Abstract
AIM
To characterize the effects of lamotrigine on QT interval in healthy subjects.
METHODS
Healthy subjects received a single oral dose of moxifloxacin (400 mg) or placebo in crossover design, followed by a dose-escalating regimen of lamotrigine (n = 76) over a 77-day period, or matched placebo (n = 76). Blood samples were taken for determination of moxifloxacin and lamotrigine concentrations and digital 12-lead ECGs were recorded. The relationships between individual QT values and respective individual moxifloxacin or lamotrigine concentrations were explored using population pharmacokinetic–pharmacodynamic (PK–PD) modelling.
RESULTS
Moxifloxacin was associated with a maximum mean increase from baseline in QTcF of 14.81 ms [90% confidence interval (CI) 13.50, 16.11] 2.5 h after dosing. Steady-state exposure to lamotrigine (50, 150 or 200 mg b.d.) was not associated with an increase in QTc interval. Small reductions in QTcF (maximum mean difference from placebo −7.48 ms, 90% CI −10.49, −4.46) and small increases in heart rate (maximum mean difference from placebo 5.94 bpm, 90% CI 3.81, 8.06) were observed with lamotrigine 200 mg b.d. vs. placebo. No effect of lamotrigine on QRS duration or blood pressure was observed. No outliers with QTcF > 450 ms, or with an increase from baseline of >60 ms were observed in the lamotrigine group. PK–PD modelling indicated statistically significant decreases in individually corrected QT intervals for lamotrigine and statistically significant increases in individually corrected QT intervals for moxifloxacin over the concentration ranges studied.
CONCLUSIONS
Therapeutic doses of lamotrigine (50–200 mg b.d.) were not associated with QT prolongation in healthy subjects.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Drugs that inhibit the human cardiac delayed rectifier potassium current may lead to prolongation of the cardiac QT interval and are associated with a fatal, polymorphic, ventricular tachycardia known as torsades de pointes.
Lamotrigine is indicated in the treatment of epilepsy and the prevention of mood episodes in patients with bipolar disorder.
Lamotrigine inhibits the human cardiac delayed rectifier potassium current in vitro, and it has been hypothesized that QT prolongation may contribute to the risk of sudden unexpected death in epilepsy patients.
WHAT THIS STUDY ADDS
This is the first reported thorough QT/QTc study with lamotrigine conducted to International Conference on Harmonization guidelines.
The mean QTc interval was not prolonged by lamotrigine in healthy subjects, as assessed by the standard heart rate correction methods (Fridericia's and Bazett's).
The in vitro inhibition of the delayed rectifier potassium current does not translate into an effect on QT in man.
Keywords: clinical trial, healthy subject, lamotrigine, moxifloxacin, QT
Introduction
Lamotrigine is indicated in the treatment of epilepsy and the prevention of mood episodes in patients with bipolar disorder. Lamotrigine appears to stabilize neuronal membranes and thus block voltage-sensitive channels that inhibit the release of the excitatory amino acid neurotransmitters glutamate and aspartate. The effect of lamotrigine on the human cardiac delayed rectifier potassium current (IKr), which is involved in cardiac repolarization and coded by the human ether-a-gogo-related gene (hERG) [1], has been studied by Danielsson et al. [2]. Lamotrigine, in common with phenytoin, inhibited the IKr; the concentration (unbound) of lamotrigine at which 50% inhibition of the IKr was observed in vitro (IC50) was 229 µM or 58 645 ng ml−1 (compared with 240 µM or 65 832 ng ml−1 for phenytoin) [2, 3]. Drugs that inhibit the IKr may lead to prolongation of the cardiac QT interval and be proarrhythmic, as they are associated with a fatal, polymorphic, ventricular tachycardia, known as torsades de pointes [4].
Therapeutic concentrations of lamotrigine in epilepsy range from 10 to 60 µM (2561 to 15 366 ng ml−1) [5] and, assuming 55% protein binding of lamotrigine (Lamictal US Prescribing Information), unbound concentrations equate to 4.5–27 µM (1152.5–6914.7 ng ml−1). The ratio of the IC50 value in the hERG to unbound plasma lamotrigine concentration at the higher end of the therapeutic range is approximately 8.5, giving a reasonable safety margin. In vitro studies of lamotrigine have demonstrated no evidence of prolongation of the cardiac action potential in dog Purkinje fibres. In fact, dose-dependent reductions in action potential duration, similar to those produced by phenytoin, were observed [GlaxoSmithKline (GSK) data on file]. There is also evidence that lamotrigine causes small but consistent reductions in the rate of spontaneous contraction of isolated cardiac tissue and slows the rate of membrane depolarization of guinea pig ventricular myocytes (GSK data on file). Intravenous bolus doses of up to 10 and 20 mg kg−1 lamotrigine in rat and cynomolgous monkey in vivo cardiovascular studies were associated with small decreases in blood pressure and heart rate, but did not identify QT prolongation (GSK data on file).
People with epilepsy have a higher risk of sudden unexpected death (SUDEP), which can be 20–40 times more prevalent compared with the healthy population [6–8]. Although the exact cause of death often remains unclear, as SUDEP is usually unwitnessed, most deaths seem to be seizure-related [9–11]. However, multiple factors including cardiac arrhythmias may contribute to SUDEP [9]. QTc prolongation has been observed on simultaneously recorded ECG during brief periods of epileptiform activity [12]. Given the effect of lamotrigine on the IKr, the question has been raised as to whether lamotrigine could contribute to SUDEP by prolonging the QT interval, leading to cardiac arrhythmias [13].
