Abstract
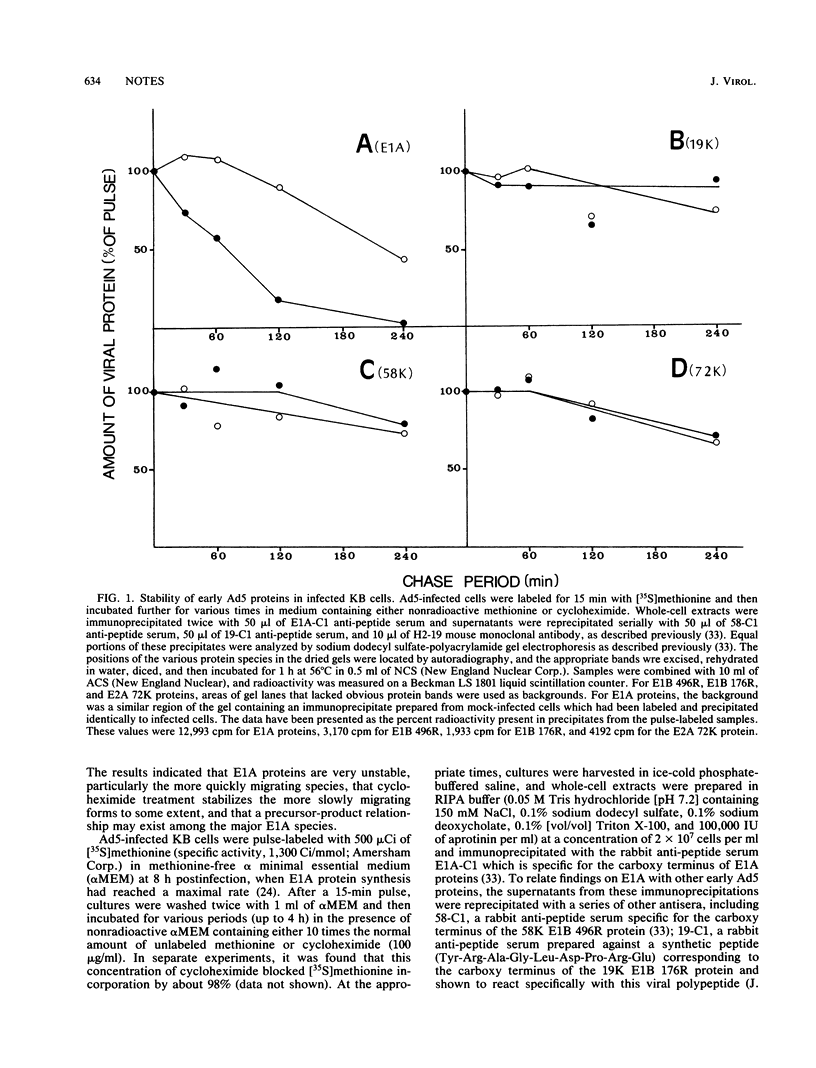
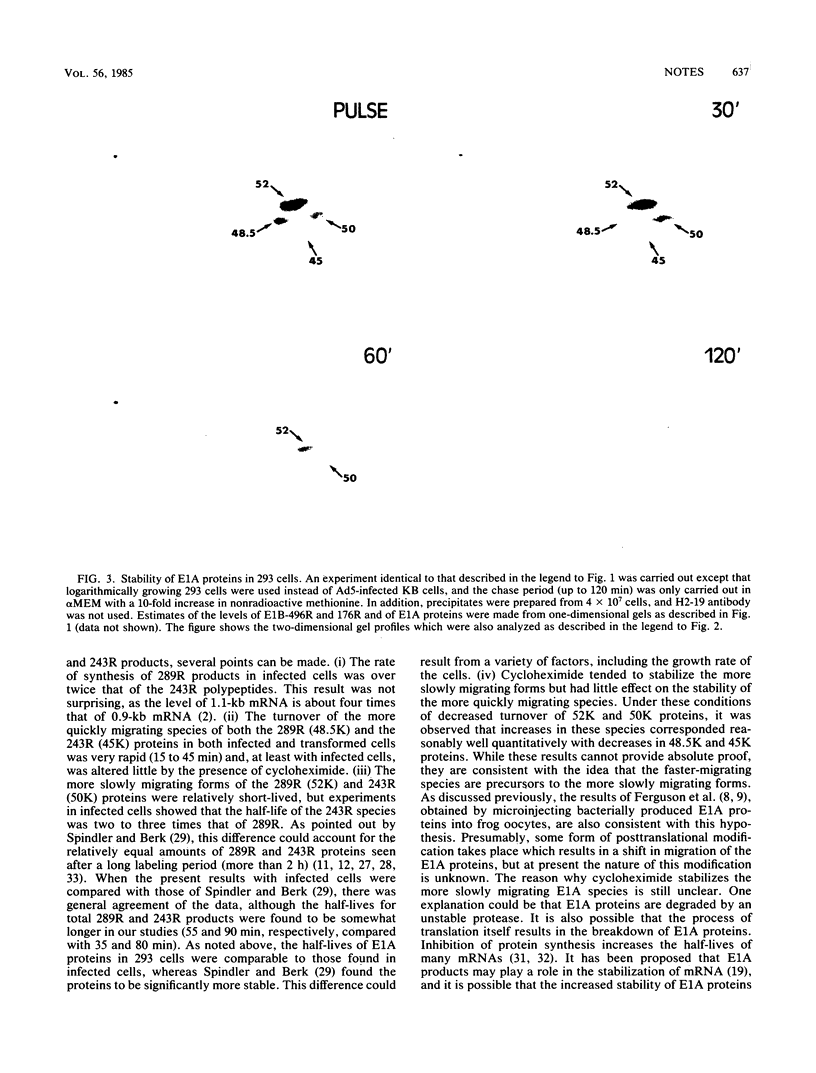
Early region 1A (E1A) of human adenovirus type 5 (Ad5) produces two mRNAs coding for phosphoproteins of 289 and 243 residues (289R and 243R). Each of these products has been shown to migrate on sodium dodecyl sulfate gels as two major and two minor species. In the present study, the stabilities of E1A polypeptides, as well as those of some other early Ad5 proteins, were studied in infected KB cells that were pulse-labeled with [35S]methionine and then chased in the presence or absence of cycloheximide. The E1B 58,000- and 19,000-molecular-weight proteins (58K and 19K proteins; 496R and 176R) as well as the E2A 72K DNA-binding protein were relatively stable over the 4-h chase period; turnover was less than 30%. The E1A species were considerably more unstable, with an overall half-life of about 60 min. Interestingly, it was found that when cycloheximide was present during the chase, E1A proteins were much more stable, and the half-life increased to about 240 min. Analysis of the stabilities of individual E1A species indicated that the products of the 1.1-kilobase mRNA (289R) had half lives (about 55 min) somewhat shorter than those (about 90 min) of the 0.9-kilobase mRNA products (243R). In addition, the faster-migrating species produced from each mRNA (molecular weights, 48,500 and 45,000) had significantly shorter half-lives than did the slower-migrating species (52,000 and 50,000). In the presence of cycloheximide, the faster-migrating species were still quite short-lived, but the half-lives of the 52K and 50K species were considerably increased. An examination of the kinetics of turnover of the various E1A species suggested that the faster-migrating forms may be precursors to the slower-migrating ones. Somewhat similar stabilities were also found for the various E1A species in Ad5-transformed 293 cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Structure of the adenovirus 2 early mRNAs. Cell. 1978 Jul;14(3):695–711. doi: 10.1016/0092-8674(78)90252-0. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Hen R., Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984 Dec 13;312(5995):608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- Dijkema R., Dekker B. M., van der Feltz M. J., van der Eb A. J. Transformation of primary rat kidney cells by DNA fragments of weakly oncogenic adenoviruses. J Virol. 1979 Dec;32(3):943–950. doi: 10.1128/jvi.32.3.943-950.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey J. F., Evelegh C. M., Branton P. E., Bayley S. T. Peptide maps and N-terminal sequences of polypeptides from early region 1A of human adenovirus 5. J Virol. 