Abstract
T box bacterial genes utilize a riboswitch mechanism to regulate gene expression at the transcriptional level. Complementary base pairing of the 5′-untranslated mRNA with uncharged cognate tRNA stabilizes formation of an antiterminator element and permits complete transcription. In the absence of tRNA, a mutually exclusive RNA terminator element forms and results in transcription termination. This regulatory mechanism requires divalent metal ion at the antitermination event The structural effects of Mg2+ binding to antiterminator model RNA were investigated to ascertain if this requirement is due to the presence of a specific metal ion binding site in the antiterminator. Spectroscopic analysis identified the presence of a hydrated, diffuse Mg2+ binding site The results indicate that the mechanistic requirement for divalent metal ion is not due to Mg2+-induced pre-formation of a functional antiterminator receptor; rather, Mg2+ binds in a helical region of high phylogenetic sequence conservation adjacent to the tRNA binding site.
Keywords: RNA, divalent metal ion, binding, T box, transcription, riboswitch
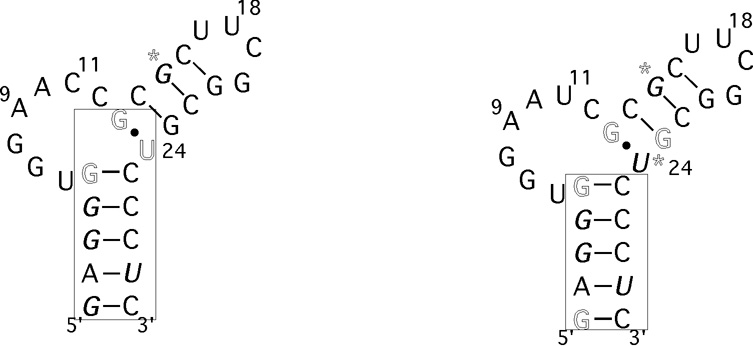
Bacterial regulation of gene expression utilizes a variety of mechanisms including RNA structural rearrangement [1]. A riboswitch mechanism is an example of this type of control where nascent mRNA directly senses metabolites and other effector molecules leading to RNA structural rearrangements which regulate transcription [2–4] The T box genes constitute an important class of riboswitches and over 700 examples have been identified to date [5–9]. These genes utilize a unique method to regulate the expression of aminoacyl-tRNA synthetase, amino acid biosynthesis, and amino acid transport genes by responding to the aminoacyl charging ratio of tRNA [3,10] Uncharged cognate tRNA is recognized by the 5′-untranslated mRNA (leader region) resulting in transcription antitermination [5] This RNA molecular switch can function in the absence of additional cofactors [11]. The leader region contains a complex set of RNA elements, conserved at the primary and secondary structural level, which recognize the tRNA by base pairing with the anticodon and the acceptor end nucleotides [3, 10]. Anticodon base pairing occurs with a specifier sequence located near the beginning of the leader region and provides the cognate specificity for the riboswitch [3] The acceptor end nucleotide base pairing occurs with a portion of the highly conserved T box sequence in the bulge of an antiterminator element and stabilizes its structure [7, 12] As a consequence, this precludes formation of the mutually exclusive, more thermodynamically stable, terminator element and results in transcription antitermination [13] At low levels of Mg2+ the uncharged cognate tRNA binds the leader region via the anticodon-specifier sequence base pairing, but no efficient transcription antitermination occurs until a threshold level of Mg2+ is reached [14] In addition, the requirement for higher concentrations of Mg2+ is specific to the antitermination event strongly implicating that the requirement is related to the event of tRNA binding the antiterminator element [15] In this study the structural effects of divalent metal ion probes on a functionally relevant [7, 16] antiterminator model RNA (AM1A) were determined and compared to effects on a reduced function [16, 17] model RNA (C11U) in order to ascertain whether a specific metal ion binding site is present in the antiterminator element. The characterization of the metal ion binding will contribute to a better mechanistic understanding of this biologically significant riboswitch.