A study of mortality in epilepsy, based on pooled data from applications submitted to the US Food and Drug Administration for four antiepileptic drugs approved during the 1990s, suggests that the incidence of sudden death falls in the range of 3.4–4.3 per 1000 person-years [14]. The rate of SUDEP in patients exposed to lamotrigine during clinical trials (3.5 per 1000 patient-years of exposure) was found to be similar to the expected rate of SUDEP in young adults with severe epilepsy [15] and similar (eight per 1000 patient-years) to that observed with gabapentin [16]. No conclusive data are reported in the literature to suggest that any specific antiepileptic drug is more implicated than others in sudden death. The available data appear only to suggest rates similar to those recorded for lamotrigine [12, 17, 18].
Although preclinical, clinical and post-marketing data have not shown any evidence of an association between exposure to lamotrigine and QT prolongation, given the potential risk of SUDEP within the population generally exposed to lamotrigine it was considered important to characterize its effects on QTc in accordance with current regulatory guidelines [International Conference on Harmonization (ICH) E14 guideline [19]].
Methods
Subjects
The study population consisted of 152 healthy, nonsmoking, male and female subjects aged 18–55 years, with a body mass index in the range 18.5–29.9 kg m−2. Eligible subjects had normal ECG, vital signs and laboratory tests, had no clinically significant medical history and were taking no medication that would interfere with the procedures or compromise subject safety. All subjects provided written informed consent prior to any study-specific procedures being undertaken. The study was approved by the local ethics committee (Ravenscourt Ethics Committee, London, UK) and the Medicines and Healthcare products Regulatory Authority and was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki.
Study design
This was a single-centre, five-session, sequential-treatment, parallel-group study. Following a screening examination, eligible subjects first participated in a randomized, two-period, single-blind, crossover comparison of moxifloxacin and placebo during which they received a single oral dose of moxifloxacin 400 mg (Avelox®; Bayer, Leverkeusen, Germany) and a non-identical placebo (supplied by GlaxoSmithKline) in random order with a 7-day wash-out period between doses. Pharmacokinetic and ECG profiling was performed over a 24-h period after each dose of study medication, during which subjects were resident in the clinical pharmacology unit. Following a further 7-day wash-out period, the same subjects were randomized (1 : 1) into the double-blind, parallel-group phase of the study. Subjects randomized to group 1 received increasing doses of lamotrigine (Lamictal®; GlaxoSmithKline) from 25 mg day−1 to 200 mg b.d., from day 1 to day 77, followed by a brief down-titration period from day 78 to day 85. Subjects randomized to group 2 received matched placebo (also supplied by GlaxoSmithKline) on each corresponding study day. Subjects were dosed largely on an outpatient basis during this phase, but were resident from before dosing until 12 h postdose on each dose escalation day (days 15, 29, 43, 50) as well as on day 42 (50 mg b.d.) and from predose on day 63 until 12 h postdose on day 77 (other doses). Because of the slow up-titration required to achieve therapeutic plasma concentrations of lamotrigine, the comparison of QTc on lamotrigine vs. placebo was achieved using a parallel group design. The duration of the study precluded a crossover design for these treatments. Moxifloxacin was compared with placebo using a crossover design, because single doses could be used.
Electrocardiogram assessments and QTc evaluation
A profile of 12-lead ECG recordings was collected using digital format GE Marquette 12-Lead Digital Recorder equipment (GE Marquette, Milwaukee, WI, USA) for 24 h after dosing with moxifloxacin/placebo and for 12 h after dosing with lamotrigine/placebo on day 42 (50 mg b.d.), day 63 (150 mg b.d.) and day 77 (200 mg b.d.). ECG recordings were taken at three time points within 1 h before dosing to form the baseline, then at 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10 and 12 h postdose, plus at 24 h for the moxifloxacin/placebo single doses. To account for intrinsic variability, triplicate recordings were taken 1 min apart at each of the assessment time points. All ECGs were recorded after the subject had been resting in the supine position for at least 15 min. On ECG assessment days, dosing was conducted in the fasting state (at least 10 h) and subjects continued fasting for 4 h after dosing. Food and fluid intake were strictly controlled and hot and cold drinks and food were avoided 30 min before each scheduled ECG recording.
The digital ECG recordings were transmitted electronically to a specified ECG core laboratory for computer-based, manually verified, digital calliper measurement of conduction intervals (RR, PR, QRS and QT) using the tangent method [20, 21]. Where possible, measurement of intervals for each session was performed on three consecutive ECG complexes from a single lead, preferably lead II. Where possible, the same lead was used throughout the study for a given subject. All ECGs for a given subject were read by the same person.
Sample size considerations
With 50 evaluable subjects in each treatment group (lamotrigine and placebo) and a between-subject standard deviation (SD) of 10 ms (from GlaxoSmithKline's previous experience), it was estimated that the half-width of the 90% confidence interval (CI) for the difference between lamotrigine and placebo in mean QTcF at each time point would have been approximately 3.3 ms. The moxifloxacin comparison with placebo was a within-subject comparison. Based on a within-subject SD of 10 ms and 100 evaluable subjects, it was estimated that the half-width of the 90% CI for the difference between moxifloxacin and placebo would have been approximately 2.4 ms.
Data analysis and statistical methods
Manually read QT interval values were corrected using Fridericia's and Bazett's corrections to obtain QTcF and QTcB. For each subject, at each time point, QTc interval values were then calculated as the average of triplicate measures. The primary end-point was prospectively defined as QTcF, with QTcB being a secondary end-point. Scatter plots of manually read, uncorrected QT, QTcF and QTcB against RR were produced to check the efficiency of each of the correction factors in correcting for heart rate.
QTcF and QTcB were analysed using repeat measures analysis of covariance (ancova) separately for day 42 (50 mg b.d.), day 63 (150 mg b.d.) and day 77 (200 mg b.d.). The ancova model included the covariates regimen, time point and regimen × time point as fixed effect terms and pretreatment baseline, gender, pretreatment baseline × time and subject as random effect terms. Point estimates and corresponding 90% CI were calculated for the difference between each dose of lamotrigine and the corresponding placebo at each time point. The same type of analysis was used to determine the difference between moxifloxacin and placebo taking into account the crossover design. All statistical analyses were performed using SAS 8.2 software (SAS Institute, Cary, NC, USA).