1984 Apr;50(1):30–37. doi: 10.1128/jvi.50.1.30-37.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B., Jones N., Richter J., Rosenberg M. Adenovirus E1a gene product expressed at high levels in Escherichia coli is functional. Science. 1984 Jun 22;224(4655):1343–1346. doi: 10.1126/science.6374895. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Gaynor R. B., Berk A. J. Cis-acting induction of adenovirus transcription. Cell. 1983 Jul;33(3):683–693. doi: 10.1016/0092-8674(83)90011-9. [DOI] [PubMed] [Google Scholar]
- Gaynor R. B., Tsukamoto A., Montell C., Berk A. J. Enhanced expression of adenovirus transforming proteins. J Virol. 1982 Oct;44(1):276–285. doi: 10.1128/jvi.44.1.276-285.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Abrahams P. J., Mulder C., Heijneker H. L., Warnaar S. O., De Vries F. A., Fiers W., Van Der Eb A. J. Studies on in vitro transformation by DNA and DNA fragments of human adenoviruses and simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):637–650. doi: 10.1101/sqb.1974.039.01.077. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Harter M. L., Lewis J. B. Adenovirus type 2 early proteins synthesized in vitro and in vivo: identification in infected cells of the 38,000- to 50,000- molecular-weight protein encoded by the left end of the adenovirus type 2 genome. J Virol. 1978 Jun;26(3):736–749. doi: 10.1128/jvi.26.3.736-749.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houweling A., van den Elsen P. J., van der Eb A. J. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology. 1980 Sep;105(2):537–550. doi: 10.1016/0042-6822(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Katze M. G., Persson H., Philipson L. Control of adenovirus early gene expression: posttranscriptional control mediated by both viral and cellular gene products. Mol Cell Biol. 1981 Sep;1(9):807–813. doi: 10.1128/mcb.1.9.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J. L., Harter M. L. Plasmid-directed synthesis of genuine adenovirus 2 early-region 1A and 1B proteins in Escherichia coli. Mol Cell Biol. 1984 Aug;4(8):1427–1439. doi: 10.1128/mcb.4.8.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell. 1982 Jul;29(3):913–919. doi: 10.1016/0092-8674(82)90453-6. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Perricaudet M., Akusjärvi G., Virtanen A., Pettersson U. Structure of two spliced mRNAs from the transforming region of human subgroup C adenoviruses. Nature. 1979 Oct 25;281(5733):694–696. doi: 10.1038/281694a0. [DOI] [PubMed] [Google Scholar]