Materials and methods
RNA Synthesis
All RNAs were transcribed in vitro using T7 RNA polymerase [18–20] RNAs were purified on 20% polyacrylamide gels (19:1 acrylamide/bisacrylamide) containing 7 M urea The RNA was isolated by electroelution and ethanol precipitation followed by dialysis An extensive dialysis procedure was utilized to ensure complete removal of trace metal ions prior to experimentation [21] RNA samples were initially dialyzed at 4 °C against 3 × 1 L of 5 mM EDTA pH 6.5 for 24 hours followed by 10–12 × 1 L of 5 mM sodium phosphate buffer, pH 6.5, .005 mM EDTA for 4–5 days with buffer changes occurring every 8–12 hours The dialyzed RNA was lyophilized following dialysis and resuspended in half the original volume resulting in a final buffer concentration of 10 mM sodium phosphate pH 6.5, 0.01 mM EDTA.
Circular Dichroism
CD spectra were acquired using a Jasco J700 spectropolarimeter coupled with a Peltier temperature regulator. RNA spectra were acquired and analyzed using standard methods [22]. Samples initially contained 200 µL of 10 µM RNA in 50 mM sodium phosphate buffer, pH 6.5, 0.01 mM EDTA, 200 mM NaCl and were thermally renatured prior to use followed by thermal equilibration in a 0.1 cm pathlength cell at 4 °C. Data were acquired over a spectral range of 200–320 nm with each spectra consisting of an average of three scans. After each addition of metal ion the RNA sample was re-equilibrated at 4 °C prior to acquisition Each spectrum was baseline corrected (relative to buffer) and the molar circular dichroism calculated based on the total sample concentration at each titration step using established methods [22].
Terbium(III) Footprinting
A stock solution (100 mM) of TbCl3 (Sigma-Aldrich) was prepared in 5 mM cacodylate buffer (pH 5.5) as previously described [23] Antiterminator model RNAs were 5′-32+P-end-labeled as previously described [16]. Footprinting reactions were conducted according to literature procedure [23] and contained labeled (2 pmol) and unlabeled (25 pmol) antiterminator model RNA in 10 µL of buffer with 15 mM MgCl2 and a range of TbCl3 (0–20 mM). Reaction mixtures were separated on a 20% denaturing polyacrylamide gel and the bands visualized by autoradiography. RNA treated with alkaline hydrolysis conditions or RNase T1 was also separated on the same gel to aid in band identification.
NMR
NMR samples (600 µL) contained 50 mM sodium phosphate pH 6.5, 0.01 mM EDTA, 200 mM NaCl with RNA (1 to 2 mM) in 90% H2O, 10% D2O with DSS as a reference. All samples were renatured prior to use and equilibrated at 4 °C for 10 min prior to acquisition. All NMR spectra were collected using a Bruker DRX 800 MHz spectrometer at 4 °C. For 1D spectra, the water peak was suppressed during acquisition using excitation sculpting [24] and spectra were acquired with 256 scans and a 15 ppm sweep width. For the 1D titration experiments, MgCl3 was added up to 15 mM at 1 mM increments; Co(NH3)6Cl3 was added to final concentrations of 0.01, 0.025, 0.05, 0.10, 0.25, 0.50, 1.0, 1.5, and 2.0 mM; MnCl2 was added to final concentrations of 0.05 and 0.1 mM since only micromolar amounts of Mn2+ are necessary to see the relaxation effects on proton resonances [25] and higher millimolar concentrations caused the RNA to precipitate (data not shown) Two dimensional H2O-NOESYs were acquired with excitation sculpting water suppression [24] and TPPI phase sensitive detection [26] in the standard NMR buffer containing 15 mM MgCl2+ or 2 mM Co(NH3)6Cl3 Spectra consisted of 512 increments of 128 scans and 300 ms mixing time or 256 increments of 96 scans and 500 ms mixing time and a 15 ppm sweep width in both dimensions.