Categorical analyses were performed to summarize the number of subjects per regimen who had a maximum increase from baseline in manually read QTcF of ≤30, >30 and >60 ms. This was undertaken using the average of the replicate ECGs at each postdose time point. Individual subjects who had a maximum QTcF value ≤450, >450 and >500 ms were also summarized for each regimen.
Pharmacokinetic assessments
Pharmacokinetic samples were taken to quantify the steady-state lamotrigine and single-dose moxifloxacin concentrations during ECG assessments and to investigate any relationship between concentration and QT for lamotrigine and moxifloxacin.
Blood samples (approximately 2 ml) were taken predose and 0.25, 0.5, 1, 2, 3, 4, 6, 8, 10 and 12 h after dosing with lamotrigine on day 42 (50 mg b.d.), day 63 (150 mg b.d.) and day 77 (200 mg b.d.), and predose and 0.25, 0.5, 0.75, 1, 1.25, 1.5, 2, 3, 4, 6, 12 and 24 h postdose for moxifloxacin. Serum was obtained by centrifugation in a refrigerated centrifuge and frozen for storage. Samples were analysed for moxifloxacin and lamotrigine concentrations using validated analytical methods based on protein precipitation, followed by liquid chromatography with tandem mass spectrometry analyses. The standard curve concentration range for lamotrigine from a 50-µl aliquot of human serum encompassed a lower limit of quantification (LLQ) of 4 ng ml−1 and higher limit of quantification (HLQ) of 4000 ng ml−1. The standard curve concentration range for moxifloxacin from a 50-µl aliquot of human serum encompassed an LLQ of 25 ng ml−1 and an HLQ of 5000 ng ml−1. For lamotrigine, the accuracy and intraday precision were 0.9%, %bias < 6.2% and percent coefficient of variation (CV) <5.7%. For moxifloxacin, the accuracy and intraday precision were −4.6%, %bias < 1.9% and %CV < 3.1%. Noncompartmental pharmacokinetic analyses were used to derive pharmacokinetic parameters including observed maximum plasma concentration (Cmax), time to observed Cmax (tmax) and area under the plasma concentration–time curve over a 24-h (AUC0–24) and a 12-h (AUC0–12) dosing period, for moxifloxacin and lamotrigine, respectively.
Pharmacokinetic/pharmacodynamic modelling
A population pharmacokinetic model was developed for moxifloxacin and lamotrigine in order to predict the drug concentration at the time of the QT measurements. Factors influencing QT interval were explored, including the RR interval and circadian rhythm of QT. The relationships between individual QT values and their respective individual predicted moxifloxacin or lamotrigine concentrations were explored, taking into account the covariates identified in the placebo analysis (shown below):
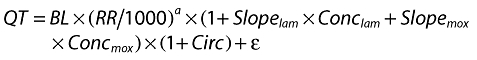 |
where QT is the QT interval, BL is the baseline QT, RR is the RR interval, α is the power exponent on RR interval in seconds, CIRC is a complex function that described the systematic variation in QT interval within the study day for an individual subject (i), and ε is the residual error.
Safety assessments
All adverse events (AEs) were recorded from the first dose of study medication until the follow-up visit. Vital signs and clinical laboratory tests were monitored at intervals during the study, and any clinically relevant abnormalities in these measures were to be reported as AEs.
Results
Subject demographics and disposition
One hundred and fifty-two subjects were enrolled into the study. Seventy-six subjects (33 female and 43 male) with a mean age of 27.1 years were randomized to receive lamotrigine (group 1) and 76 subjects (22 female and 54 male) with a mean age of 26.9 years were randomized to receive placebo (group 2). Most subjects were of White/Caucasian/European heritage (72% in group 1 and 76% in group 2). No subject took concomitant medication that could have confounded the interpretation of the pharmacokinetic or ECG data. The mean baseline ECG interval data were similar between the two groups. During the course of the study, 26 subjects (34%) from group 1 and 19 subjects (25%) from group 2 discontinued treatment. The primary reasons for withdrawal were AEs and the subject's decision (Table 1).
Table 1.
Subject disposition
| Moxifloxacin single-dose | Placebo single-dose | Lamotrigine day 1–day 85 | Placebo day 1–day 85 | |
|---|---|---|---|---|
| Number of subjects entered | 143 | 147 | 62 | 70 |
| Number of subjects completed | 134 (94%) | 137 (93%) | 50 (81%) | 57 (80%) |
| Number of subjects withdrawn | 9 (6%) | 10 (7%) | 13 (21%) | 13 (18%) |
| Reasons for withdrawal: | ||||
| Adverse event | 2 (1%) | 1 (<1%) | 7 (11%) | 2 (3%) |
| Protocol violation | 0 | 0 | 1 (2%) | 2 (3%) |
| Subject's decision | 6 (4%) | 9 (6%) | 4 (6%) | 7 (10%) |
| Other | 1 (<1%) | 0 | 1 (2%) | 2 (3%) |
Descriptive and statistical analysis of QT interval data was comprised of 149 subjects with data from at least one session for moxifloxacin/placebo, 114 subjects for day 42 (50 mg lamotrigine), 109 subjects for day 63 (150 mg lamotrigine) and 106 subjects for day 77 (200 mg lamotrigine).
QT data
Scatter plots of QT vs. RR interval clearly demonstrated an increase in QT with increasing RR interval, demonstrating the need to correct QT for heart rate (Figure 1a). A scatter plot of QTcF vs. RR interval showed a random scatter of data points, suggesting that Fridericia's formula adequately corrected the data for heart rate (Figure 1b). In contrast, a scatter plot of QTcB vs. RR interval showed slightly overcorrected QT values at lower RR intervals and slightly undercorrected QT values at higher RR intervals, indicating that Bazett's formula did not adequately correct for heart rate (Figure 1c).