- Rowe D. T., Branton P. E., Graham F. L. The kinetics of synthesis of early viral proteins in KB cells infected with wild-type and transformation-defective host-range mutants of human adenovirus type 5. J Gen Virol. 1984 Mar;65(Pt 3):585–597. doi: 10.1099/0022-1317-65-3-585. [DOI] [PubMed] [Google Scholar]
- Rowe D. T., Branton P. E., Yee S. P., Bacchetti S., Graham F. L. Establishment and characterization of hamster cell lines transformed by restriction endonuclease fragments of adenovirus 5. J Virol. 1984 Jan;49(1):162–170. doi: 10.1128/jvi.49.1.162-170.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. T., Graham F. L., Branton P. E. Intracellular localization of adenovirus type 5 tumor antigens in productively infected cells. Virology. 1983 Sep;129(2):456–468. doi: 10.1016/0042-6822(83)90183-6. [DOI] [PubMed] [Google Scholar]
- Rowe D. T., Yee S. P., Otis J., Graham F. L., Branton P. E. Characterization of human adenovirus type 5 early region 1A polypeptides using antitumor sera and an antiserum specific for the carboxy terminus. Virology. 1983 Jun;127(2):253–271. doi: 10.1016/0042-6822(83)90142-3. [DOI] [PubMed] [Google Scholar]
- Smart J. E., Lewis J. B., Mathews M. B., Harter M. L., Anderson C. W. Adenovirus type 2 early proteins: assignment of the early region 1A proteins synthesized in vivo and in vitro to specific mRNAs. Virology. 1981 Jul 30;112(2):703–713. doi: 10.1016/0042-6822(81)90315-9. [DOI] [PubMed] [Google Scholar]
- Spindler K. R., Berk A. J. Rapid intracellular turnover of adenovirus 5 early region 1A proteins. J Virol. 1984 Nov;52(2):706–710. doi: 10.1128/jvi.52.2.706-710.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R., Ziff E. B. HeLa cell beta-tubulin gene transcription is stimulated by adenovirus 5 in parallel with viral early genes by an E1a-dependent mechanism. Mol Cell Biol. 1984 Dec;4(12):2792–2801. doi: 10.1128/mcb.4.12.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. C., Nevins J. R., Blanchard J. M., Ginsberg H. S., Darnell J. E., Jr Metabolism of mRNA from the transforming region of adenovirus 2. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):447–455. doi: 10.1101/sqb.1980.044.01.048. [DOI] [PubMed] [Google Scholar]
- Wilson M. C., Sawicki S. G., Salditt-Georgieff M., Darnell J. E. Adenovirus type 2 mRNA in transformed cells: map positions and difference in transport time. J Virol. 1978 Jan;25(1):97–103. doi: 10.1128/jvi.25.1.97-103.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee S. P., Rowe D. T., Tremblay M. L., McDermott M., Branton P. E. Identification of human adenovirus early region 1 products by using antisera against synthetic peptides corresponding to the predicted carboxy termini. J Virol. 1983 Jun;46(3):1003–1013. doi: 10.1128/jvi.46.3.1003-1013.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]