Results and discussion
Divalent Metal Ion Probes
The binding site and structural effects of Mg2+ on T box antiterminator RNA were investigated using the Mg2+ RNA binding site probes Mn2+ [27, 28], Co(NH3)63+ [29, 30] and Tb3+ [29–32] All metal ion concentrations used were within typical literature ranges. In addition, all experiments were conducted in the presence of excess monovalent cation (200 mM NaCl) to ensure that any observed effects would be due to a specific requirement for divalent metal ion and not simply due to a non-specific electrostatic effect.
Circular Dichroism
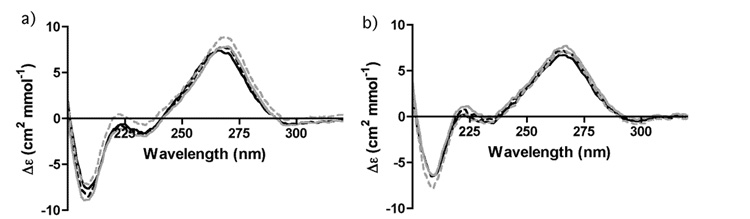
Only a slight change in the CD of AM1A was observed upon addition of Mg2+ up to a final concentration of 15 mM (Figure 1a) The peak at 266 nm, a region sensitive to base stacking differences [22], increased by 16% at 15 mM Mg2+. There were no spectral changes observed when Mg2+ was added to C11U (Figure 1b). In addition, no significant spectral differences were observed for either antiterminator RNA upon addition of Co(NH3)63+ (See Supplementary Material). The data indicate that a small increase in base stacking occurs with AM1A in the presence of Mg2+ and that there is no significant divalent metal ion-dependent structural reorganization occurring with either antiterminator model RNA.
Figure 1.
CD spectra of a) AM1A and b) C11U in the presence of 0 (black solid line), 5 (black dashed line), 10 (grey solid line) and 15 (grey dashed line) mM Mg2+ in 50 mM sodium phosphate buffer, pH 6.5, 0.01 mM EDTA, 200 mM NaCl.
NMR chemical shift differences
At the higher concentrations of monovalent ions used in this study, new imino proton peaks were observed in the NMR spectra (Supplemental Material). The newly observed imino protons all corresponded to an alternate structure (AM1A*) differing from AM1A in the region of helix A2 (J. Means, J. Hines, unpublished results) This is consistent with the NMR-derived solution structure of AM1A at lower salt concentrations where structural flexibility was observed in the bulge 5′ region and non-A-form character in helix A2 [33] The higher salt concentrations begin to stabilize one or more of these alternate conformations on the NMR time-scale. Analysis of the previous NMR structure led to the hypothesis that the partial flexibility of the antiterminator facilitated tRNA acceptor end recognition and binding [33]. Consequently, the NMR imino proton peaks associated with the alternate structure were also monitored to determine whether the alternate structure, AM1A*, interacted differently with divalent metal ions.
The imino proton chemical shifts of the antiterminator model RNAs were monitored upon addition of Mg2+ or Co(NH3)63+. In addition, the imino proton peak line broadening (relaxation) effects were monitored upon addition of Mn2+ (Figure 2 and Supplementary Material). The most significant chemical shift effects observed for AM1A were at U24 (maximum upfield shifts of 0.04 and 0.1 ppm for Mg2+ and Co(NH3)63+ respectively). An upfield shift was also observed for G13 and significant downfield shifts (0.04 ppm) were observed for G1, G3, G4, G15 and G28. No change was observed with the UUCG tetraloop, G21 and G23 imino protons (Δδ < 0.007 ppm, data not shown). For AM1A*, the Mg2+ and Co(NH3)63+ chemical shift differences were very small (Δδ < 0.01 ppm) and the general trends were the same as those observed with the major AM1A peaks (data not shown) The small magnitude of the chemical shift changes in AM1A indicate that there is no significant structural reorganization occurring in the presence of divalent metal ions. Additional NMR spectra indicate that the bulge structure does not change significantly in the presence of the Mg2+ (J. Means, J. Hines, unpublished results), indicating that there is not a specific divalent metal ion binding site located in the bulge. The localization of the majority of the AM1A shift differences within helix A1 and the hinge (U24) region along with the similar trends in shift differences for the hydrated Mg2+ probe, Co(NH3)63+, points to a diffuse binding site for hydrated Mg2+ along helix A1 that is coupled with a minor structural reorganization in the hinge region. This is consistent with the CD data that indicated only a slight increase in base stacking in the presence of Mg2+. No significant chemical shift change was observed at G3/G4 in AM1A beyond 12 mM Mg2+ (Supplementary Material) This value is similar to the range identified in in vitro antitermination experiments where the antitermination event required 12–15 mM Mg2+ for maximal efficiency [15].