Figure 1.
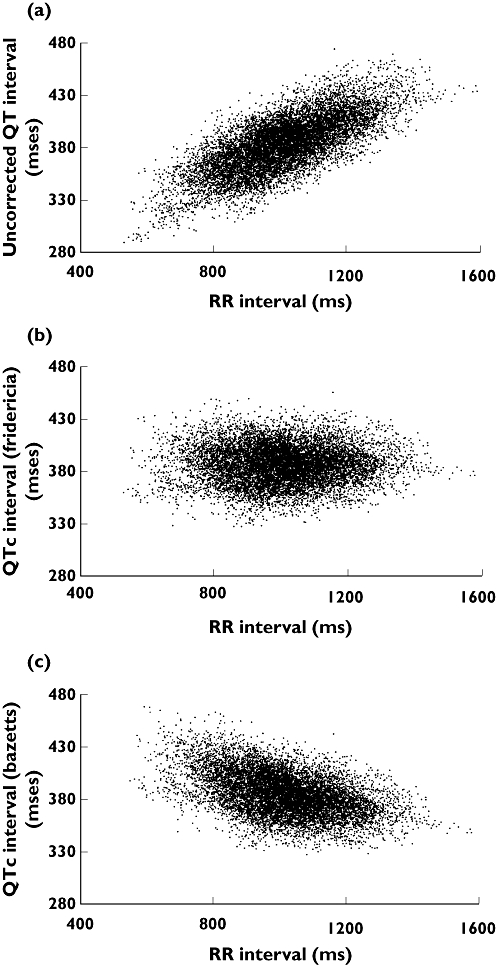
Scatter plots of (a) uncorrected QT, (b) QTcF and (c) QTcB vs. the RR interval
Effect of moxifloxacin on QTc
Mean QTcF was significantly prolonged in subjects receiving moxifloxacin compared with placebo (Table 2, Figure 2). From 0.5 h onwards, the point estimate of the difference from placebo in mean QTcF ranged from 6.00 to 14.81 ms. The largest difference in QTcF for moxifloxacin compared with placebo was observed at 2.5 h postdose (mean 14.81 ms, 90% CI 13.50, 16.11).
Table 2.
Point estimates and 90% confidence intervals (CI) for the adjusted mean difference in QTcF (lamotrigine 200 mg b.d. vs. placebo and single dose of moxifloxacin 400 mg vs. single-dose placebo)
| Time point (h) | Lamotrigine 50 mg b.d. vs. placebo (day 42) (ms) | Lamotrigine 100 mg b.d. vs. placebo (day 63) (ms) | Lamotrigine 200 mg b.d. vs. placebo (day 77) (ms) | Single-dose moxifloxacin 400 mg vs. single-dose placebo (ms) | ||||
|---|---|---|---|---|---|---|---|---|
| Point estimate | 90% CI | Point estimate | 90% CI | Point estimate | 90% CI | Point estimate | 90% CI | |
| 0.25 | −5.86 | −8.76, −2.96 | −3.74 | −6.47, −1.00 | −7.48 | −10.49, −4.46 | 0.45 | −0.86, 1.75 |
| 0.5 | −5.55 | −8.44, −2.66 | −4.26 | −6.99, −1.52 | −6.64 | −9.65, −3.62 | 6.00 | 4.69, 7.31 |
| 1 | −6.76 | −9.66, −3.86 | −3.79 | −6.53, −1.06 | −5.31 | −8.33, −2.30 | 10.86 | 9.55, 12.17 |
| 1.5 | −4.97 | −7.86, −2.08 | −3.11 | −5.84, −0.37 | −6.77 | −9.79, −3.76 | 12.07 | 10.76, 13.37 |
| 2 | −5.61 | −8.50, −2.72 | −3.31 | −6.04, −0.57 | −6.85 | −9.87, −3.84 | 12.56 | 11.25, 13.87 |
| 2.5 | −6.16 | −9.06, −3.27 | −2.63 | −5.37, 0.10 | −5.60 | −8.62, −2.59 | 14.81 | 13.50, 16.11 |
| 3 | −4.20 | −7.10, −1.31 | −2.65 | −5.39, 0.08 | −4.55 | −7.56, −1.54 | 12.74 | 11.43, 14.04 |
| 4 | −4.79 | −7.68, −1.90 | −4.41 | −7.15, −1.68 | −6.38 | −9.39, −3.36 | 14.09 | 12.78, 15.40 |
| 6 | −3.44 | −6.33, −0.5 | −0.73 | −3.47, 2.01 | −6.00 | −9.01, −2.98 | 11.34 | 10.03, 12.65 |
| 8 | −1.50 | −4.39, 1.39 | −0.45 | −3.19, 2.29 | −2.81 | −5.82, 0.20 | 9.53 | 8.23, 10.84 |
| 10 | −2.67 | −5.56, 0.22 | −2.54 | −5.28, 0.19 | −4.86 | −7.88, −1.85 | 11.31 | 10.00, 12.62 |
| 12 | −4.35 | −7.24, −1.46 | −3.50 | −6.24, −0.77 | −4.32 | −7.34, −1.31 | 8.81 | 7.50, 10.12 |
| 24 | – | – | – | 7.07 | 5.77, 8.38 | |||
Figure 2.
