louis M, Maryse LM. Incidence rate of adverse drug reactions during long-term follow-up of patients newly treated with amiodarone. Am J Ther. 2006;13:315–9. doi: 10.1097/00045391-200607000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Bateman DN, Good AM, Afshari R, Kelly CA. Effects of licence change on prescribing and poisons enquiries for antipsychotic agents in England and Scotland. Br J Clin Pharmacol. 2003;55:596–603. doi: 10.1046/j.1365-2125.2003.01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasquet I, Negre-Pages L, Fourrier A, Nachbaur G, El-Hasnaoui A, Kovess V, Lepine JP. Psychotropic drug use and mental psychiatric disorders in France; results of the general population ESEMeD/MHEDEA 2000 epidemiological study. Encephale. 2005;31:195–206. doi: 10.1016/s0013-7006(05)82386-3. [DOI] [PubMed] [Google Scholar]
- 15.Barbey JT, Lazzara R, Zipes DP. Spontaneous adverse event reports of serious ventricular arrhythmias, QT prolongation, syncope, and sudden death in patients treated with cisapride. J Cardiovasc Pharmacol Ther. 2002;7:65–76. doi: 10.1177/107424840200700202. [DOI] [PubMed] [Google Scholar]
- 16.Camm AJ. The design and conduct of human studies to detect and quantify QT interval prolongation induced by new chemical entities. Fundam Clin Pharmacol. 2002;16:141–5. doi: 10.1046/j.1472-8206.2002.00088.x. [DOI] [PubMed] [Google Scholar]
- 17.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogne K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le MH, Bennett V. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–9. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 18.Splawksi. OMIM 2004. [8 April 2008]. Available at http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=600919.
- 19.Tawil R, Ptacek LJ, Pavlakis SG, DeVivo DC, Penn AS, Ozdemir C, Griggs RC. Andersen's syndrome: potassium-sensitive periodic paralysis, ventricular ectopy, and dysmorphic features. Ann Neurol. 1994;35:326–30. doi: 10.1002/ana.410350313. [DOI] [PubMed] [Google Scholar]
- 20.Paulussen AD, Gilissen RA, Armstrong M, Doevendans PA, Verhasselt P, Smeets HJ, Schulze-Bahr E, Haverkamp W, Breithardt G, Cohen N, Aerssens J. Genetic variations of KCNQ1, KCNH2, SCN5A, KCNE1, and KCNE2 in drug-induced long QT syndrome patients. J Mol Med. 2004;82:182–8. doi: 10.1007/s00109-003-0522-z. [DOI] [PubMed] [Google Scholar]
- 21.Yang P, Kanki H, Drolet B, Yang T, Wei J, Viswanathan PC, Hohnloser SH, Shimizu W, Schwartz PJ, Stanton M, Murray KT, Norris K, George AL, Jr, Roden DM. Allelic variants in long-QT disease genes in patients with drug-associated torsades de pointes. Circulation. 2002;105:1943–8. doi: 10.1161/01.cir.0000014448.19052.4c. [DOI] [PubMed] [Google Scholar]