Figure 2.
Summary of divalent metal ion probe effects on imino proton chemical shift and line broadening for antiterminator model RNA a) AM1A and b) C11U. Imino proton chemical shift effects for Mg2+ are indicated by bases in bold italic (downfield) or outline (upfield). Stars indicate sites where the upfield vs. downfield shift for the Co(NH3)63+ differed from that of Mg2+. Sites of specific Mn2+ line broadening effects on imino protons are indicated by a box.(See Supplementary Material for data).
The different effects seen with Co(NH3)63+ at G15 point to the possibility of an inner sphere Mg2+ binding site in this region, however, neither the Mn2+ nor the NOESY data support this possibility for AM1A (see below). The more likely explanation for the different effects seen at G15 is that Co(NH3)63+ has a different long-range induced structural effect than hydrated Mg2+ when bound along helix A1. This is consistent with the CD data that showed only Mg2+ led to changes in the stacking of AM1A while Co(NH3)63+ did not.
The Mg2+ and Co(NH3)63+ chemical shift effects for C11U (Figure 2b and Supplementary Material) followed the same trends as observed for AM1A with the exception of U24 (shifted downfield by Mg2+ and upfield by Co(NH3)63+) and G23 (shifted upfield by both probes). The most significant effects where still with imino protons in helix A1, but the effects in helix A2 were more pronounced than those observed with AM1A.
Mn2+relaxation
The most significant relaxation effects observed for AM1A were with the imino protons of G1, U28, G3, G4 and U24 (> 50% peak reduction at 0.05 mM Mn2+) In C11U, the significant effects were at the same positions with the exception that U24 was not as affected (<50% reduction at 0.05 mM Mn2+) In both AM1A and C11U no relaxation effects were observed for imino protons in either helix A2 or the UUCG tetraloop until higher concentrations of Mn2+ were present. Since the Mn2+ induced relaxation effects are distance dependent, these data indicate that Mn2+ is binding along helix A1 (G1–G5) in both model RNAs and only in close proximity to U24 in AM1A. This is consistent with the chemical shift difference data. In addition, the data indicate that the C11U mutation in the bulge region likely affects the bulge structure and hinge angle between the helices, thus changing the distance between U24 and helix A1. This is consistent with NMR and molecular modeling studies of C11U. The extensive stacking of the bases at the 3′ end of the bulge induces the kink between the helices in AM1A and is disrupted in C11U leading to a change in the bend angle between the helices ([33] and J. Means, M. Gerdeman, J. Hines unpublished results).