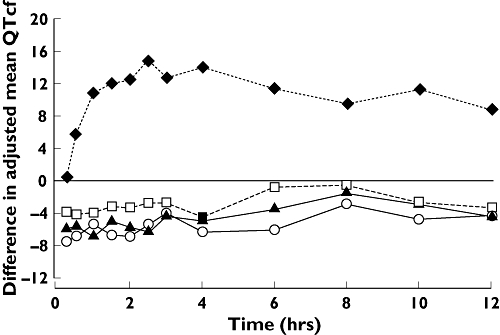
Difference from placebo in adjusted mean QTcF by time. Lamotrigine 50 mg bd vs. Placebo (□), Lamotrigine 150 mg bd vs. Placebo (▴), Lamotrigine 200 mg bd vs. Placebo (○), Moxifloxzcin SD vs. Placebo SD (♦)
Effect of lamotrigine on QTc
No prolongation of QTcF interval at steady-state lamotrigine 50, 150 or 200 mg b.d. compared with placebo was observed at any time point. Lamotrigine was, in fact, associated with a small reduction in QTcF relative to placebo at all doses and across all time points tested (Figure 2). The largest mean decrease was −7.48 ms after the 200-mg b.d. dose of lamotrigine (Table 2). Analysis of the QTcB interval confirmed that of the QTcF.
Categorical analysis
No subject at any time in the study had a QTcF exceeding 500 ms (Table 3). One female subject had a QTcF of 454.1 ms following a single 400-mg dose of moxifloxacin (representing an increase of +36 ms from her mean baseline value of 417.8 ms), whereas no subjects had a QTcF exceeding 450 ms in the lamotrigine group. Twenty-three subjects had an increase from baseline in QTcF which exceeded 30 ms following a single 400-mg dose of moxifloxacin (16%) compared with one subject on placebo (<1%). During the dosing periods with lamotrigine or placebo, only one or two subjects per dose group had an increase in QTcF exceeding 30 ms (Table 3). No subject had an increase from baseline of >60 ms.
Table 3.
Summary of categorical analysis of outliers (n %) as absolute values of QTcF and increase from baseline in QTcF on lamotrigine, moxifloxacin and their respective placebos
| n (%) | QTcF value (ms) | Increase from baseline in QTcF (ms) | ||||
|---|---|---|---|---|---|---|
| ≤450 | >450 | >500 | ≤30 | >30 | >60 | |
| Moxifloxacin 400 mg single dose | 140 (>99) | 1 (<1) | 0 | 118 (84) | 23 (16) | 0 |
| Placebo single dose | 145 (100) | 0 | 0 | 144 (>99) | 1 (<1) | 0 |
| Lamotrigine 50 mg b.d. | 54 (100) | 0 | 0 | 53 (98) | 1 (2) | 0 |
| Placebo | 60 (100) | 0 | 0 | 59 (98) | 1 (2) | 0 |
| Lamotrigine 150 mg b.d. | 51 (100) | 0 | 0 | 49 (96) | 2 (4) | 0 |
| Placebo | 58 (100) | 0 | 0 | 57 (98) | 1 (2) | 0 |
| Lamotrigine 200 mg b.d. | 49 (100) | 0 | 0 | 48 (98) | 1 (2) | 0 |
| Placebo | 57 (100) | 0 | 0 | 55 (96) | 2 (4) | 0 |
QRS, heart rate and blood pressure
No trends in mean QRS duration were observed with any treatment (Figure 3).
Figure 3.
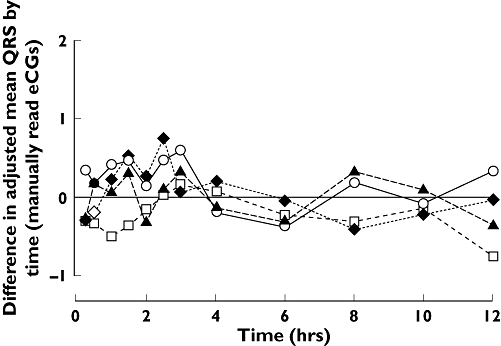
Difference from placebo in adjusted mean QRS by time. Lamotrigine 50 mg bd vs. Placebo (□), Lamotrigine 150 mg bd vs. Placebo (▴), Lamotrigine 200 mg bd vs. Placebo (○), Moxifloxacin SD vs. Placebo SD (♦)
Lamotrigine treatment was associated with a small, dose-related increase in heart rate compared with placebo (Figure 4). The largest mean difference compared with placebo was 5.94 bpm (90% CI 3.81, 8.46) observed with the 200-mg b.d. lamotrigine dose. Plots of standing and supine blood pressure vs. time for each treatment did not reveal any consistent changes that would indicate a drug effect.
Figure 4.
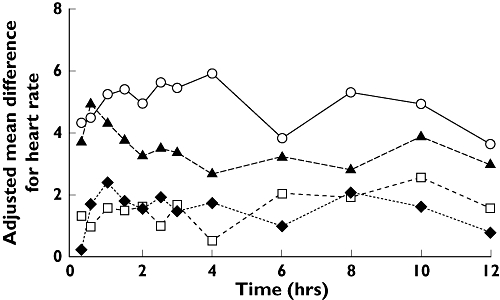
Difference from placebo in adjusted mean heart rate by time. Lamotrigine 50 mg bd vs. Placebo (□), Lamotrigine 150 mg bd vs. Placebo (▴), Lamotrigine 200 mg bd vs. Placebo (○), Moxifloxzcin SD vs. Placebo SD (♦)
Pharmacokinetics of lamotrigine
Steady-state was achieved following multiple daily doses of 50, 150 and 200 mg b.d. lamotrigine on the days when QTcF was determined, and the geometric means of the AUC0–12 and Cmax values indicated an approximately dose-proportional increase in the bioavailability of lamotrigine over the dose range 50–200 mg b.d. (Table 4).
Table 4.