Co(NH3)63+ NOEs
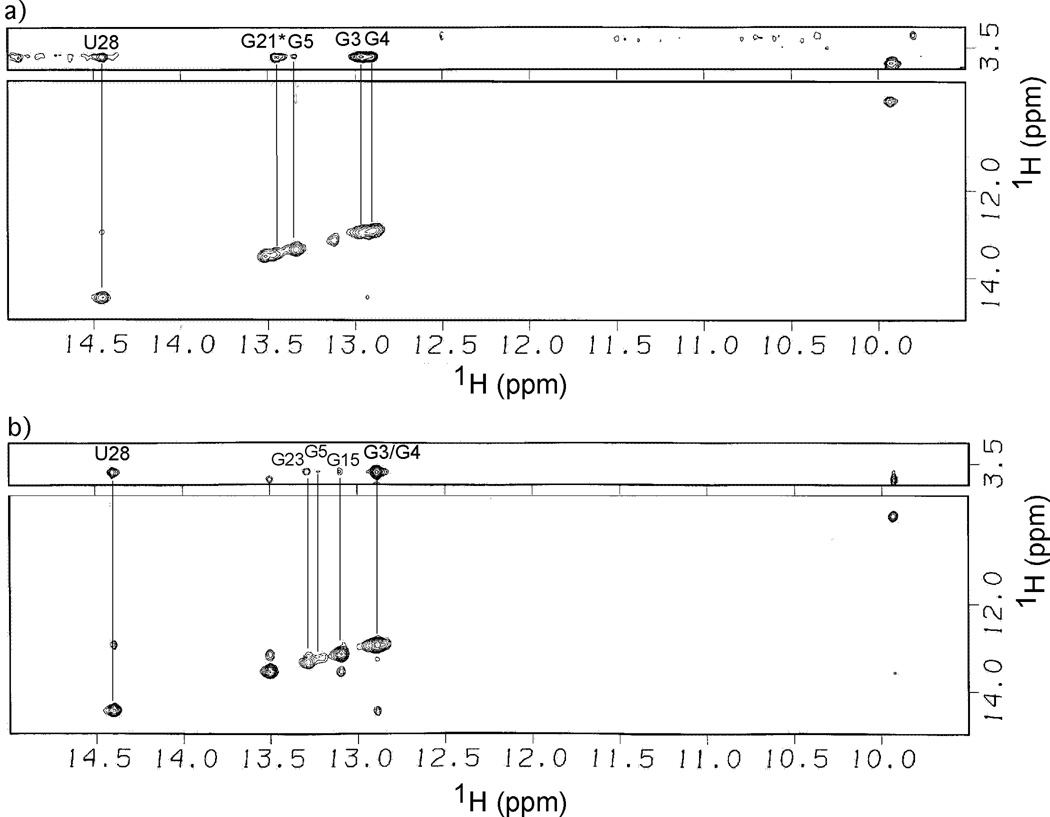
The NOESY data for AM1A and C11U in the presence of Co(NH3)63+ are consistent with the rest of the spectroscopic data (Figure 3). In AM1A, the largest NOE cross peaks to the Co(NH3)63+ NH3 protons where with the imino protons of U28, G3 and G4. An NOE was also present for G5 but was weaker. No conclusions could be drawn regarding U24, as it was not readily observed along the diagonal. An additional NOE to the NH3 protons was observed and tentatively assigned to G21* of AM1A* (the alternate structure observed at high concentrations of Na+, see above) but the peak was distorted leading to difficulties in making a definitive assignment (i.e., between G21* of AM1A* and G23 of AM1A) and subsequent interpretation. The NOESY data for AM1A support the interpretation that the multivalent metal ion binds along helix A1. The self-consistency between the Co(NH3)63+, Mg2+ and Mn2+ data indicate that the Mg2+ binds helix A1 as a hexahydrate via diffuse binding.
Figure 3.
H2O-NOESY (800 MHz) imino proton region of a) AM1A and b) C11U in the presence of Co(NH3)6Cl3 (2 mM). Lines indicate the intermolecular NOEs between Co(NH3)63+ protons with antiterminator imino protons. Spectra were obtained at 277 K with a 300 ms mixing time in 50 mM sodium phosphate buffer, pH 6.5, 0.01 mM EDTA, 200 mM NaCl.
The strongest NOEs to Co(NH3)63+ in C11U were with U28, G3 and G4 while the NOE for G5 was significantly weaker. The data indicate that Co(NH3)63+ binds along helix A1. Weak NOEs were also observed with G15 and G23. This is in contrast to AM1A and indicates that there is a weak association of Co(NH3)63+ in the middle of helix A2. However, the previously discussed mixed titration effects, lack of specific Mn2+ effects and the significantly weaker NOEs to Co(NH3)63+ in helix A2 are consistent indicators that there is no significant Mg2+ binding in this region compared to the affinity of the divalent metal ion binding site along helix A1.
Preferred residence site
Divalent magnesium is the premier metal ion involved in RNA structure formation and stability in both catalytic and noncatalytic molecules [34–37] Specific metal ion binding, in the classical sense, refers strictly to high affinity binding sites in particular positions. Specific metal ion binding in this type of site is typically required for the RNA molecule to fold into its tertiary structure and maintain its functional conformation [29, 38]. For this reason, the involvement of specific binding metal ions in the three-dimensional fold of RNA molecules dominates the literature, however, divalent metal ions are capable of binding at not only the tertiary level but also the secondary [39]. The Mg2+ binding site in helix A1 is likely an example of a preferred residence site previously observed with other G-rich, stacked regions [28] While Mg2+ binding does not structurally rearrange the antiterminator, as would be expected with a high affinity, specific metal ion binding site, the site is in a location where a divalent metal ion would help reduce electrostatic repulsion once the tRNA acceptor end binds.
Interestingly, the primary sequence in this region of helix A1 (5′ AGGG-3′) is highly conserved, but the reason for the high degree of phylogenetic conservation has not been identified [7] Some compensatory mutations are tolerated within this region, however, a compensatory mutation of the nucleotide corresponding to G5 in AM1A reduced antitermination efficiency in vivo threefold [7]. The presence of a Mg2+ binding site along helix A1 is one possible reason for a selective advantage that resulted in the high degree of sequence conservation in this region.
In this paper we reported on the divalent metal ion binding site of T box antiterminator model RNA. The results confirm that antiterminator model RNAs bind Mg2+ via diffuse, hydrated metal ion binding at the highly conserved stacked G residues in helix A1. In addition, no significant metal ion binding differences were observed between the functional and reduced function variant indicating that the reduced antitermination efficiency induced by the mutation corresponding to C11U is not due to differences in binding Mg2+. In conclusion, a divalent metal ion binding site was identified in a region of high phylogenetic sequence conservation along helix A1 of the T box antiterminator model RNA suggesting that Mg2+-induced facilitation of antiterminator function may have contributed to the sequence conservation in this region.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01-GM61048 and the Office of the Vice President for Research, Ohio University. We would like to thank Dr C. Cottrell at OSUCCIC for NMR assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grundy FJ, Henkin TM. From ribosome to riboswitch: control of gene expression in bacteria by RNA structural rearrangements. Critical Reviews in Biochemistry and Molecular Biology. 2006;41:329–338. doi: 10.1080/10409230600914294. [DOI] [PubMed] [Google Scholar]
- 2.Grundy FJ, Henkin TM. Regulation of gene expression by effectors that bind to RNA. Curr. Opin. Microbiol. 2004;7:126–131. doi: 10.1016/j.mib.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Henkin TM, Grundy FJ. Cold Spring Harbor Symp. Quant Biol. Vol. 71. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2006. pp. 1–7. [DOI] [PubMed] [Google Scholar]
- 4.Tucker BJ, Breaker RR. Riboswitches as versatile gene control elements. Curr. Op. Struct. Biol. 2005;15:342–348. doi: 10.1016/j.sbi.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Grundy FJ, Henkin TM. tRNA as a positive regulator of transcription antitermination in B. subtilis. Cell. 1993;74:475–482. doi: 10.1016/0092-8674(93)80049-k. [DOI] [PubMed] [Google Scholar]