Summary of pharmacokinetic parameters for lamotrigine and moxifloxacin
| Treatment | n | Geometric mean (% coefficient of variation) | Median (range) | |
|---|---|---|---|---|
| AUC0–12 (µg h−1 ml−1) | Cmax (µg ml−1) | tmax (h) | ||
| Lamotrigine 50 mg b.d. | 54 | 25.7 (40.7) | 2.59 (38.3) | 1.12 (0, 4.12) |
| Lamotrigine 150 mg b.d. | 52 | 69.6 (27.4) | 7.22 (24.7) | 1.08 (0.33, 3.08) |
| Lamotrigine 200 mg b.d. | 51 | 93.0 (23.5) | 9.61 (20.5) | 1.58 (0.33, 4.08) |
| Moxifloxacin 400 mg | 142 | 24 100* (18.5) | 2280 (26.0) | 1.60 (0.35, 6.10) |
AUC0–24 (ng h−1 ml−1).
Relationship between QT interval and moxifloxacin or lamotrigine concentrations
The population pharmacokinetic/pharmacodynamic model showed that there was a statistically significant decrease in individually corrected QT interval over the range of serum concentrations of lamotrigine studied. The slope predicted a reduction in QTc of 1.01 ms per 1000 ng ml−1 in men (Figure 5a) and of 1.05 ms per 1000 ng ml−1 in women. This corresponded to a decrease in QT of approximately 14.9 ms at the upper end of the concentration range studied (0–14 200 ng ml−1). Over the concentration range of moxifloxacin studied (0–3842 ng ml−1), the slope predicted a QTc prolongation of 6.1 ms per 1000 ng ml−1 in men (Figure 5b) and of 6.3 ms per 1000 ng ml−1 in women, corresponding to a 24.2-ms increase in QTcF at the upper end of the moxifloxacin concentration range. For QTci values calculated by NONMEM, the median RR exponent was similar to Fridericia's correction (0.255 vs. 0.33) and was associated with a 17% CV intersubject variability.
Figure 5.
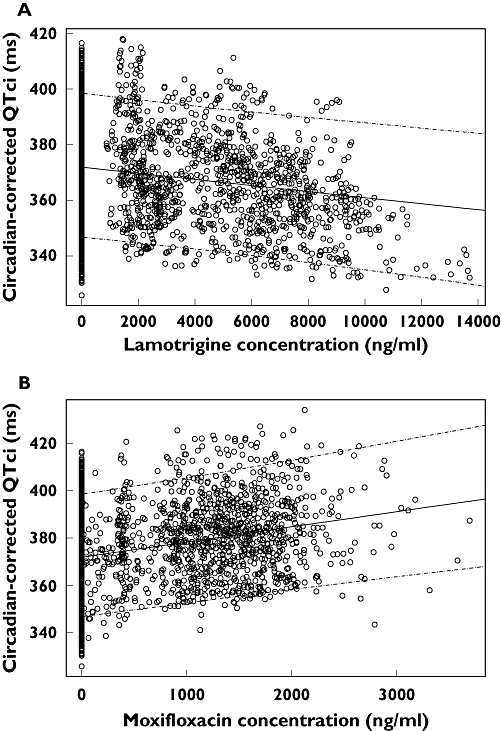
Scatter plot of circadian-corrected individual heart rate corrected QT interval (QTci) vs. plasma concentration for lamotrigine (a) and moxifloxacin (b) in male subjects. The solid lines represent the population medians and the dashed lines the 5th and 95th percentiles of predicted QTci vs. concentration relationship. Similar results were obtained in female subjects
Safety and tolerability results
Lamotrigine was well tolerated in the healthy population using this slow up-titration regimen. The most frequent drug-related AEs (reported by >5% of subjects) were headache, dizziness, nausea, abdominal pain, diarrhoea, insomnia and acne. The AEs reported by subjects receiving lamotrigine were generally rated as mild or moderate in intensity. The overall incidence of AEs was slightly higher with lamotrigine at 200 mg b.d. than with the lower doses or placebo.
Twelve subjects were withdrawn from the study because of AEs: two subjects after single-dose moxifloxacin (both with moderate events of rash), one after single-dose placebo (arrhythmia of Mobitz Type I second degree, atrioventricular block), two from the placebo repeat dosing period [pulmonary tuberculosis (the only serious AE reported during the study) and mild rash] and seven during the lamotrigine dosing period. Of the seven withdrawals during lamotrigine dosing, four were due to drug-related AEs: two cases of rash, one subject with four AEs of severe intensity (abdominal pain, collapse, dyspnoea and sweating) occurring on day 77 of dosing with lamotrigine 200 mg b.d., and one case of a laboratory abnormality. A male subject presented during lamotrigine treatment with elevated levels of alanine aminotransferase and aspartate aminotransferase to approximately four and three times, respectively, the upper limit of the laboratory normal range. Levels subsequently resolved and were not accompanied by rises in bilirubin or alkaline phosphatase. The subject had preceding symptoms of a viral infection and serological evidence suggested that Epstein–Barr virus early antigen antibody (IgM) was weakly positive.
Discussion
From ICH-E14 guidance a negative ‘thorough QT/QTc study’ would be achieved where the largest time-matched mean difference between the drug and placebo for QTcF was not greater than approximately 5 ms, with CI that excluded an effect of ≥10 ms. In this study investigating therapeutic dose levels of lamotrigine, the mean QTc interval was not prolonged using the standard heart rate correction methods (Fridericia's or Bazett's), since the upper 90% CIs of the estimated differences in adjusted means between lamotrigine and placebo were all <10 ms. In fact, lamotrigine was associated with small decreases, compared with placebo, in mean QTcF at all doses. Although lamotrigine appeared to cause small mean increases in heart rate, this did not affect the analysis of QTcF, because Fridericia's formula corrected the QT interval adequately for heart rate. Bazett's formula did not adequately correct for heart rate, so the results from this analysis are confounded and of lesser value. Because of the long duration of the study, the withdrawal rate was relatively high. However, sufficient subjects completed the study to achieve the objectives. Although men and women were not represented equally in the study population, the statistical analysis adjusted for gender.
No outliers were observed in the lamotrigine-treated group in terms of subjects with QTcF > 450 ms or an increase from baseline in QTcF > 60 ms. One or two subjects had increases in QTcF from baseline between 30 and 60 ms, but the numbers on lamotrigine and placebo were very similar. These findings provide evidence that lamotrigine at doses up to 200 mg b.d. is not associated with QT prolongation.
A limitation of this study was that the highest approved lamotrigine dose of 500 mg day−1 for monotherapy in epilepsy, or supratherapeutic doses were not evaluated because of tolerability concerns in healthy subjects. However, lamotrigine in overdose does not seem to be associated with QT prolongation [22–25]. There may also be other factors that contribute to the development of torsades de pointes in vivo, such as hypokalaemia, hypomagnesaemia and organic heart disease [26], which cannot be mimicked in a healthy subject study.
Lamotrigine was associated with a small reduction in QTcF, which is consistent with the effects of lamotrigine on action potential duration previously observed in the Purkinje fibre assay (data on file). In addition to its dose-dependent reduction in action potential duration, lamotrigine also decreased the maximal rate of rise of the action potential. This parameter is proportional to sodium conductance and probably reflects the effects of lamotrigine on neuronal sodium channels within cardiac fibres, as slight sodium channel block tends to shorten action potential duration before affecting the rate of rise [27]. The clinical consequences of QT shortening are unclear. Very rare familial syndromes of short QT interval (QT typically <300 ms), associated with mutations in genes encoding three different cardiac potassium-ion channels, have been described in the past few years [28]. These syndromes may be associated with sudden death and ventricular and atrial fibrillation [29]. It is not known whether these syndromes predispose individuals with genetically shorter QTc to be vulnerable to the QTc shortening effects of lamotrigine. However, QT shortening associated with lamotrigine was of small magnitude and clinical experience (including a large postmarketing database) has not identified this as a significant clinical concern.
In accordance with ICH guidelines, moxifloxacin was included in the study as a positive control, and administration of a single 400-mg dose resulted in a clinically significant increase in QTcF. The highest mean difference in QTcF between moxifloxacin 400 mg and placebo was 14.8 ms (90% CI 13.5, 16.1), slightly greater than that reported in the literature in similar studies with the same dose of moxifloxacin dose (8–11 ms) [30, 31]. These findings confirm the sensitivity of this study for testing the existence of any QT effects of lamotrigine.
In conclusion, these data demonstrate that at doses typically used in a clinical setting, lamotrigine did not prolong the QTc interval in healthy subjects. Based on this study, in addition to clinical and postmarketing experience, it is considered that there is no compelling evidence of an association between development of QT interval prolongation and associated cardiac arrhythmias and lamotrigine treatment.
The authors thank Professor John Camm and John Finkle for their advice; Lesley Clements and the staff of Richmond Pharmacology for study conduct; Jackie Greene for preclinical information; and Cardiabase for the manual ECG reading.
REFERENCES
- 1.Hoffmann P, Warner B. Are hERG channel inhibition and QT interval prolongation all there is in drug-induced torsadogenesis? A review of emerging trends. J Pharmacol Toxicol Methods. 2006;53:87–105. doi: 10.1016/j.vascn.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Danielsson BR, Lansdell K, Patmore L, Tomson T. Effects of antiepileptic drugs lamotrigine, topiramate and gabapentin on hERG potassium currents. Epilepsy Res. 2005;63:17–25. doi: 10.1016/j.eplepsyres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Danielsson BR, Lansdell K, Patmore L, Tomson T. Phenytoin and phenobarbital inhibit human hERG potassium channels. Epilepsy Res. 2003;55:147–57. doi: 10.1016/s0920-1211(03)00119-0. [DOI] [PubMed] [Google Scholar]
- 4.Zünkler BJ. Human ether-a-go-go-related (HERG) gene and ATP-sensitive potassium channels as targets for adverse drug effects. Pharmacol Ther. 2006;112:12–37. doi: 10.1016/j.pharmthera.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Johannessen SI, Battino D, Berry DJ, Bialer M, Krämer G, Tomson T, Patsalos PN. Therapeutic drug monitoring of the newer antiepileptic drugs. Ther Drug Monit. 2003;25:347–63. doi: 10.1097/00007691-200306000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Nashef L, Sander JW. Sudden unexpected deaths in epilepsy – where are we now? Seizure. 1996;5:235–8. doi: 10.1016/s1059-1311(96)80042-2. [DOI] [PubMed] [Google Scholar]
- 7.Ficker DM, So EL, Shen WK, Annegers JF, O’Brien PC, Cascino GD, Belau PG. Population-based study of the incidence of sudden unexplained death in epilepsy. Neurology. 1998;51:1270–4. doi: 10.1212/wnl.51.5.1270. [DOI] [PubMed] [Google Scholar]
- 8.Annegers JF, Coan SP. SUDEP: overview of definitions and review of incidence data. Seizure. 1999;8:347–52. doi: 10.1053/seiz.1999.0306. [DOI] [PubMed] [Google Scholar]