- 6.Henkin TM. tRNA-directed transcription antitermination. Mol. Microbiol. 1994;13:381–387. doi: 10.1111/j.1365-2958.1994.tb00432.x. [DOI] [PubMed] [Google Scholar]
- 7.Grundy FJ, Moir TR, Haldeman MT, Henkin TM. Sequence requirements for terminators and antiterminators in the T box transcription antitermination system: disparity between conservation and functional requirements. Nucl. Acids Res. 2002;30:1646–1655. doi: 10.1093/nar/30.7.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panina EM, Vitreschak AG, Mironov AA, Gelfand MS. Regulation of biosynthesis and transport of aromatic amino acids in low-GC Gram-positive bacteria. FEMS Microbiol. Lett. 2003;222:211–220. doi: 10.1016/S0378-1097(03)00303-3. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths-Jones S, Moxon S, Marshall M, Khanna A, Eddy SR, Bateman A. Rfam: annotating non-coding RNAs in complete genomes. Nucl. Acids Res. 2005;33:D121–D124. doi: 10.1093/nar/gki081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grundy FJ, Henkin TM. The T box and S box transcription termination control systems. Front. Biosci. 2003;8:d20–d31. doi: 10.2741/908. [DOI] [PubMed] [Google Scholar]
- 11.Grundy FJ, Winkler WC, Henkin TM. tRNA-mediated transcription antitermination in vitro: codon-anticodon pairing independent of the ribosome. Proc. Natl. Acad. Sci. USA. 2002;99:11121–11126. doi: 10.1073/pnas.162366799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grundy FJ, Rollins SM, Henkin TM. Interaction between the acceptor end of tRNA and the T box stimulates antitermination in the Bacillus subtilis tyrS gene: a new role for the discriminator base. J. Bacteriol. 1994;176:4518–4526. doi: 10.1128/jb.176.15.4518-4526.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundy FJ, Hodil SE, Rollins SM, Henkin TM. Specificity of tRNA-mRNA interactions in Bacillus subtilis tyrS antitermination. J. Bacteriol. 1997;179:2587–2594. doi: 10.1128/jb.179.8.2587-2594.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grundy FJ, Yousef MR, Henkin TM. Monitoring uncharged tRNA during transcription of the Bacillus subtilis glyQS gene. J. Mol. Biol. 2005;346:73–81. doi: 10.1016/j.jmb.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 15.Yousef MR, Grundy FJ, Henkin TM. Structural transitions induced by the interaction between tRNAGly and the Bacillus subtilis glyQS T box leader RNA. J. Mol. Biol. 2005;349:278–287. doi: 10.1016/j.jmb.2005.03.061. [DOI] [PubMed] [Google Scholar]
- 16.Gerdeman MS, Henkin TM, Hines JV. In vitro structure-function studies of the Bacillus subtilis tyrS mRNA antiterminator: evidence for factor independent tRNA acceptor stem binding specificity. Nucl. Acids Res. 2002;30:1065–1072. doi: 10.1093/nar/30.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rollins SM, Grundy FJ, Henkin TM. Analysis of cis-acting sequence and structural elements required for antitermination of the Bacillus subtilis tyrS gene. Mol. Microbiol. 1997;25:411–421. doi: 10.1046/j.1365-2958.1997.4851839.x. [DOI] [PubMed] [Google Scholar]
- 18.Milligan JF, Uhlenbeck OC. Synthesis of small RNAs using T7 RNA polymerase. Methods Enzymol. 1989;180:51–62. doi: 10.1016/0076-6879(89)80091-6. [DOI] [PubMed] [Google Scholar]
- 19.Milligan JF, Groebe DR, Witherell GW, Uhlenbeck OC. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987;15:8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kao C, Zheng M, Rudisser S. A simple and efficient method to reduce nontemplated nucleotide addition at the 3' terminus of RNAs transcribed by T7 RNA polymerase. RNA. 1999;5:1268–1272. doi: 10.1017/s1355838299991033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puglisi JD, Tinoco I., Jr. Absorbance melting curves of RNA. Methods, Enzymol. 1989;180:304–325. doi: 10.1016/0076-6879(89)80108-9. [DOI] [PubMed] [Google Scholar]