- 9.Nashef L, Hindocha N, Makoff A. Risk factors in sudden death in epilepsy (SUDEP): the quest for mechanisms. Epilepsia. 2007;48:859–71. doi: 10.1111/j.1528-1167.2007.01082.x. [DOI] [PubMed] [Google Scholar]
- 10.Langan Y, Nashef L, Sander JW. Sudden unexpected death in epilepsy: a series of witnessed deaths. J Neurol Neurosurg Psychiatry. 2000;68:211–3. doi: 10.1136/jnnp.68.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langan Y, Nashef L, Sander JW. Case–control study of SUDEP. Neurology. 2005;64:1131–3. doi: 10.1212/01.WNL.0000156352.61328.CB. [DOI] [PubMed] [Google Scholar]
- 12.Tavernor SJ, Brown SW, Tavernor RME, Gifford C. Electrocardiograph QT lengthening associated with epileptiform EEG discharges – a role in sudden unexplained death in epilepsy? Seizure. 1996;5:79–83. doi: 10.1016/s1059-1311(96)80067-7. [DOI] [PubMed] [Google Scholar]
- 13.Aurlien D, Taubøll E, Gjerstad L. Lamotrigine in idiopathic epilepsy – increased risk of cardiac death? Acta Neurol Scand. 2007;115:199–203. doi: 10.1111/j.1600-0404.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 14.Racoosin JA, Feeney J, Burkhart G, Boehm G. Mortality in antiepileptic drug programs. Neurology. 2001;56:514–9. doi: 10.1212/wnl.56.4.514. [DOI] [PubMed] [Google Scholar]
- 15.Leestma JE, Annegers JF, Brodie MJ, Brown S, Schraeder P, Siscovick D, Wannamaker BB, Tennis PS, Cierpial MA, Earl NL. Sudden unexplained death in epilepsy: observations from a large clinical development program. Epilepsia. 1997;38:47–55. doi: 10.1111/j.1528-1157.1997.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 16.Wong IC, Mawer GE, Sander JW. Adverse event monitoring in lamotrigine patients: a pharmacoepidemiologic study in the United Kingdom. Epilepsia. 2001;42:237–44. doi: 10.1046/j.1528-1157.2001.254001.x. [DOI] [PubMed] [Google Scholar]
- 17.Walczak T. Do antiepileptic drugs play a role in sudden unexpected death in epilepsy? Drug Saf. 2003;26:673–83. doi: 10.2165/00002018-200326100-00001. [DOI] [PubMed] [Google Scholar]
- 18.Tomson T, Beghi E, Sundqvist A, Johannessen SI. Medical risks in epilepsy: a review with focus on physical injuries, mortality, traffic accidents and their prevention. Epilepsy Res. 2004;60:1–16. doi: 10.1016/j.eplepsyres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 19.The E14 Implementation Working Group. The clinical evaluation of QT/QTc interval, prolongation and proarrhythmic potential for non-antiarrhythmic drugs. [3 July 2008]. ICH Guidelines E14 Step 5; 2005. Available at http://www.ich.org/LOB/media/MEDIA1476.pdf.
- 20.Moss AJ. Measurement of the QT interval and the risk associated with QTc interval prolongation: a review. Am J Cardiol. 1993;72:23B–25B. doi: 10.1016/0002-9149(93)90036-c. [DOI] [PubMed] [Google Scholar]
- 21.Locati EH. QT interval duration and adaptation to heart rate. In: Zareba W, Maison Blanche P, Locati EH, editors. Non-Invasive Electrocardiography in Clinical Practice. Armonk, NY: Futura Publishing Company Inc; 2001. pp. 71–96. [Google Scholar]
- 22.Lofton AL, Klein-Schwartz W. Evaluation of lamotrigine toxicity reported to poison centers. Ann Pharmacother. 2004;38:1811–5. doi: 10.1345/aph.1E192. [DOI] [PubMed] [Google Scholar]
- 23.O'Donnell J, Bateman DN. Lamotrigine overdose in an adult. J Toxicol Clin Toxicol. 2000;38:659–60. doi: 10.1081/clt-100102017. [DOI] [PubMed] [Google Scholar]
- 24.Braga AJ, Chidley K. Self-poisoning with lamotrigine and pregabalin. Anaesthesia. 2007;62:524–7. doi: 10.1111/j.1365-2044.2006.04913.x. [DOI] [PubMed] [Google Scholar]
- 25.Buckley NA, Whyte IM, Dawson AH. Self-poisoning with lamotrigine. Lancet. 1993;342:1552–3. doi: 10.1016/s0140-6736(05)80120-5. [DOI] [PubMed] [Google Scholar]
- 26.Yap YG, Camm J. Risk of torsades de pointes with non-cardiac drugs. Doctors need to be aware that many drugs can cause qt prolongation. BMJ. 2000;320:1158–9. doi: 10.1136/bmj.320.7243.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coraboeuf E, Deroubaix E, Coulombe A. Effect of tetrodotoxin on action potentials of the conducting system in the dog heart. Am J Physiol. 1979;236:H561–7. doi: 10.1152/ajpheart.1979.236.4.H561. [DOI] [PubMed] [Google Scholar]
- 28.Gaita F, Giustetto C, Bianchi F, Wolpert C, Schimpf R, Riccardi R, Grossi S, Richiardi E, Borggrefe M. Short QT syndrome: a familial cause of sudden death. Circulation. 2003;108:965–70. doi: 10.1161/01.CIR.0000085071.28695.C4. [DOI] [PubMed] [Google Scholar]
- 29.Brugada R, Hong K, Cordeiro JM, Dumaine R. Short QT syndrome. CMAJ. 2005;173:1349–54. doi: 10.1503/cmaj.050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morganroth J, Ilson BE, Shaddinger BC, Dabiri GA, Patel BR, Boyle DA, Sethuraman VS, Montague TH. Evaluation of vardenafil and sildenafil on cardiac repolarization. Am J Cardiol. 2004;93:1378–83. doi: 10.1016/j.amjcard.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 31.Serra DB, Affrime MB, Bedigian MP, Greig G, Milosavljev S, Skerjanec A, Wang Y. QT and QTc interval with standard and supratherapeutic doses of darifenacin, a muscarinic M3 selective receptor antagonist for the treatment of overactive bladder. J Clin Pharmacol. 2005;45:1038–47. doi: 10.1177/0091270005279010. [DOI] [PubMed] [Google Scholar]