- 22.Sosnick TR, Fang X, Shelton VM. Application of circular dichroism to study of RNA folding transitions. Methods in Enzymol. 2000;317:393–408. doi: 10.1016/s0076-6879(00)17026-0. [DOI] [PubMed] [Google Scholar]
- 23.Harris DA, Walter NG. Current Protocols in Nucleic Acid Chemistry. John Wiley and Sons, Inc; 2003. pp. 6.8.1–6.8.8. [DOI] [PubMed] [Google Scholar]
- 24.Hwang TL, Shaka AJ. Water suppression that works-excitation sculpting using arbitrary wave-forms and pulsed-field gradients. J. Magn. Reson., Ser. A. 1995;112:275–279. [Google Scholar]
- 25.Butcher SE, Allain FHT, Feigon J. Determination of metal ion binding sites wihtin the hairpin ribozyme domains by NMR. Biochemistry. 2000;39:2174–2182. doi: 10.1021/bi9923454. [DOI] [PubMed] [Google Scholar]
- 26.Marion D, Wüthrich K. Application of phase-sensitive two-dimensional correlated spectroscopy COSY for measurements of proton-proton spin-spin couplings in proteins. Biochem. Biophys. Res. Commun. 1983;113:967–974. doi: 10.1016/0006-291x(83)91093-8. [DOI] [PubMed] [Google Scholar]
- 27.Bock CW, Katz AK, Markham GD, Glusker JP. Manganese as a replacement for magnesium and zinc: Functional comparison of the divalent ions. J. Am. Chem. Soc. 1999;121:7360–7372. [Google Scholar]
- 28.Ott G, Arnold L, Limmer S. Proton NMR studies of manganese ion binding to tRNA-derived acceptor arm duplexes. Nucl. Acids Res. 1993;21:5859–5864. doi: 10.1093/nar/21.25.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tinoco I, Jr., Gonzalez RL. Solution structure and thermodynamics of a divalent metal ion binding site in an RNA pseudoknot. J. Mol. Biol. 1999;289:1267–1282. doi: 10.1006/jmbi.1999.2841. [DOI] [PubMed] [Google Scholar]
- 30.Kieft JS, Tinoco I., Jr Solution structure of a metal-binding site in the major groove of RNA complexed with cobalt(III)hexamine. Struct. 1997;5:713–721. doi: 10.1016/s0969-2126(97)00225-6. [DOI] [PubMed] [Google Scholar]
- 31.Saito H, Suga H. Outersphere and innersphere coordinated metal ions in an aminoacyl-tRNA synthetase ribozyme. Nucl. Acids Res. 2002;30:5151–5159. doi: 10.1093/nar/gkf641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walter N, Yang N, Burke J. Probing non-selective cation binding in the hairpin ribozyme with Tb(III) J. Mol. Biol. 2000;298:539–555. doi: 10.1006/jmbi.2000.3691. [DOI] [PubMed] [Google Scholar]
- 33.Gerdeman MS, Henkin TM, Hines JV. Solution structure of the B. subtilis T box antiterminator RNA: Seven-nucleotide bulge characterized by stacking and flexibility. J. Mol. Biol. 2003;326:189–201. doi: 10.1016/s0022-2836(02)01339-6. [DOI] [PubMed] [Google Scholar]
- 34.Misra VK, Draper DE. On the role of magnesium ions in RNA stability. Biopolymers. 1998;48:113–135. doi: 10.1002/(SICI)1097-0282(1998)48:2<113::AID-BIP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 35.Pyle AM. Role of metal ions in ribozymes. Met. Ions Biol. Syst. 1996;32:479–520. [PubMed] [Google Scholar]
- 36.Pyle AM. Metal ions in the structure and function of RNA. J. Biol. Inorg. Chem. 2002;7:679–690. doi: 10.1007/s00775-002-0387-6. [DOI] [PubMed] [Google Scholar]
- 37.Serra MJ, Baird JD, Dale T, Fey BL, Retatagos K, Westhof E. Effects of magnesium ions on the stabilization of RNA oligomers of defined structures. RNA. 2002;8:307–323. doi: 10.1017/s1355838202024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cate JH, Hanna RL, Doudna JA. A magnesium ion core at the heart of a ribozyme domain. Nat. Struct. Biol. 1997;4:553–558. doi: 10.1038/nsb0797-553. [DOI] [PubMed] [Google Scholar]
- 39.Tinoco I, Jr., Bustamante C. How RNA folds. J. Mol. Biol. 1999;293:271–281. doi: 10.1006/jmbi.1999.3001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.